SCH4U U5 Acids and Bases Equilibrium
1/32
Earn XP
Description and Tags
RECALL 6 STRONG ACIDS STRONG BASES
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
1. If you had 1.0 mol/L of hydrochloric acid and 1.0 mol/L of acetic acid, which solution would conduct electricity better?
a. | hydrochloric acid | c. | both would conduct the same |
b. | acetic acid | d. | neither would conduct electricity |
a. | hydrochloric acid |
2. When concentrated acetic acid is added to water,
a. | few of the molecules ionize. | c. | the gas dissolves. |
b. | most of the molecules ionize. | d. | nothing happens. |
a. | few of the molecules ionize. |
3. With sophisticated conductivity apparatus, it can be shown that pure water has slight electrical conductivity because
a. | its impossible to remove all the trace ions from water. |
b. | water molecules react with each other to produce hydronium ions and hydroxide ions. |
c. | it is impossible to accurately calibrate the conductivity apparatus. |
d. | pure water is a very good conductor of electricity. |
b. | water molecules react with each other to produce hydronium ions and hydroxide ions. |
4. A solution with a hydrogen ion concentration of 10-8 would have a pH of
a. 1 | b. 8 | c.10 | d.4 |
b. 8 |
pH = -log[H+]
5. A solution with a pH of 4 would have an [H+] equal to
a. 1x | b.2x100 | c.2 × 10-2 | d. 1 × 10 -4 |
d. 1 × 10 -4
10-pH=[H+]
6. A concentrated weak acid is best described as which of the following?
a. | a solution with a low pH. |
b. | a solution where the concentration of undissociated acid particles is low compared to the concentration of hydronium ions. |
c. | a solution where the concentration of hydronium ions is large compared to the concentration of undissociated acid particles. |
d. | a solution with a high pH. |
e. | a solution where the concentration of undissociated acid particles is high and the relative quantity of hydronium ions is small. |
e. | a solution where the concentration of undissociated acid particles is high and the relative quantity of hydronium ions is small. |
7. If a pH meter was placed in a 1.4 mol/L solution of nitric acid the reading would be which of the following?
a.1.4 | b.14.15 | c.–0.15 | d.0.15 | e..0 |
c.–0.15
Step 1: Understand the acid
Nitric acid is a strong acid, meaning it completely dissociates in water:
HNO₃ → H⁺ + NO₃⁻
So, the concentration of H⁺ ions is the same as the concentration of the acid:
[H⁺] = 1.4 mol/L
Step 2: Use the pH formula
pH = –log[H⁺]
pH = –log(1.4)
Use a calculator:
pH ≈ –0.15
Final Answer:
c. –0.15 ✅
8. Which of the following salts acts like a base when added to water?
a. | sodium perchlorate | d. | both a) and b) |
b. | potassium nitrite | e. | both b) and c) |
c. | lithium sulfite | ||
e. | both b) and c) |
b. | potassium nitrite |
c. | lithium sulfite |
a. Sodium perchlorate (NaClO₄)
ClO₄⁻ is the conjugate base of perchloric acid (HClO₄), a strong acid.
Conjugate bases of strong acids are very weak bases (essentially neutral).
→ Does NOT act like a base
b. Potassium nitrite (KNO₂)
NO₂⁻ is the conjugate base of nitrous acid (HNO₂), a weak acid.
So NO₂⁻ acts as a base in water.
→ ✅ Acts like a base
c. Lithium sulfite (Li₂SO₃)
SO₃²⁻ is the conjugate base of HSO₃⁻, which is itself the conjugate base of H₂SO₃ (sulfurous acid), a weak acid.
SO₃²⁻ is a basic anion.
→ ✅ Acts like a base
d. both a) and b)
❌ Incorrect, because sodium perchlorate is neutral.
e. both b) and c)
✅ Correct, both potassium nitrite and lithium sulfite act like bases.
9. Which of the following salts could be combined with HC2H3O2 to form a buffer?
a. | sodium oxalate | c. | sodium acetate | e. | both c) and d) |
b. | iron(III) gluconate | d. | manganous cyanate | ||
c. | sodium acetate |
10. A solution which conducts electricity is a(n)
a.acid | b.base | c.salt | d.electrolyte | e.saturated |
d.electrolyte
11. When 45 mL of 0.25 mol/L sulfuric acid is mixed with 25 mL of 0.48 mol/L calcium hydroxide the solution is/has a
a.acidic | b.neutral | c.basic | d.pH below 7 | e.both a and d |
c.basic
12. When 45 mL of 0.65 mol/L acetic acid is added to 65 mL of 0.45 mol/L sodium hydroxide the resulting mixture is/has a(n)
a.neutral | b.basic | c.acidic | d.pH < 7 | e.both c and d |
b.basic
13. The Kb for four bases are CN1- = 1.6 ◊ 10-5, CO32- = 2.1 ◊ 10-4, NH3 = 1.8 ◊ 10-5, F1- = 1.5 ◊ 10-11.
If the pH's of 0.50 mol/L solutions of each of these weak bases were measured and they were placed in order from highest pH to lowest the result would be which of the following?
a. | CN1-, CO32-, NH3, F1- | c. | F1-, CN1-,NH3,CO32- | e. | none of the above |
b. | CO32-,NH3, CN1-, F1- | d. | CO32-,CN1-,NH3, F1- | ||
b. | CO32-,NH3, CN1-, F1- |
14. A small amount of NaOH(aq) is added to this buffer system:
HCHO2 + H2O <=====> H3O1+ + CHO21-
Which one of the following statements are true?
a. | the pH drops only a little since the equilibrium shifts right |
b. | the pH rises only a little since the equilibrium shifts left |
c. | the pH drops only a little since the equilibrium shifts left |
d. | the pH rises only a little since the equilibrium shifts right |
e. | the pH does not change since the buffer uses up all the HCl(aq) |
d. | the pH rises only a little since the equilibrium shifts right |
15. If sodium formate was dissolved in distilled water, which of the following could be added to make a functional buffer?
a. | potassium formate | c. | formic hydroxide | e. | potassium citrate |
b. | formic acid | d. | citric acid | ||
b. | formic acid |
16. Kw is which of the following?
a. | the equilibrium constant for water which is always 1.0 ◊ 10-14 |
b. | Ka ◊ Kb for conjugate acid - base partners @ 25oC |
c. | the log[H2O] @ 25oC |
d. | both a) and c) |
e. | none of the above |
b. | Ka x Kb for conjugate acid - base partners @ 25oC |
17. For phosphoric acid, H3PO4, the Ka1 =
a. | [PO43-][H1+]3 / [H3PO4] | d. | [H3PO4] / [H2PO41-][H1+] |
b. | [HPO42-][H1+] / [H3PO4] | e. | [H3PO4] / [PO43-][H1+]3 |
c. | [H2PO41-][H1+] / [H3PO4] | ||
c. | [H2PO41-][H1+] / [H3PO4] |
18. A solution of barium hydroxide has a [OH1-] = 5.6 ◊ 10-3 mol/L. The pH is which of the following?
a. | 11.8 | c. | 11.75 | e. | none of the above |
b. | 12.25 | d. | 12.3 | ||
c. | 11.75 |
19. If the Ka of a weak acid is 1.6 x 10-8, the Kb of its conjugate base partner must be which of the following?
a.6.20 | b.1.0 x 10-14 | c.6.8 x 10-7 | d.6.3 x 10-7 | e.7.80 |
d.6.3 x 10-7
20. Which of the following is NOT totally ionized when mixed with water?
a.HCl | b.HBr | c.HI | d.HF | e. HNO3 |
d.HF
21. Even if you did not know the identities of the six strong acids, it is easy to differentiate them from weak acids because strong acids
a. | have a pH>12.00 | c. | have a very large pKa | e. | do not have a pKa |
b. | have a pH<6.00 | d. | have a very small pKa | ||
e. | do not have a pKa |
22. For a weak triprotic acid, which of the following statements is NOT true?
a. | Ka3<<Ka2<<Ka1 |
b. | The pH is determined by the first ionization. |
c. | The concentration of the second stage conjugate base will be the same as Ka2. |
d. | Each successive ionization must be considered when determining pH. |
e. | The acid will donate protons in a step-wise manner. |
d. | Each successive ionization must be considered when determining pH. |
23. An aqueous solution is prepared from NH4CN. The Ka for NH4+ is 5.6x10-10 and the Ka for HCN is 4.9x10-10. The pH of the solution will be
a. | acidic | c. | neutral | e. | 1.0x10-14 |
b. | basic | d. | not enough information | ||
a. | acidic |
24. 50mL of a 0.5 M solution of HCl is titrated with 25mL of 1.0 M NaOH. The pH at the end of this titration will be
a.>7.00 | b.acidic | c.neutral | d.5.00 | e. 1.5 M |
25. In a titration of 0.10M acetic acid (Ka=1.8x10-5), the pH at the equivalence point must be somewhere around
a.0.50 | b.4 | c.7 | d.8.5 | e.13 |
d.8.5
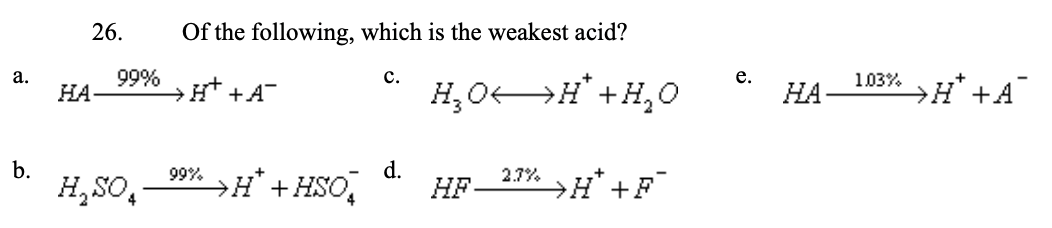
26. Of the following, which is the weakest acid?
E.
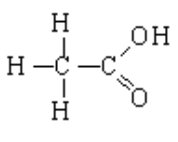
1. Acetic acid (HC2H3O2 or CH3COOH) is a common example given for a carboxylic acid. Draw a Lewis structural diagram and explain how this molecule acts as an acid and why it has a monoprotic nature. (3 marks)
the hydrogen of the hydroxide group donates its H+ ion to solution
-the other hydrogen atoms are non-acidic because they are tightly bound to the methyl carbon
2. Explain why 2.0 molar solutions of the strong acids have the same acid strength in water (the levelling effect of water), despite having different chemical formulas, but this is not the case with all the strong bases. (3 marks)
levelling effect of water: since each of the strong acids will ionize to the same extent (100%), two strong acids of the same concentration will contribute the same number of H+ ions (i.e. 1M HCl and 1M HNO3 will both release 1M of H+ and their conjugate bases into solution)
-strong acids dissociate completely in water, making solutions of H3O+
-this makes H3O+ the strongest acid that can exist in aqueous solution (all other acids will only partially dissociate in water)
-this means that the 6 strong acids have identical strengths in aqueous solution
1M HCl will completely ionize into 1M H3O+(aq) and 1M Cl-
-similarly for bases, strong bases completely dissociate in aqueous solution, so no equilibrium reaction is possible:acids stronger than H3O+ completely ionize, so all of their H+ ions are released
bases: 2 M NaOH releases 2 moles of OH-, whereas 2 M Ba(OH)2 would release 4 M of OH- into solution
3. The following Ka and Kb are provided:
Ka (HCO3-) = 5.6x10-11 Kb (C2H3O2) = 5.6x10-10
Ka (NH4+) = 5.6x10-10 Kb (HCO3-) = 2.3x10-8
Each of the following salts will dissociate completely in water. For each solution, indicate whether it will be acidic, basic or neutral, and give a simple reason (what reasoning led to this decision?). (5 marks)
a) NH4Cl in water: b) NaHCO3 in water: c) NH4C2H3O2 in water
d) NaC2H3O2 in water: e) KNO3 in water:
3. 1/2 mark for pH range, 1/2 mark for reason
a) acidic NH4+ is a conjugate acid of a weak base
b) basic Kb>Ka
c) neutral Ka = Kb
d) basic C2H3O2- conjugate base of weak acid
e) neutral salts of strong acids/bases
4. When data from titration experiments are graphed, the resulting graphs have predictable features and shapes. Draw sketch graphs for the titrations of
i. a strong acid; ii. a weak acid; and iii. a polyprotic acid.
Describe how they differ in the important features of each graph. (7 marks)
4. strong-strong: begins at low pH (<2.5)
-with slow gradual increase in pH as acid is neutralized by titrant until just before equivalence point where a single drop causes rapid increase in pH
-pH at equivalence is 7 1 mark
-transition slope from acidic to basic is quite steep and large 1 mark
weak-strong: begins at higher pH (3-6)
-slow gradual transition includes a buffering region where acid/conjugate base occur in solution together
-marked by a halfway point in the titration, where exactly half the original acid has been converted to conjugate base (so [weak acid]=[conjugate base])
-pH at equivalence is >7 (in the basic range because of the presence of conjugate base)
-transition slope is shorter
polyprotic-strong: will have 2 transitions corresponding to loss of successive H+,second one higher pH than first one
-transitions will be short
1 mark showing different starting pH
1 mark showing strong equivalence at pH=7
1 mark showing weak equivalence at pH>7
1 mark showing difference in size of transition slope
1 mark showing buffering region
1 mark showing halfway point
1 mark showing 2 small transitions for polyprotic
5. 25.0 mL of 0.100 M propanoic acid (HC3H5O2; Ka=1.3x10-5) is titrated with 0.100 M KOH. Calculate the pH’s after 12.5 mL of KOH, and 25.0 mL of KOH have been added. (12 marks) 4.89 and 8.79
5. for 12.5 mL KOH added, this is the halfway point
for the neutralization in moles: HC3H5O2 + OH- ↔H2O + C3H5O2-
HC3H5O2 | OH- | ↔H2O | C3H5O2- | |
I | 2.5 mmol | 1.25mmol | 0 | |
C | -1.25 mmol | -1.25 mmol | +1.25 mmol | |
E | 1.25 mmol | 0 | 1.25 mmol |
for the pH in molarity: HC3H5O2 ↔H+ + C3H5O2-
-the volume has changed to 37.5 mL
HC3H5O2 | ↔H+ | + C3H5O2- | |
I | 1.25 mmol/37.5mL =0.0333 M | 0 | 1.25 mmol/37.5mL =0.0333 M |
C | -x | +x | +x |
E | 0.0333 M-x | x | 0.0333 M+x |
2 marks
OR a simple statement that this is the halfway point of a weak acid titration, so pH=pKa
OR, since this is in the buffer region, we can use Henderson-Hasselbalch
for 25.0 mL of KOH added, this is the equivalence point
for the neutralization in moles: HC3H5O2 + OH- ↔H2O + C3H5O2- 1 mark
HC3H5O2 | OH- | ↔H2O | C3H5O2- | |
I | 2.5 mmol | 2.5 mmol | 0 | |
C | -2.5 mmol | -2.5 mmol | +2.5mmol | |
E | 0 | 0 | 2.5 mmol |
1 mark
for the pH in molarity: HC3H5O2 ↔H+ + C3H5O2- but there is no weak acid left, so the pH will be determined from the reaction of the conjugate base in water, thus
C3H5O2- ↔ ΟΗ− + HC3H5O2 1 mark
-the volume has changed to 50 mL 1 mark
C3H5O2- | ↔ OH- | + HC3H5O2 | |
I | 2.5mmol/50 mL =0.05 M | 0 | 0 |
C | -x | +x | +x |
E | 0.05-x | x | x |
1 mark
for the propanoic ion, we need a Kb, which we can determine from the Ka of the conjugate acid
6. One general equation for an acid/base reaction is given as: HA(aq) + H2O(l)↔ H3O+(aq) + A-(aq). The following equation is used to develop several concepts:
H2O(l) + H2O (l) ↔ H3O+ (aq) + OH- (aq)
Discuss each of: the hydronium ion, autoionization, conjugates and Kw using this reaction. (10marks)
6. hydronium ion: doubtful that free naked protons exist because of the enormous charge concentration (+1 concentrated in such a very small region of space).
-highly concentrated +ve charge is very strongly attracted to any region where -ve charges exist
-given the electron distribution of water with its two non-bonding electron pairs, we can see that the non-bonding orbitals would be susceptible or available to the naked proton, resulting in the formation of a hydronium ion H3O+ (sometimes called a hydrated proton) (3 marks)
autoionization: a small portion of water molecules will themselves be ionized (broken into H+ and OH-)
-since this is water ionizing water = self ionization or autoionization (a process that occurs on the order of a few water molecules in every billion)
the autoionization equation becomes 2H2O(l ) ↔ H3O+ (aq) + OH-(aq) (2 marks)
conjugate pairs are substances that differ by one proton (B/L will always have a pair of conjugates)
-conjugates here are water(base)/hydronium(acid) and water(acid)/hydroxide(base) (2 marks)
recall that pure liquids cannot change their concentrations
-in one litre of water, there are 1000g of water (which translates into 55.5 moles)
thus, we can say the molarity of water is 55.5 M
-even with the autoionization effect, only a very small fraction of water molecules are ionized, so the concentration of water remains virtually unchanged
autoionization equation becomes 2H2O(l ) ↔ H3O+ (aq) + OH-(aq)
and the Keq = [H3O+][OH-] and since this ion product constant is unique to water, we write Kw
-we see that the concentrations of H+ and OH- in water must be the same (one water molecule ionizes into one H+ and one OH-, thus, [H+] = [OH-]
-the experimentally determined value at 25oC of Kw is 1.0x10-14, and since [H+] = [OH-], the [H+] must be 1.0x10-7 (3 marks)
7. Hydrogen cyanide gas (HCN), a powerful respiratory inhibitor, is highly toxic. It is a very weak acid (Ka = 6.2 x 10-10) when dissolved in water. If a 50.0 mL sample of 0.100 M HCN is titrated with 0.100 M NaOH, calculate the pH of the solution
a) after 8.00 mL of 0.100 M NaOH has been added
b) at the half-way point of the titration (the logic here will be similar to the equivalence point: what is the concentration of acid halfway to the equivalence point?)
c) at the equivalence point of the titration
a) 8.49 b) 9.21 c)10.96