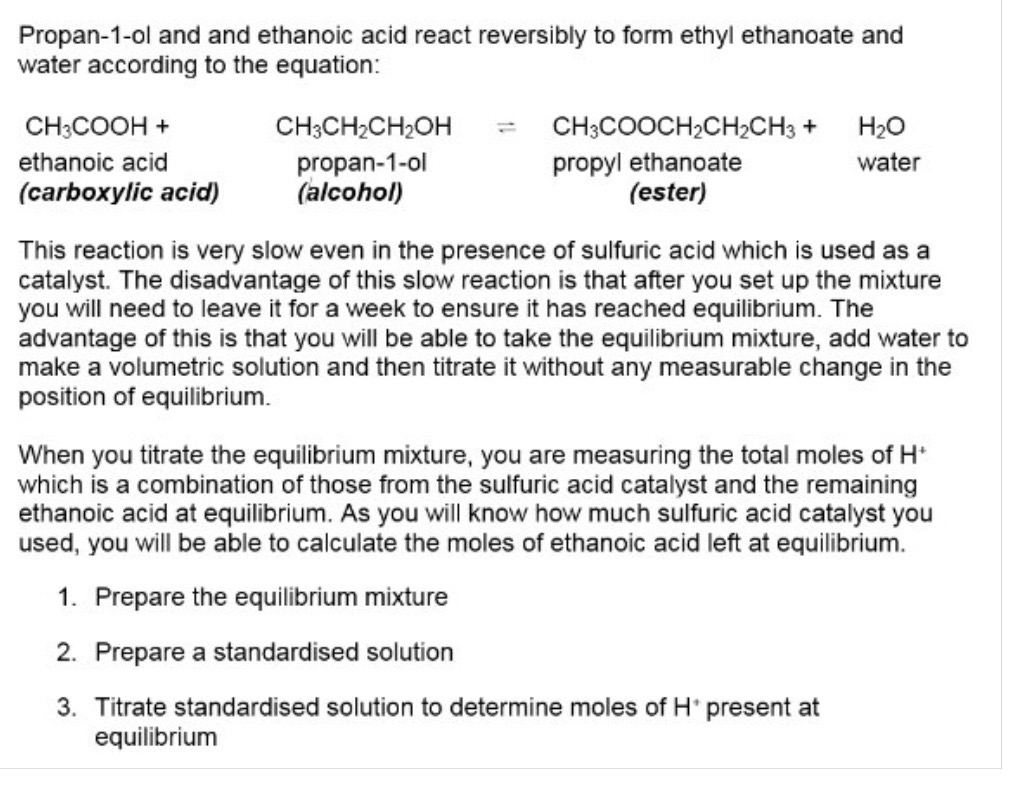
3.1.6.2 equilibrium constant, Kc, for homogeneous systems
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
19 Terms
For a homogeneous equilibrium reaction, we can write an expression for an equilibrium constant, Kc in terms of the concentration of reactants + products involved → what is Kc
A ratio of the concentration of the products to the reactants
What is homogeneous equilibrium?
A system in which the reactants + products are in the same phase
What is the formula for equilibrium constant Kc
Kc = [products] / [reactants]
*[] represents the equilibrium conc. in mol dm-³
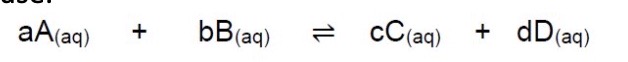
What would the general expression for Kc be for this equation?
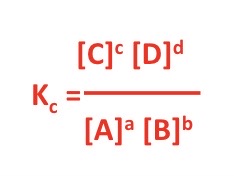
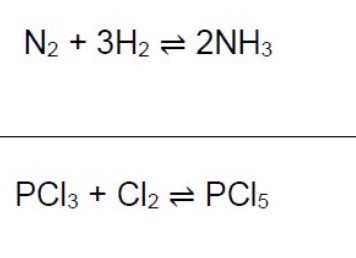
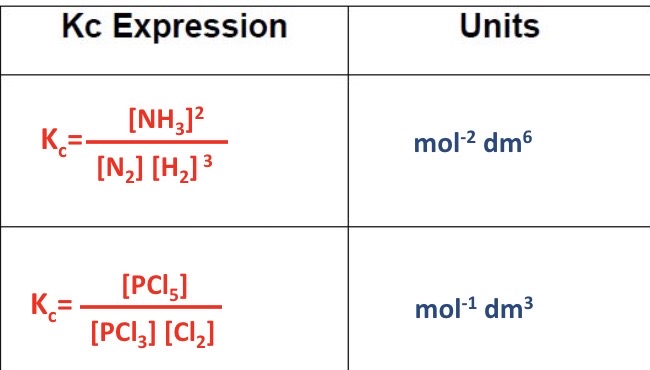
What would the Kc expression with units be for these 2 equations?

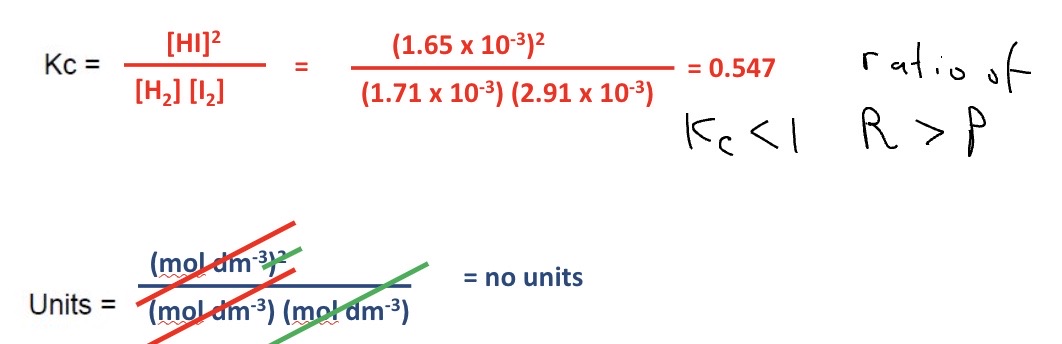
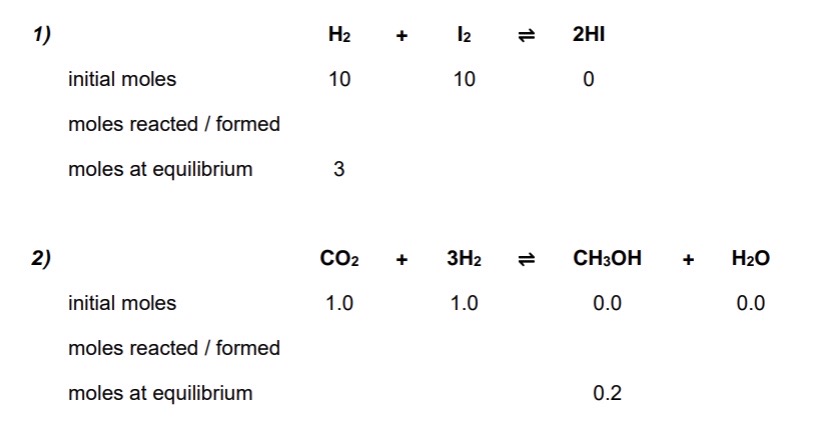
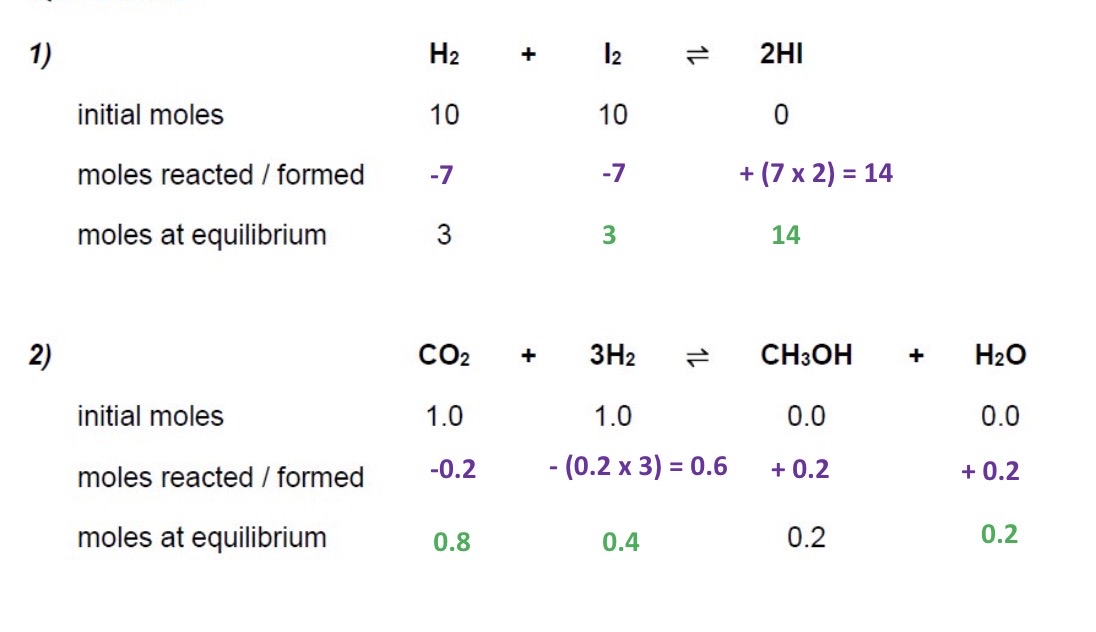
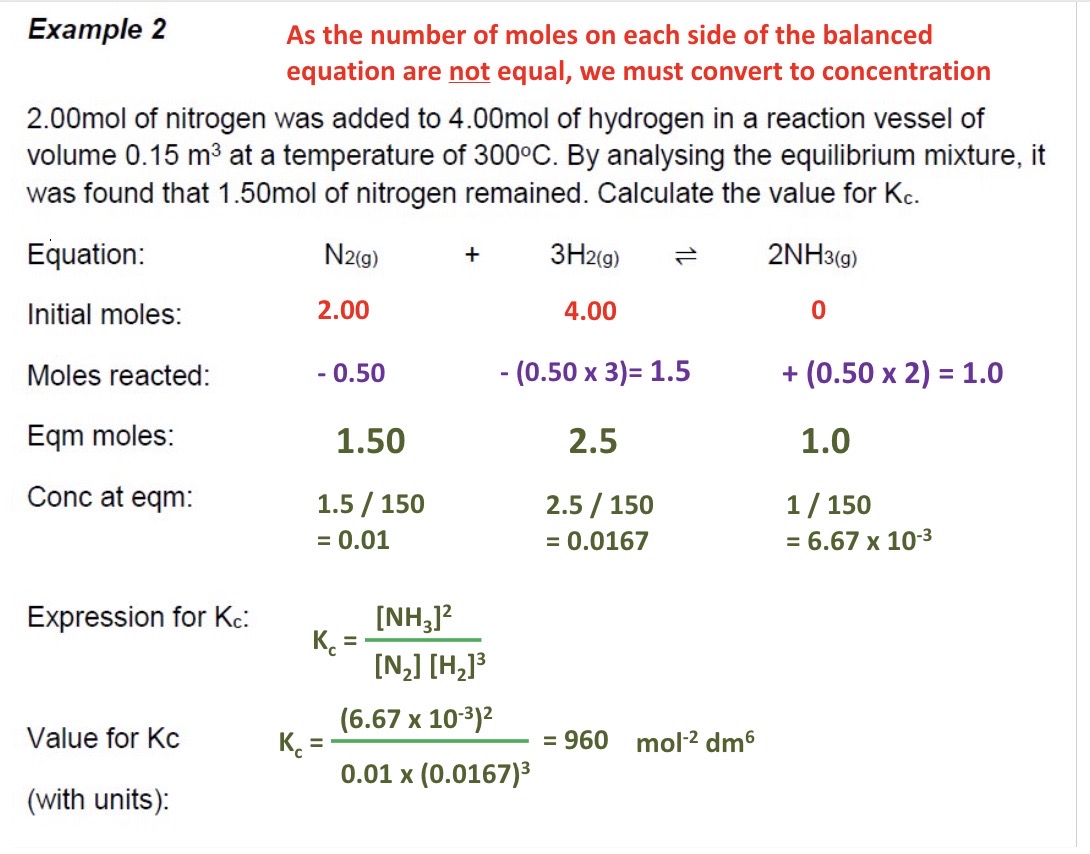
Calculate the moles at equilibrium using the initial moles + moles of one of the species at equilibrium for these 2 examples

Calculate a value for Kc

Why can you use moles in the previous questions, rather than calculating the concentration?
No. moles are equal on both sides of the equation

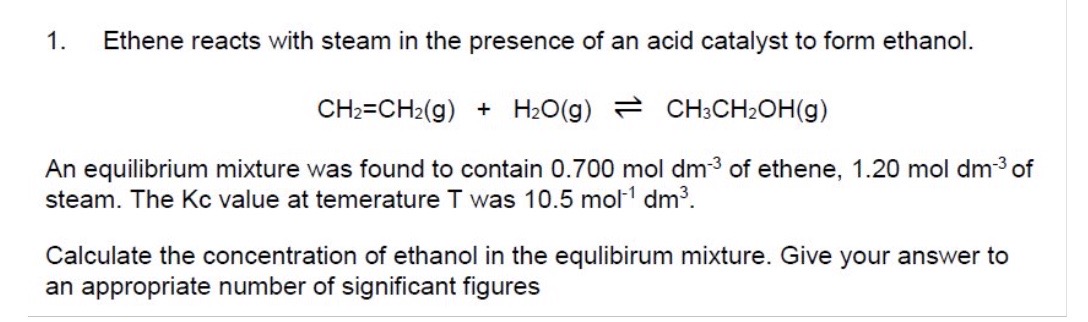
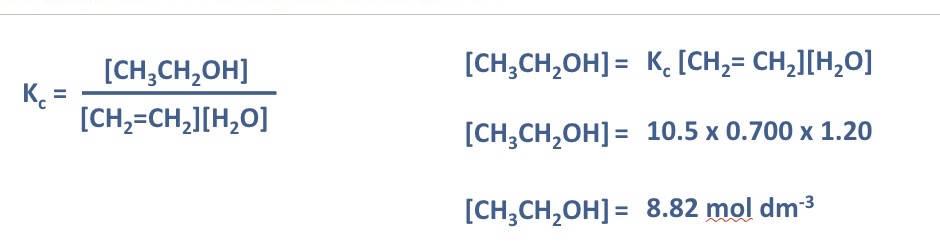
Calculate a value for Kc
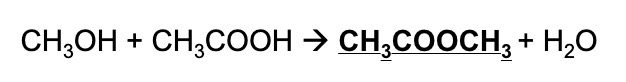
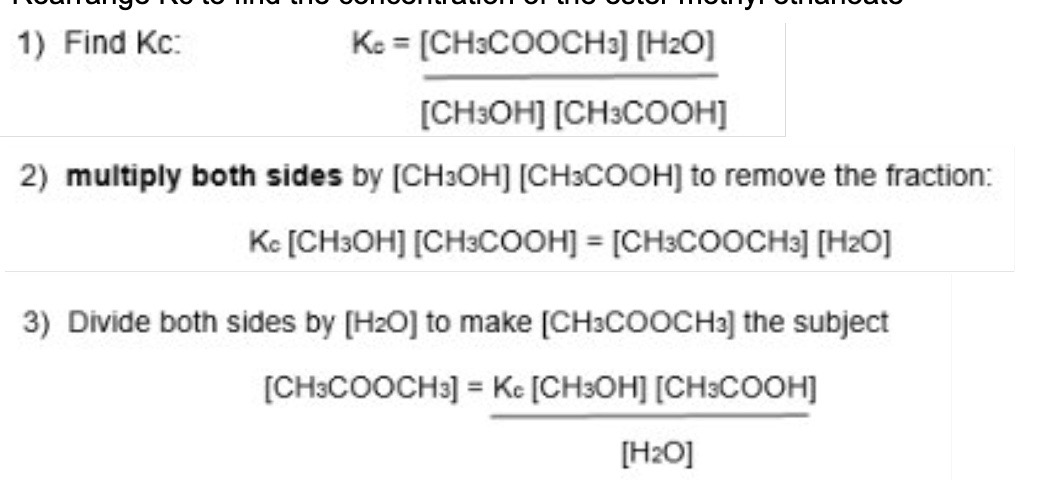
Rearrange Kc to find the concentration of the ester, methyl ethanoate
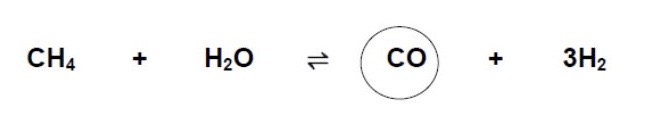
Rearrange Kc expression to find CO
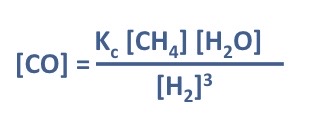
The value of KC is what dependent?
temperature - it is only affected by temperature
How does a change in temperature change the value of equilibrium constants, Kc?
Increase in temp always shifts the equilibrium in the endothermic direction
Decrease in temp always shifts the equilibrium in the exothermic direction
Why is Kc changed when the concentration changes?
If the concentration of any species involved in equilibrium is changed, then the conc. of other species will change → value of Kc remains constant
Why do catalysts not affect the yield of a chemical process?
No effect on the Kc
No effect on the position of equilibrium in a chemical reaction → increases the rates of the forwards + backwards reactions equally
Kc is unchanged when pressure is changed but only when does changing pressure cause equilibrium to shift?
When there are different no. moles on either side of the balanced equation
What practical methods can be used to find Kc → using an example of a chemical reaction