Chapter 1 Module 1 Condensed formulas and line notation
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
A condensed formula in chemistry represents a molecule’s chemical formula using the ____________ of the elements that make it up
Chemical symbols
A condensed formula is written in a ___________ without any subscripts. Basically, a shortened version of a structural formula that shows only the _____________________ in a molecule rather than their specific arrangement
Compact form; types and numbers of atoms
Example of condensed formula for propane is
CH3CH2CH3
Line notation or skeletal formula is a shorthand way of representing a chemical element or molecule using ______ and ____________ to represent atoms and their _________
Lines; symbols; bonds
In line notation, each vertex represents a ___________, while the hydrogen atoms are _______
Carbon atom; implied
The line notation for propane looks like this:
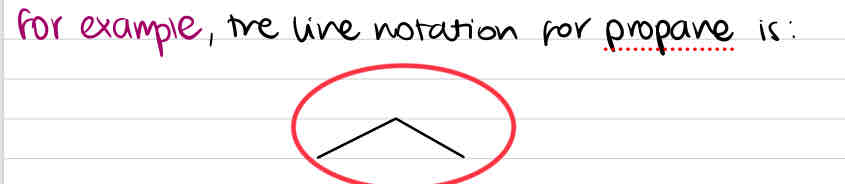
The zigzag line is used to indicate the _________ of the molecules and to signify that the molecule is either _______ or _____________, as __________ DO NOT have zigzags. Triple bonds have a ___________________
Geometry; single; double bond; triple bonds; 180 degree straight lines
Dashes or Wedges represent the bonds between atoms in a _________________. Dashes represent bonds _____________________ of the page. While Wedges represent bonds _________________________.
Three-dimensional structure; coming out of the plane; going into the page’s plane
Wedge comes _________ out of the plane of the page
Forwards/ Going into
Dashes goes _________ out of the plane of the page
Backwards/coming out
DAT tip: Any elements other than carbon and hydrogen are represented by their ____________
Chemical symbols