Chemistry Molecule Shapes
0.0(0)
0.0(0)
Card Sorting
1/24
Earn XP
Description and Tags
Last updated 5:17 PM on 1/24/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
1
New cards
EPN: 2
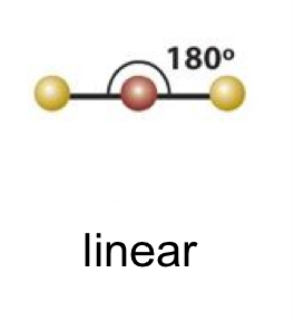
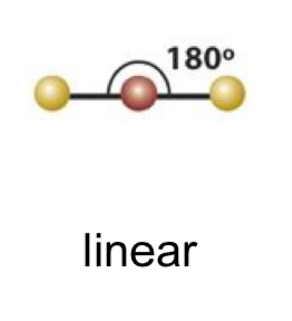
EPG: linear
2
New cards
EPN: 3
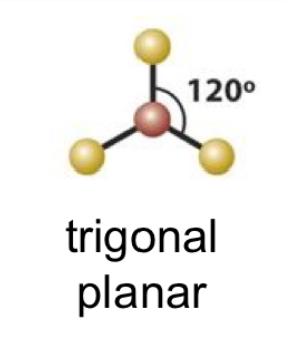
EPG: Trigonal Planar
3
New cards
EPN: 4
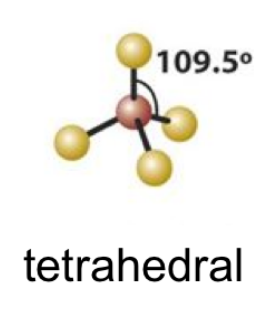
EPG: Tetrahedral
4
New cards
EPN: 5
EPG: Trigonal Bipyramidal
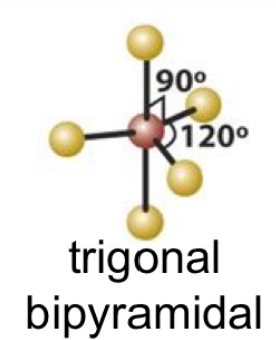
5
New cards
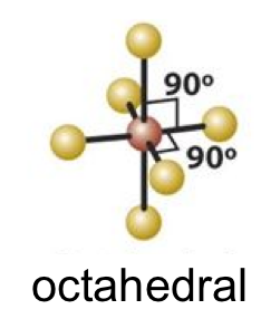
EPN: 6
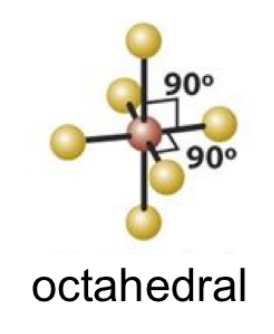
EPG: Octahedral
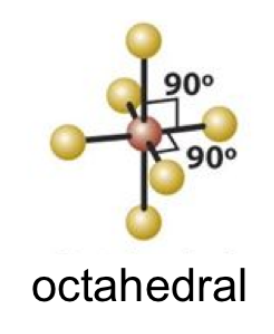
6
New cards
(2 bonds; 0 lone pairs)
linear 1
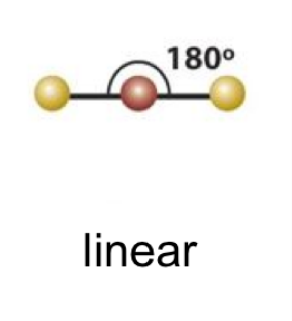
7
New cards
(3 bonds; 0 lone pairs)
trigonal planar
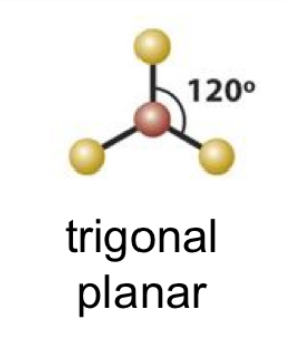
8
New cards
(2 bonds; 1 lone pair)
bent 1
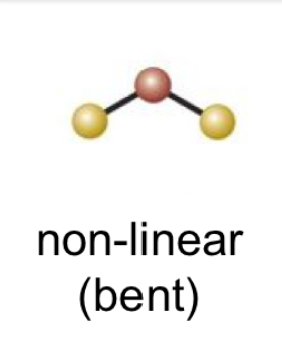
9
New cards
(4 bonds; 0 lone pairs)
tetraheadral
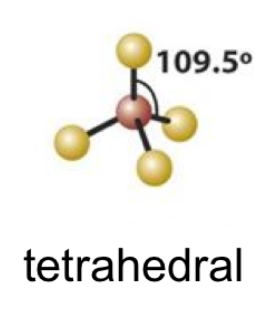
10
New cards
(3 bonds; 1 lone pair)
trigonal pyramidal
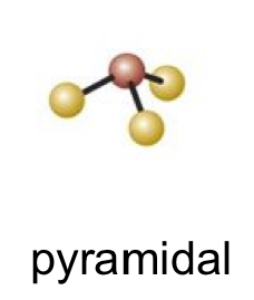
11
New cards
(2 bonds; 2 lone pairs)
bent 2
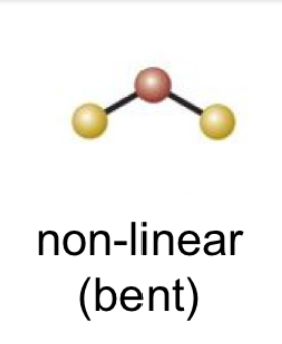
12
New cards
(5 bonds; 0 lone pairs)
trigonal bipyrimidal
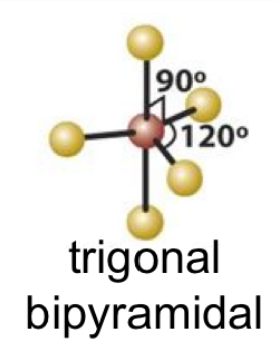
13
New cards
(4 bonds; 1 lone pair)
seesaw
14
New cards
( 3 bonds; 2 lone pairs)
t shaped
15
New cards
(2 bonds; 3 lone pairs)
linear 2
16
New cards
(6 bonds; 0 lone pairs)
octahedral
17
New cards
(5 bonds; 1 lone pair)
square pyramidal
18
New cards
(4 bonds; 2 lone pairs)
square planar
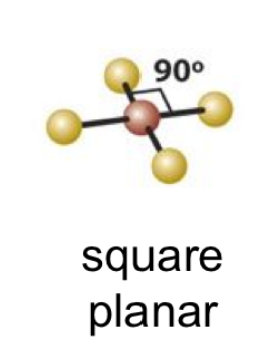
19
New cards
linear bond angle
180
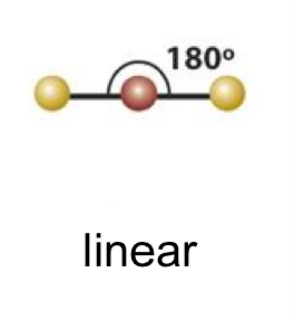
20
New cards
trigonal planar bond angle
120
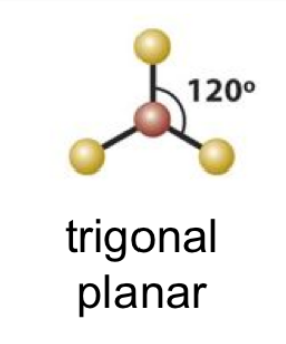
21
New cards
tetrahedral bond angle
109.5
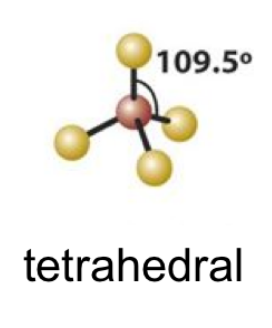
22
New cards
tigonal bipyrimadal and seesaw bond angles
90 and 120
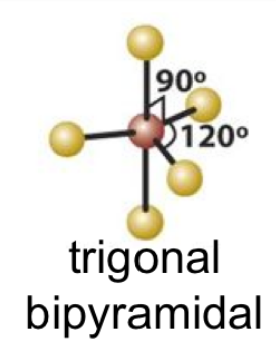
23
New cards
t shapes bond angles
90
24
New cards
octahedral bond angle
90
25
New cards
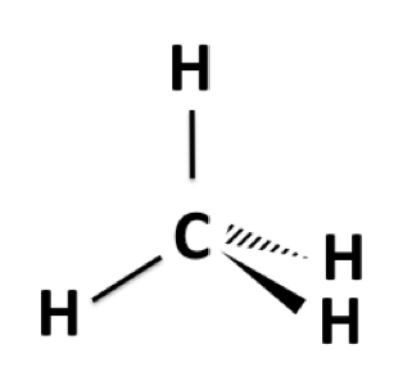
3D drawing