Organic Chemistry: Exam 1 Review
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
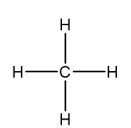
bond angle and name
tetrahedral, 107.5

bond angle and name
Linear, 180, no lone pairs on central atom
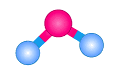
bond angle and name
Bent, 109.5, lone pairs on central atom
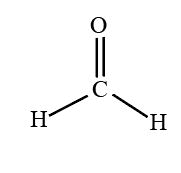
bond angle and name
trigonal planar, 120, no lone pair on central atom
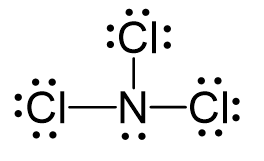
bond angle and name
trigonal pyramidial, 107.5, lone pair on central atom
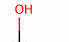
functional group name
alcohol
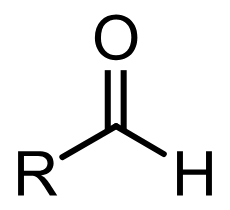
functional group name
aldehyde
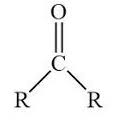
functional group name
ketone
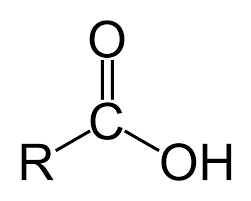
functional group name
carboxylic acid

functional group name
amine
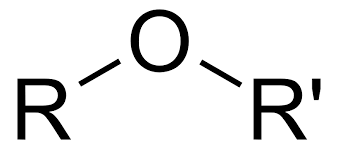
functional group name
ether
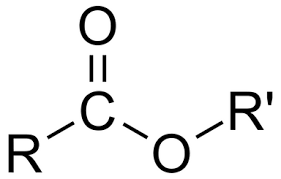
functional group name
ester
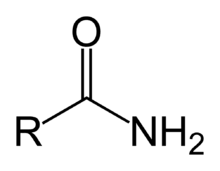
functional group name
amide
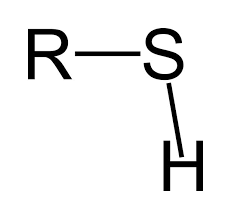
functional group name
thiol
what is a carbonyl, and which functional groups are this?
doule bonded oxygen; present in aldehydes, ketones, carboxylic acid, ester, amide functional groups
which 2 functional groups make an amino acid?
amine and carboxylic acid
london dispersion forces
weakest IMF, only for nonpolar molecules
dipole-dipole interaction
partial charges interacting, polar molecules, medium IMF
hydrogen bonding
H is bond to F, O, or N, polar molecules, strongest
11 carbons are called what
undecane
12 carbons are called what
dodecane
combustion reactions are composed of:
fuel + O2 —> CO2 + H2O and release energy
adding a halogen to an alkane:
CH4 + X2 —> CH3X + HX; reaction takes a hydrogen off