O-Chem 2 Lecture Exam 1
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
II
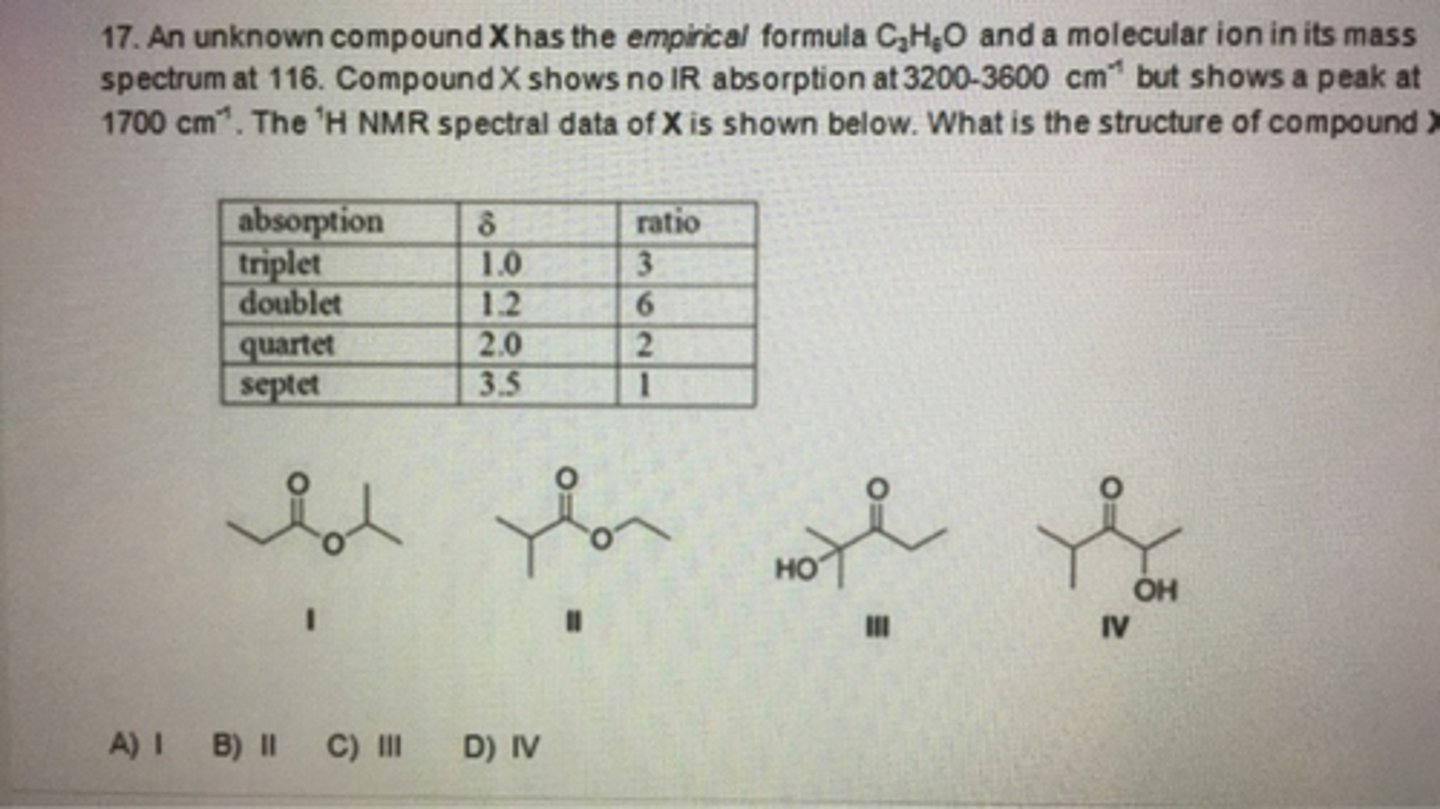
An unknown compound A has the molecular formula C12H16O. Compound A absorbs strongly in the IR at 1700 cm-1. The NMR spectral data of compound A is given below. What is the structure of compound A?
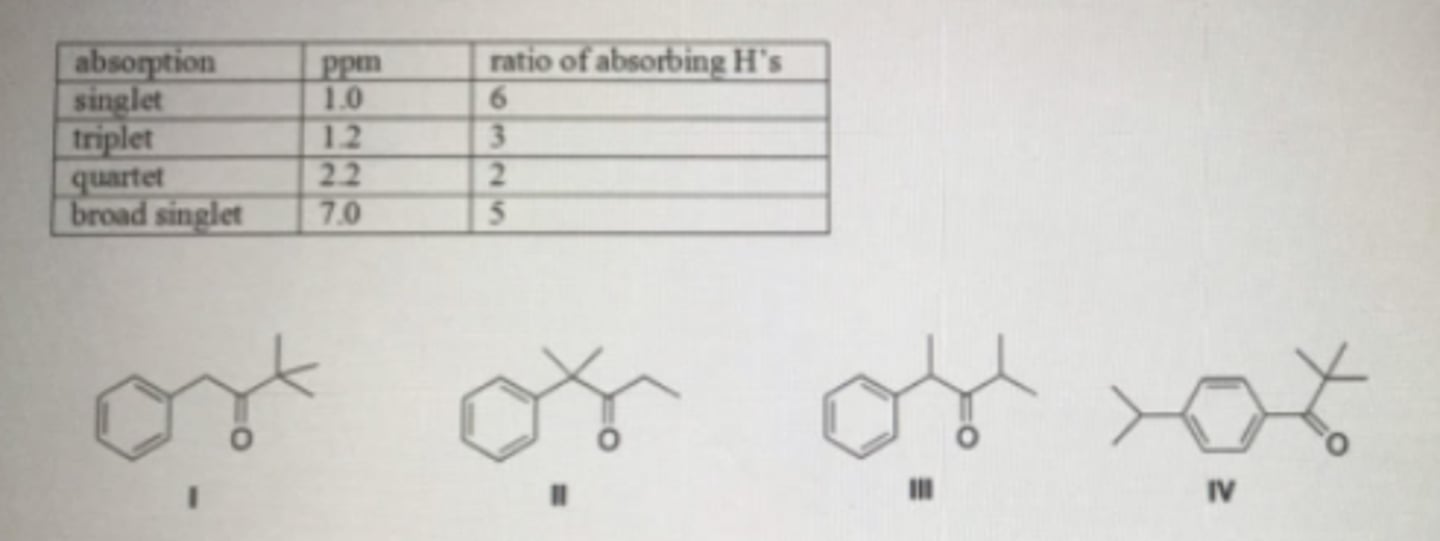
A: 4
B:3
C:5
D:3
For each of the following compounds, indicate how many 13CNMR signals will be seen.
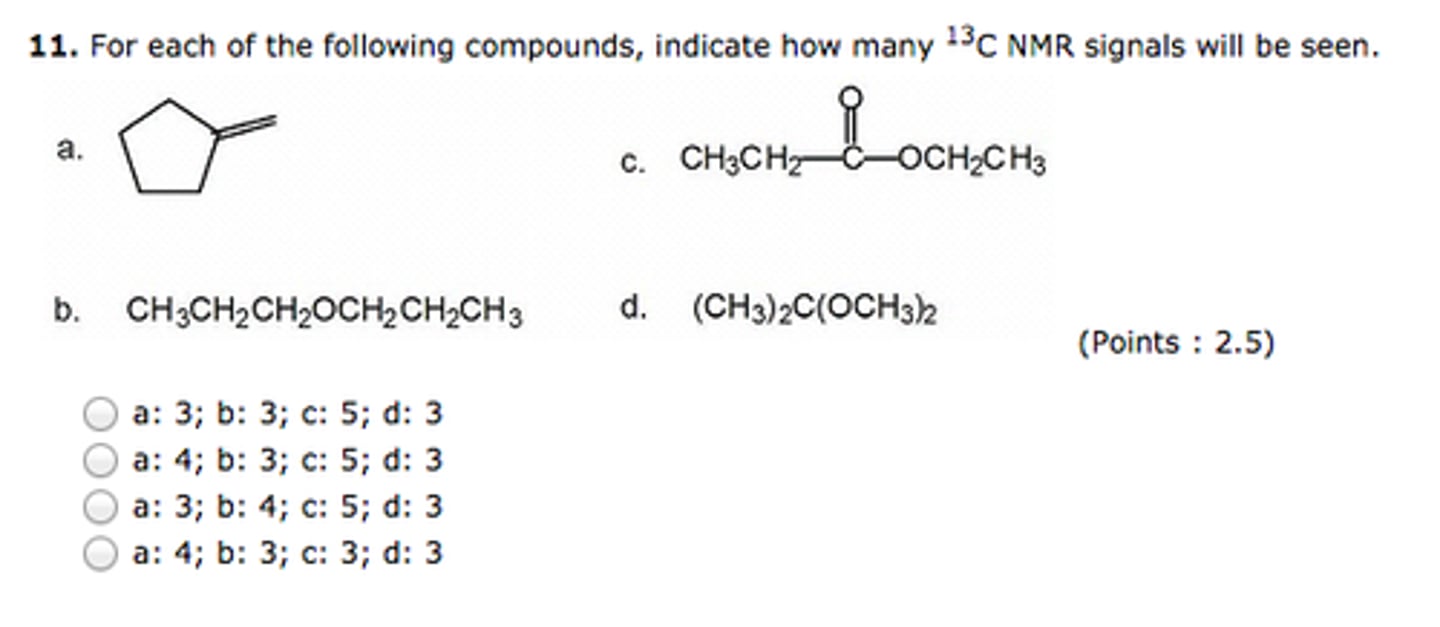
I:2
II:6
III:1
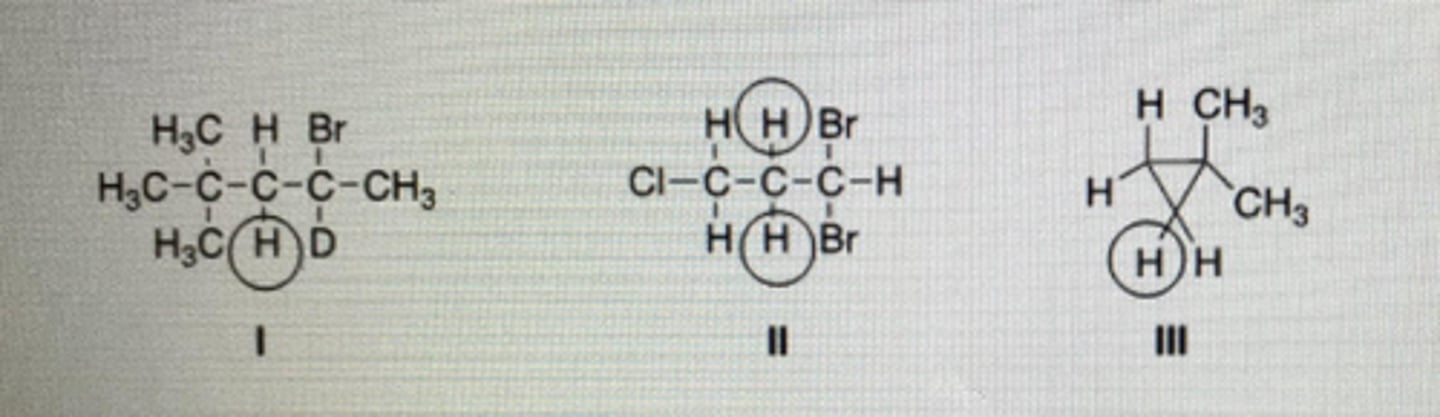
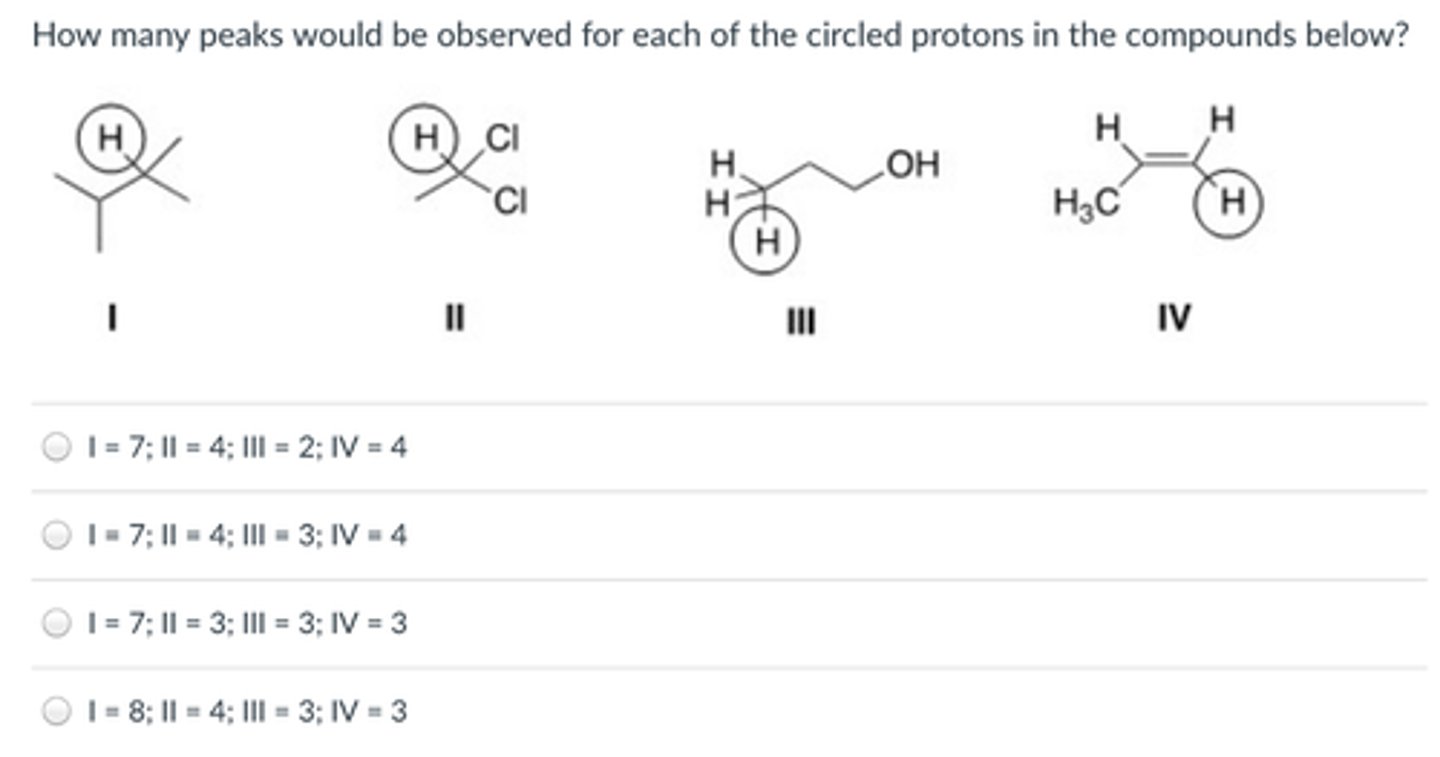
How many peaks would be observed for each of the circled protons in the compounds below?
Radio frequency
What region of the electromagnetic spectrum does nuclear magnetic resonance spectroscopy use?
B
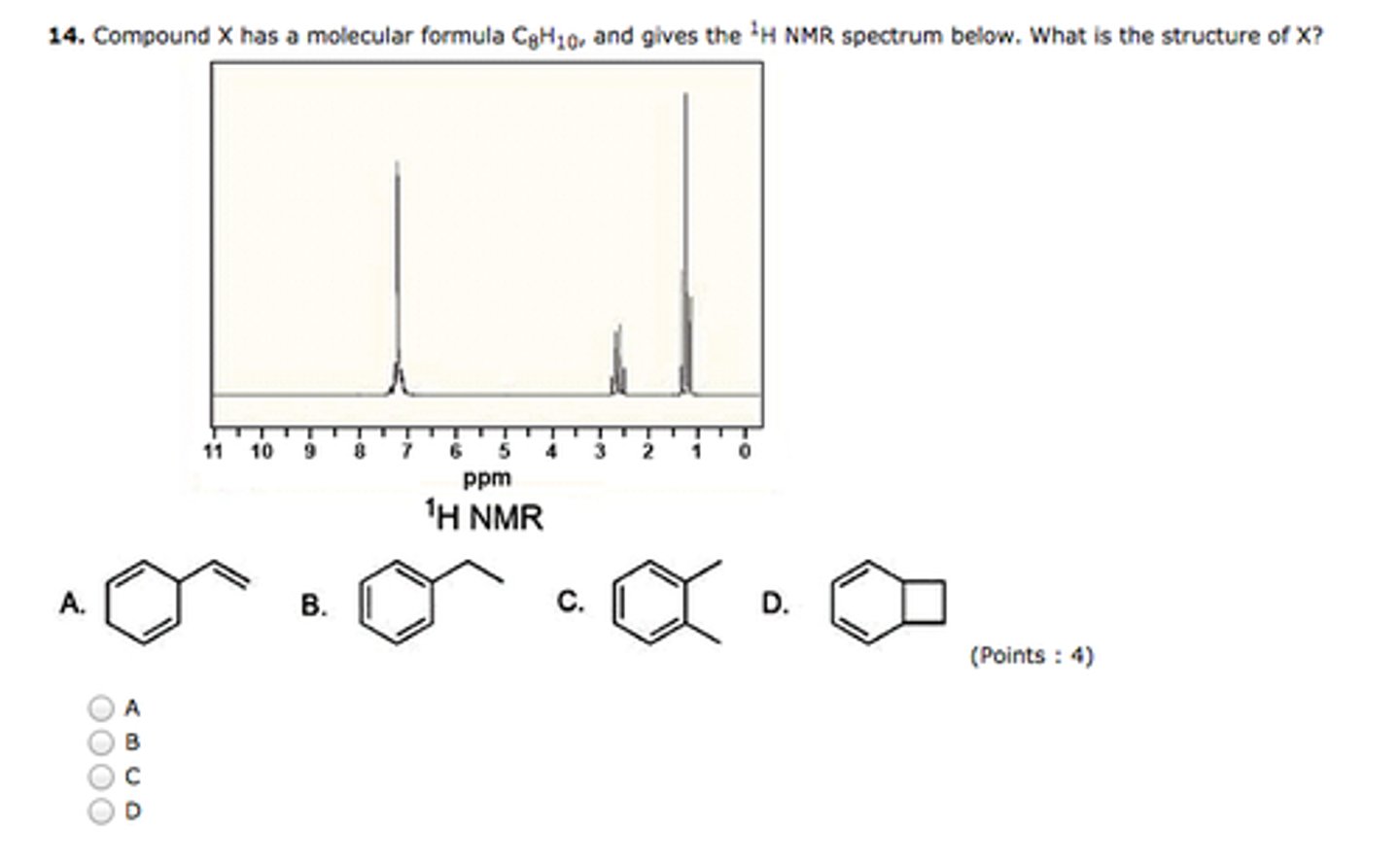
III
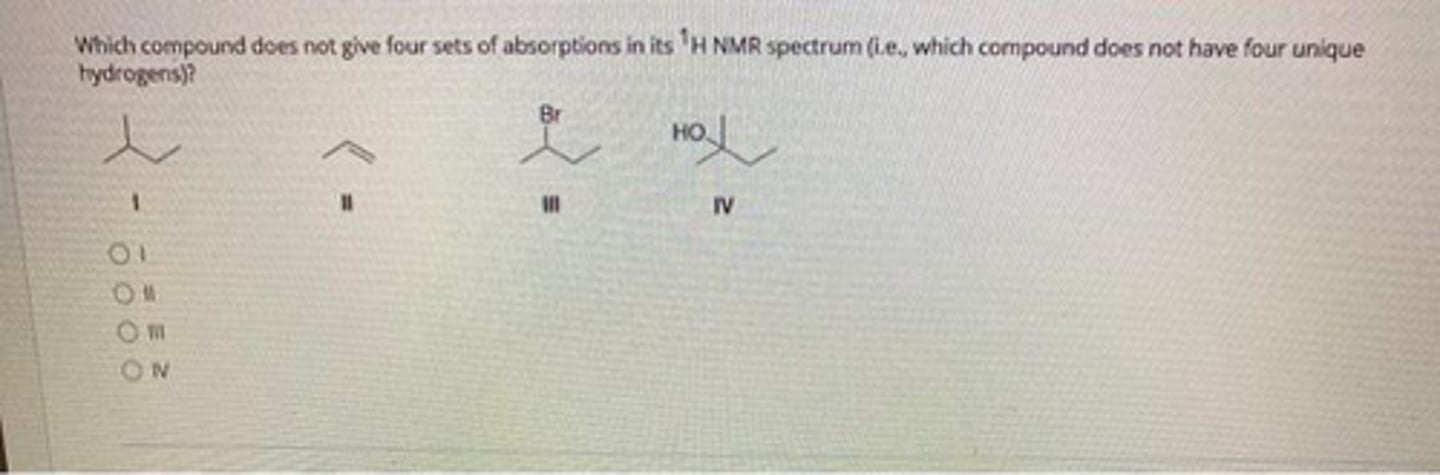
I:2
II:4
III:7
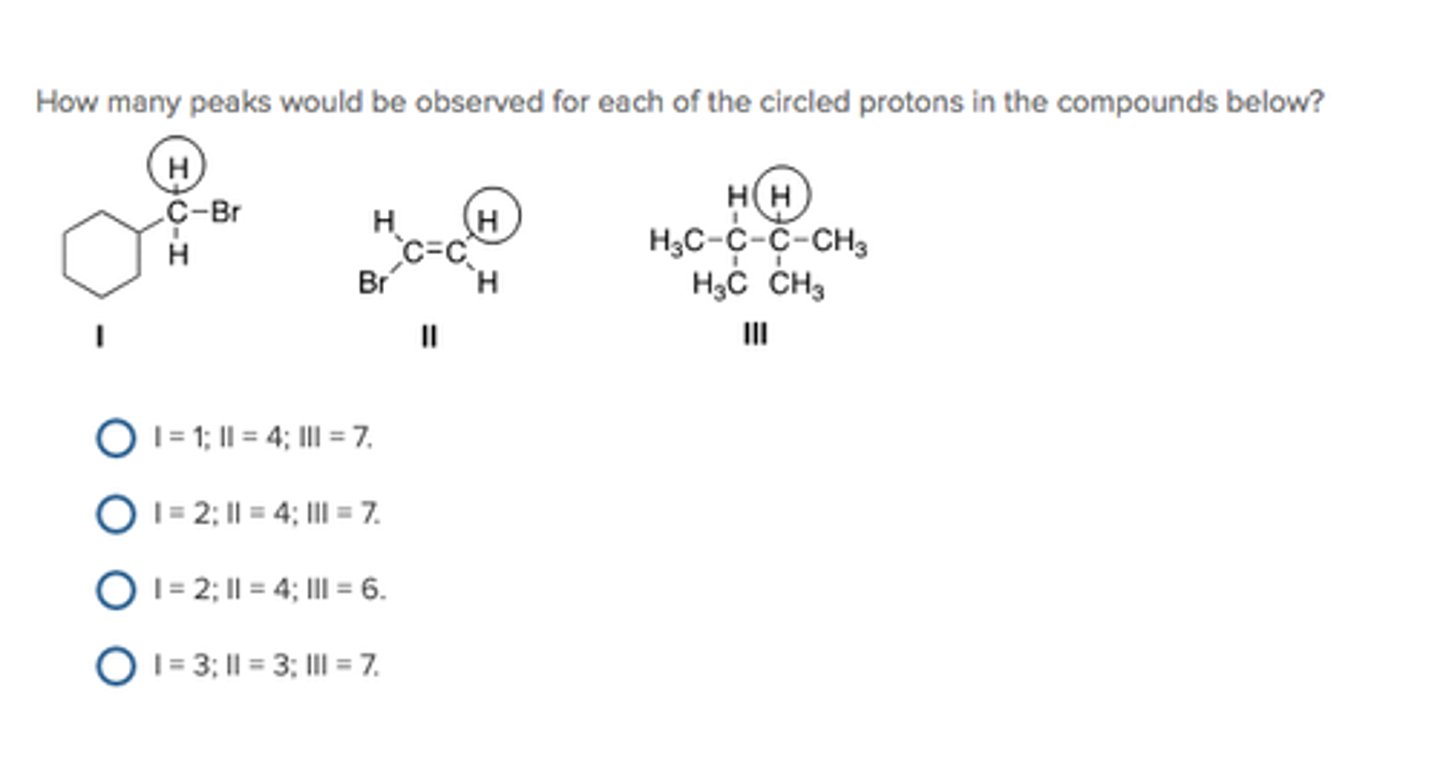
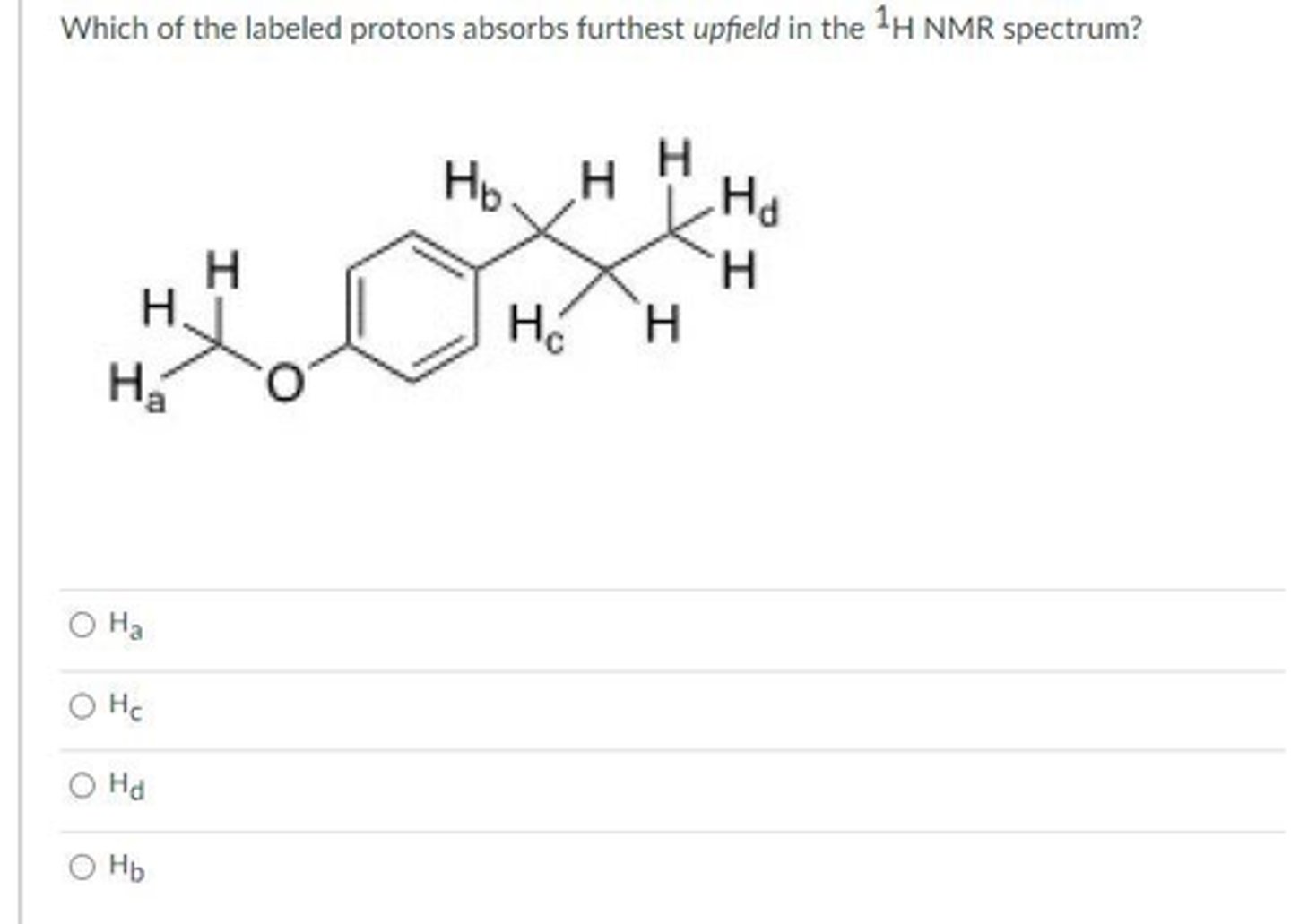
Ha
II
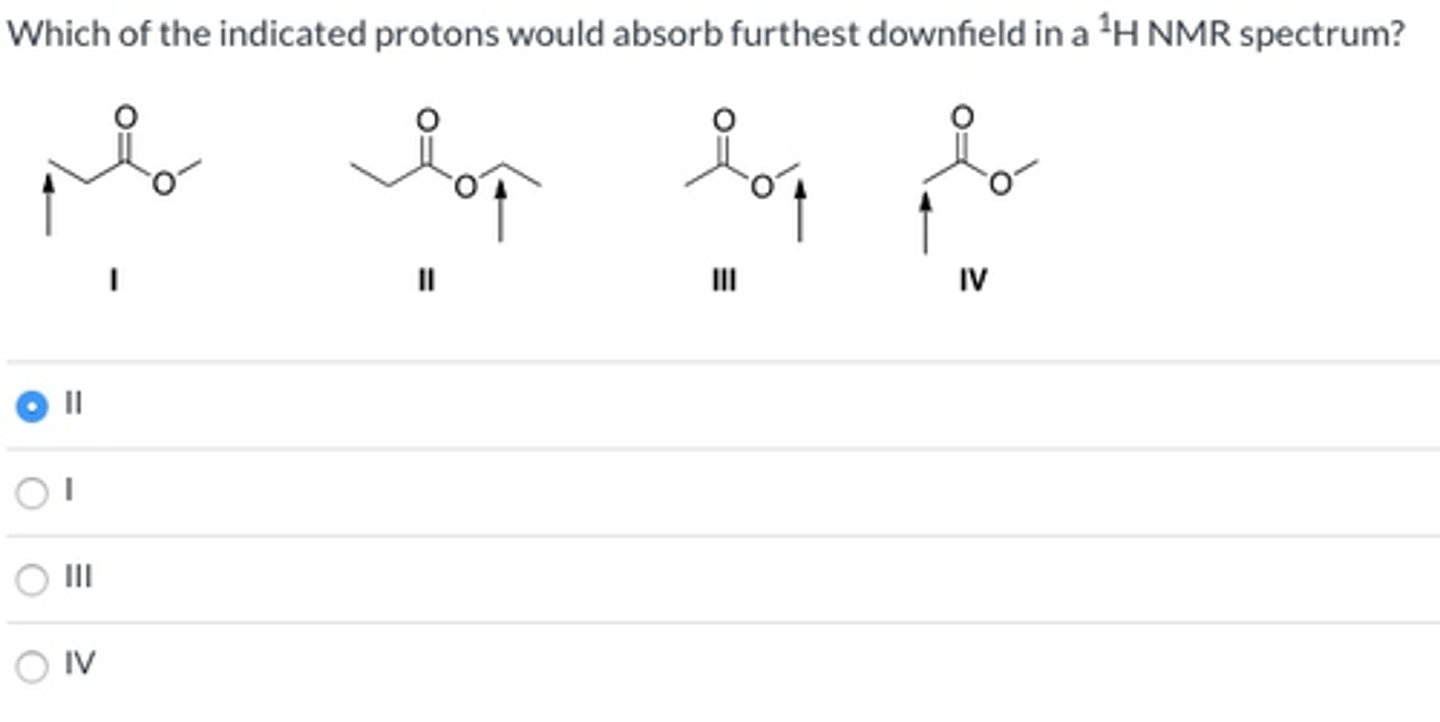
II
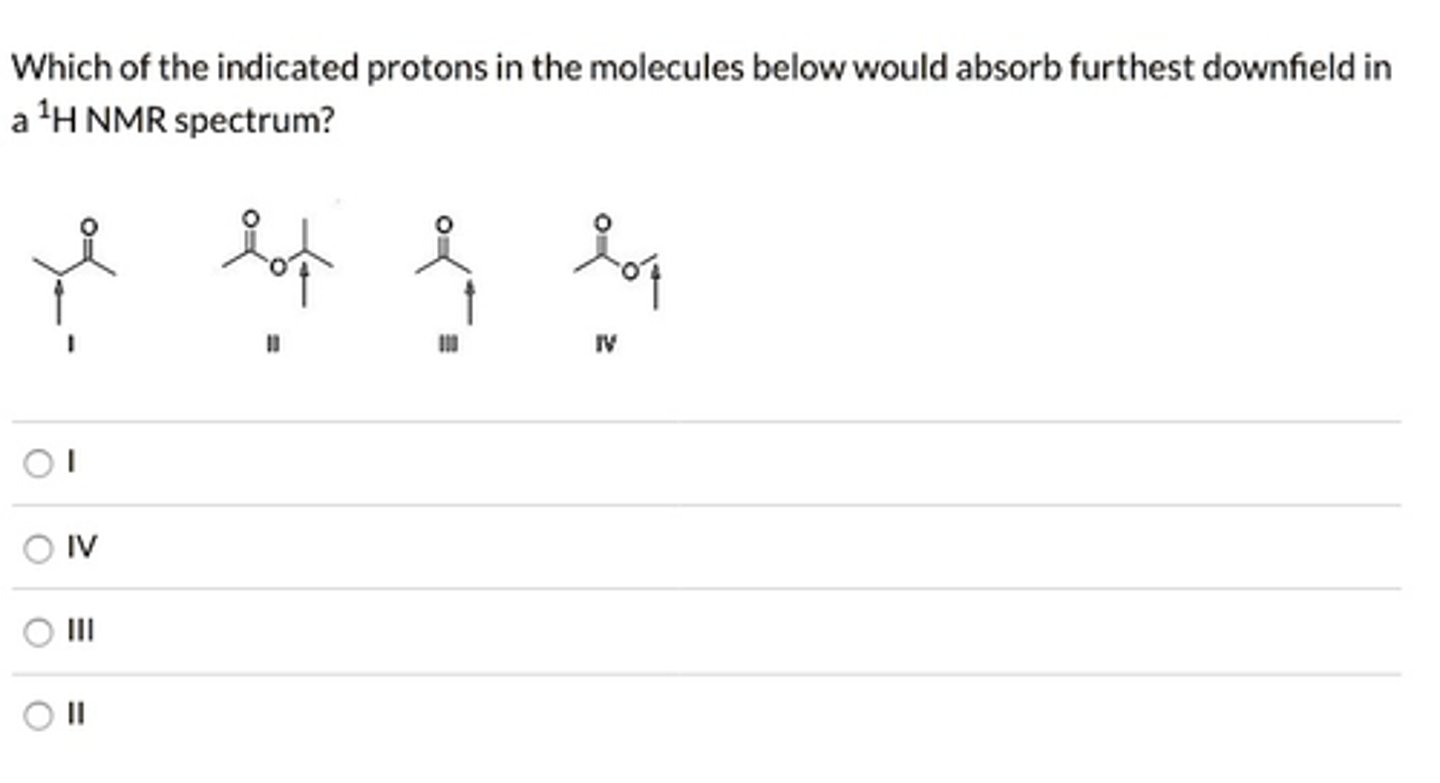
Hd
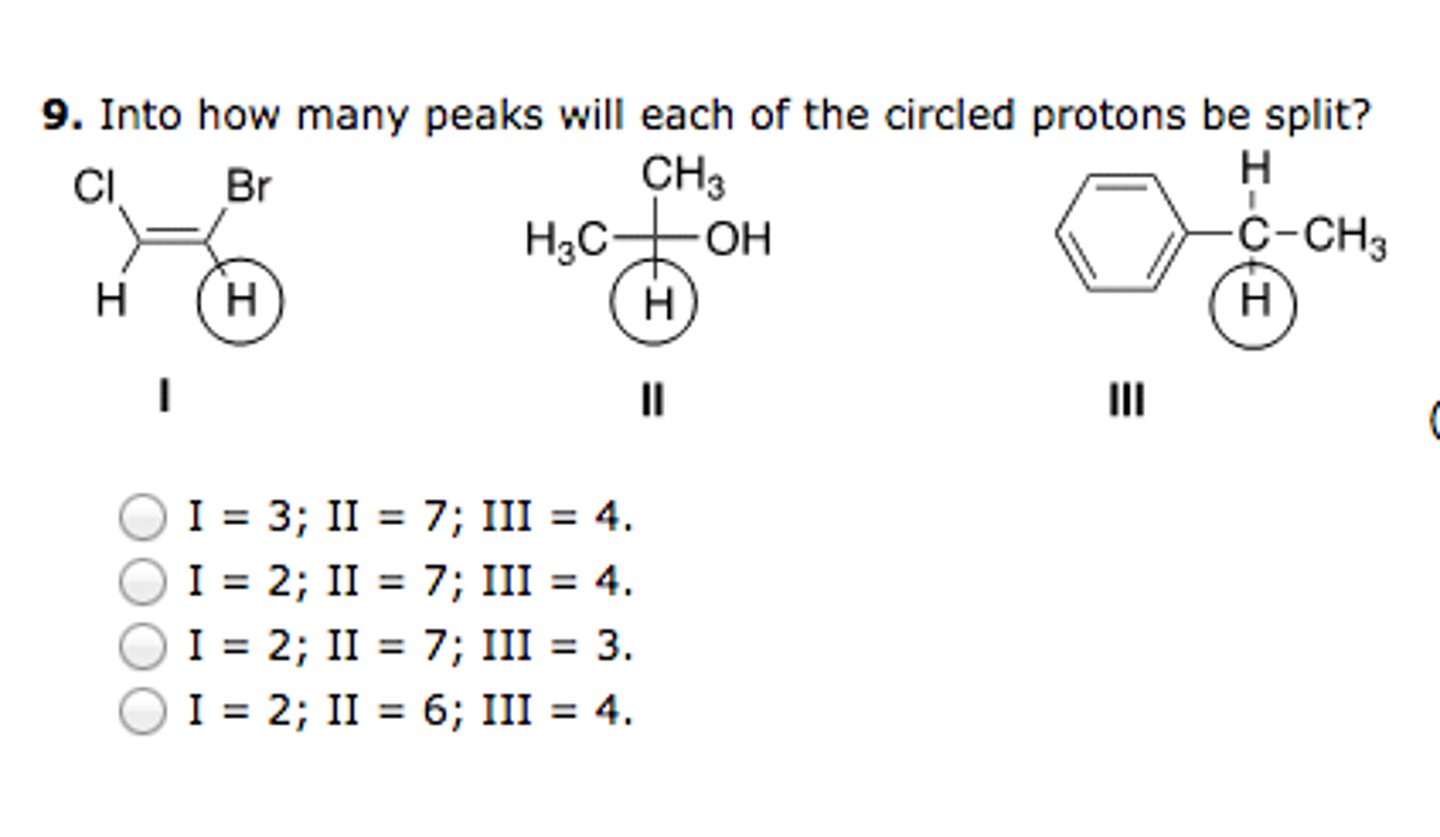
I:2
II:7
III:4
Chemical shift in Delta will remain the same
What effect does increasing the operating frequency of a 1HNMR spectrum have on the value of the chemical shift in Delta?
I:2
II:4
III:5
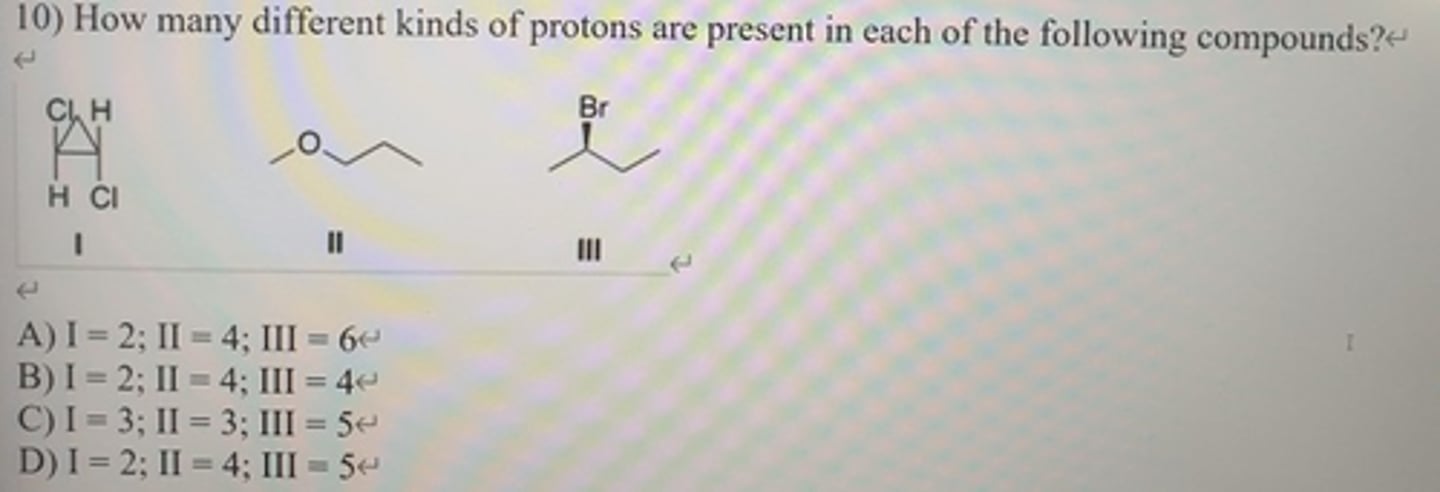
I:7
II:4
III:3
IV:4
C

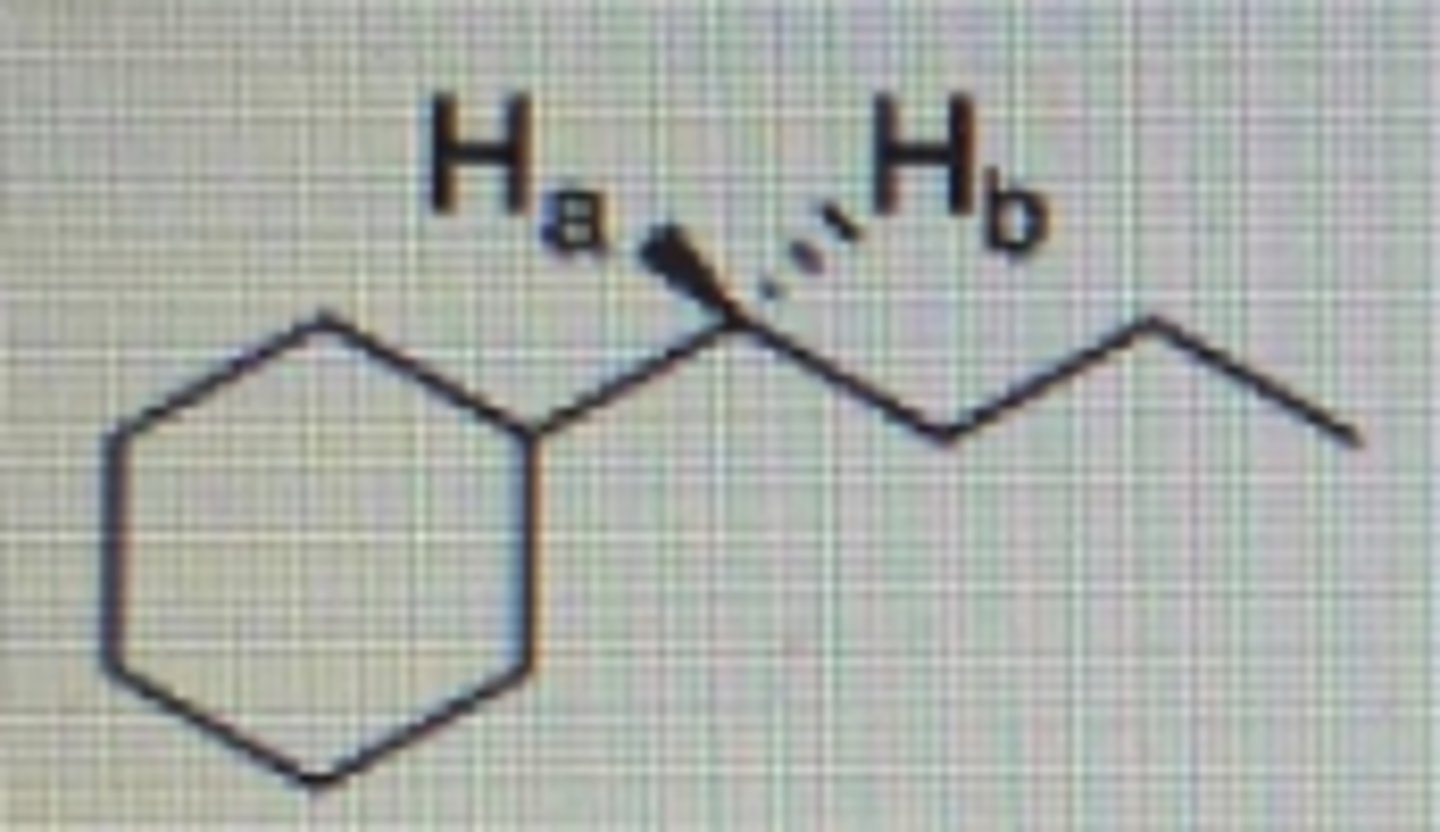
Enantiopic
What is the relationship between Ha and Hb in the following compound?
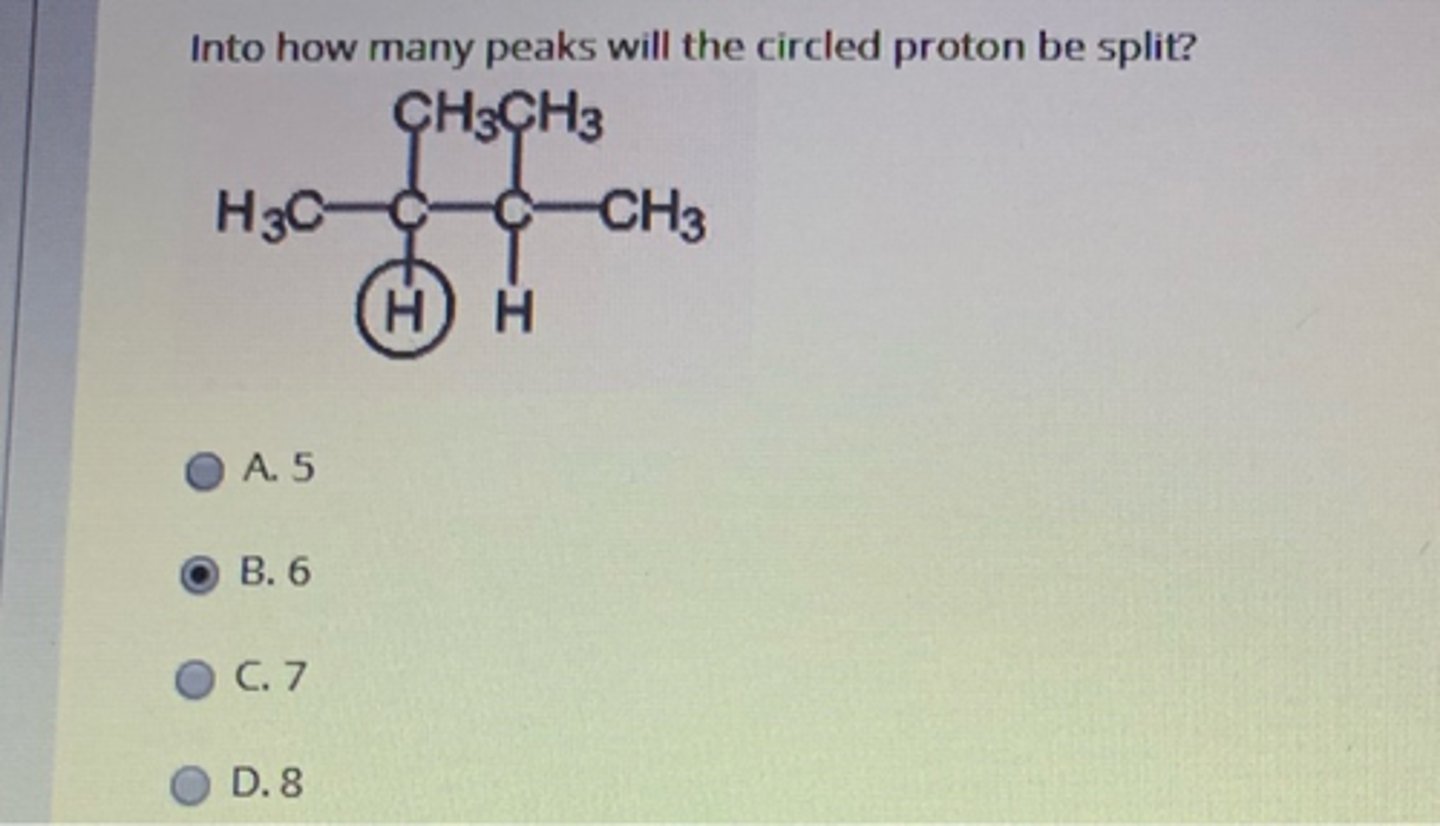
7
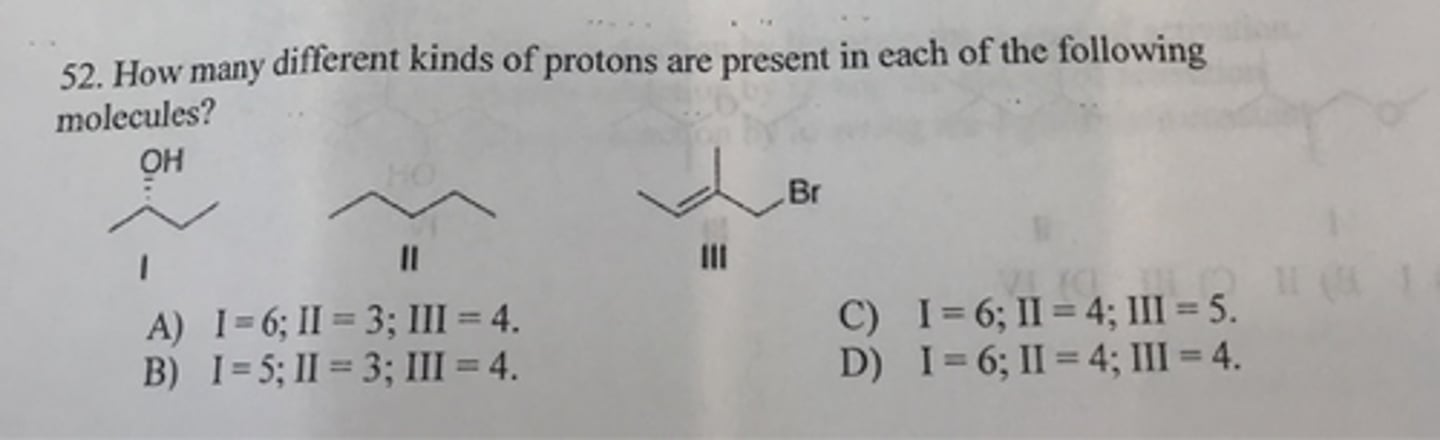
I:6
II:3
III:4
I
PrP

Only I and III
SP2

I
D
What is the major organic product of the following reaction?
Electrophilic addition
Which of the following is the appropriate term for the mechanism of the addition of HBr to 1,3 dienes?
(E)-3-Bromo-2-Pentene
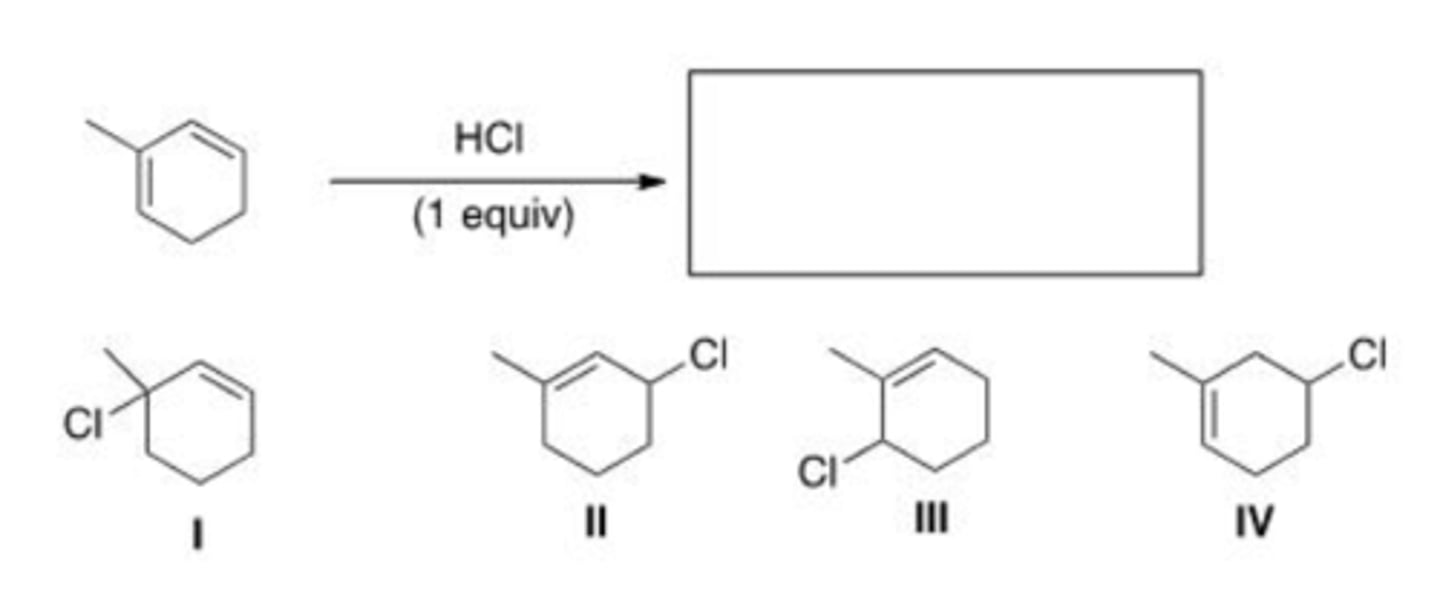
Which of the following is not a product obtained from the addition of 1 equivalent of HBr to (E)-1,3-pentadiene?
II
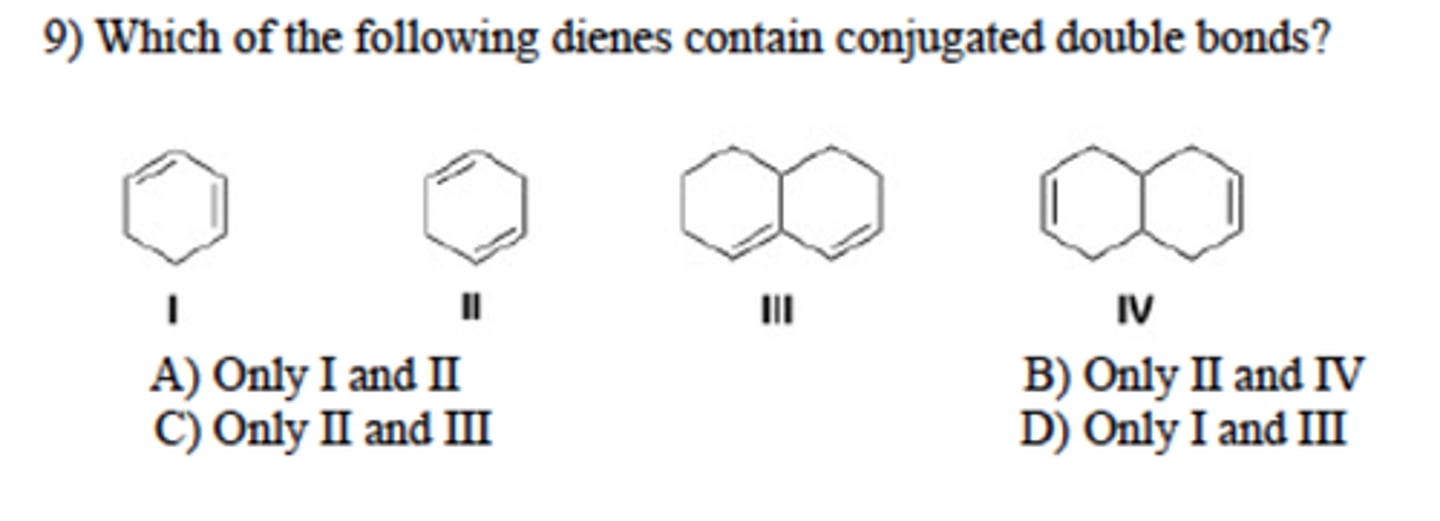
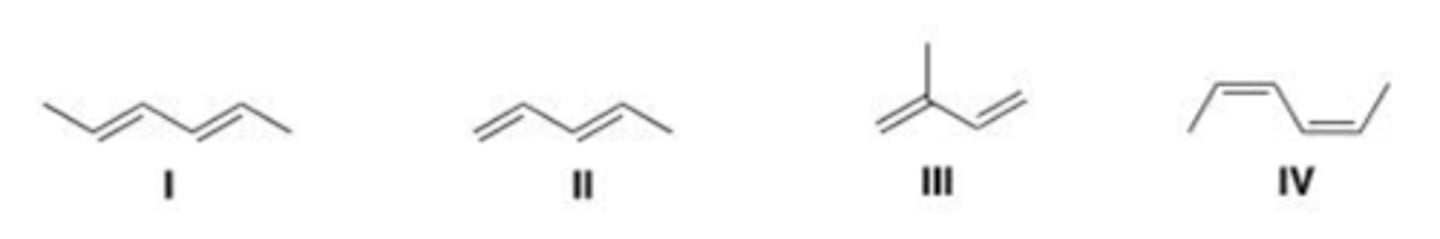
Which of the following are the conjugated dienes?
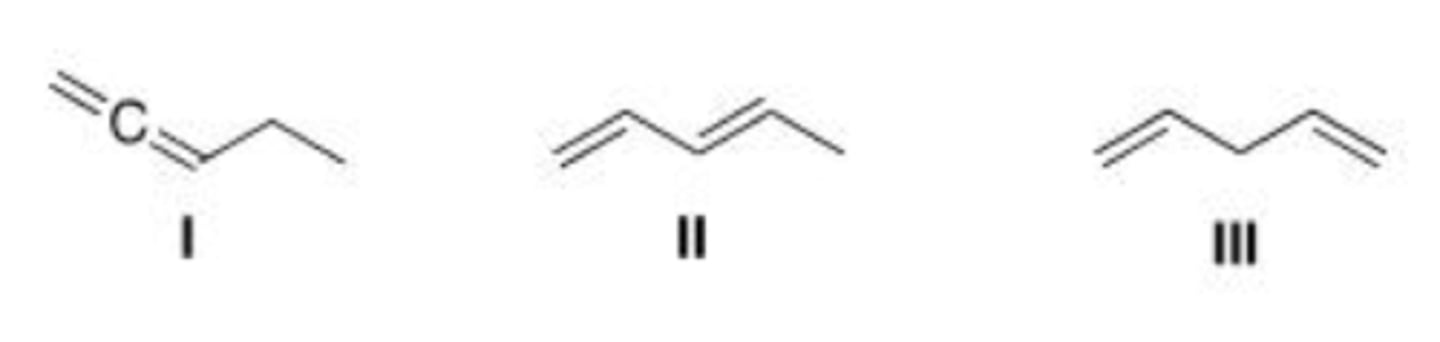
IV
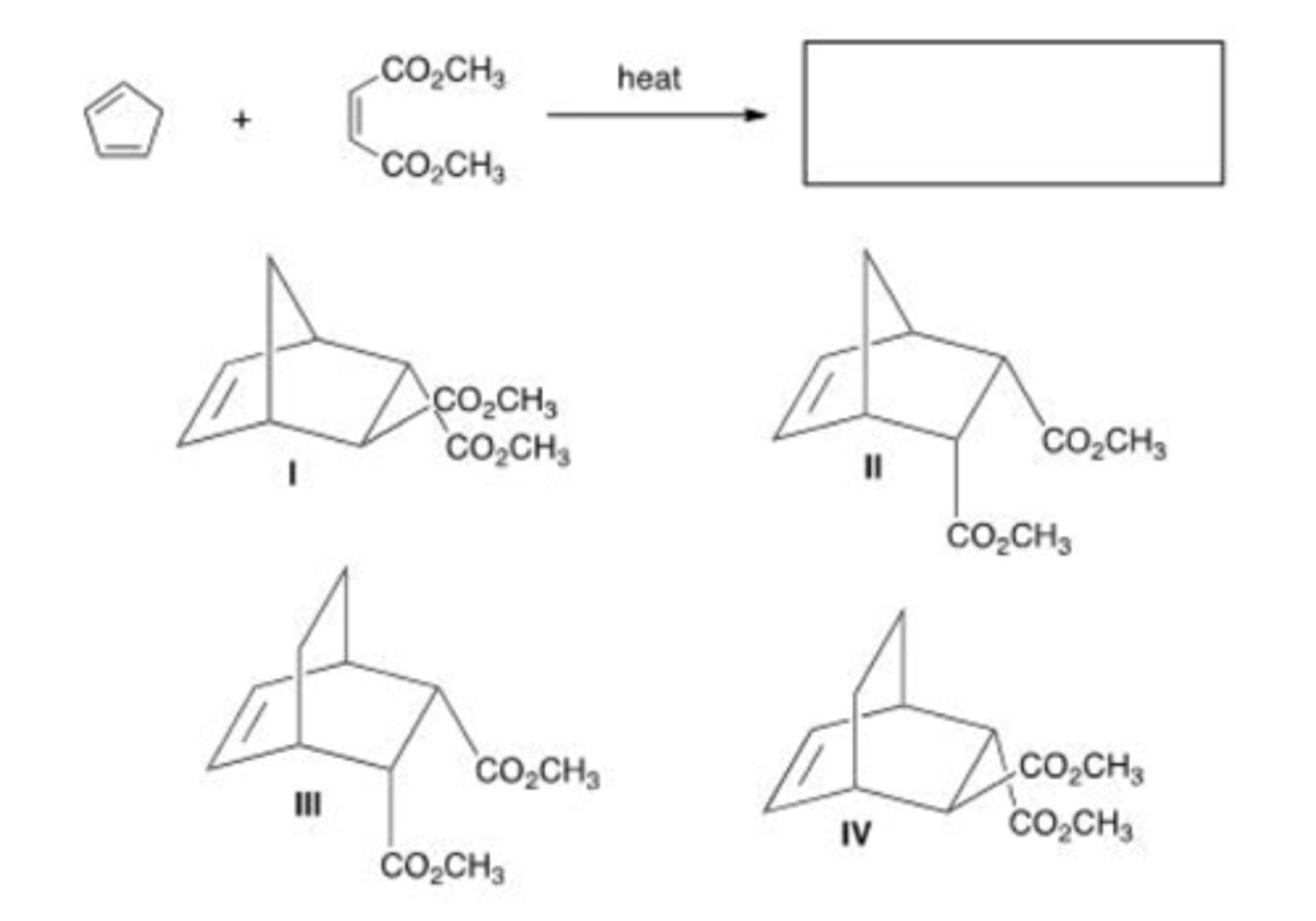
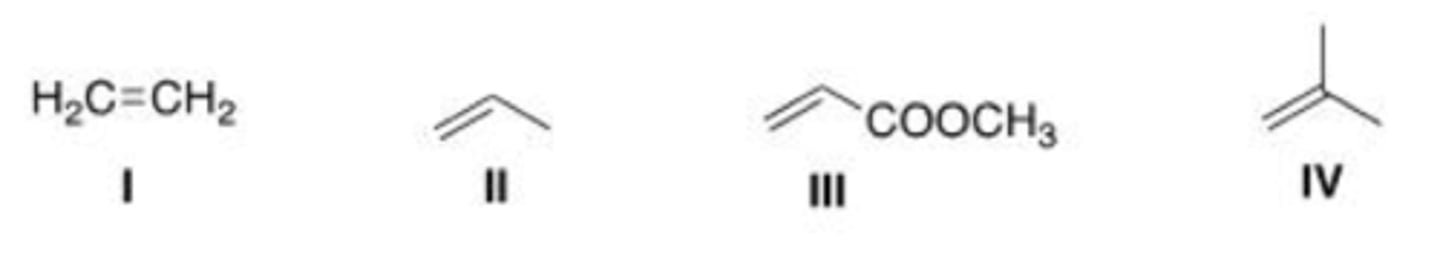
Which of the following compounds is the most reactive dienophile in a Diels-Alder reaction?
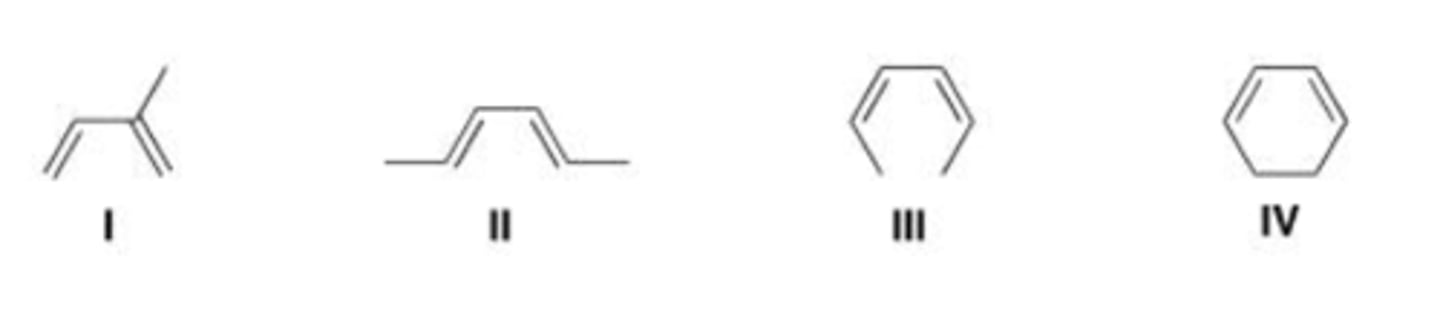
I, II, and III
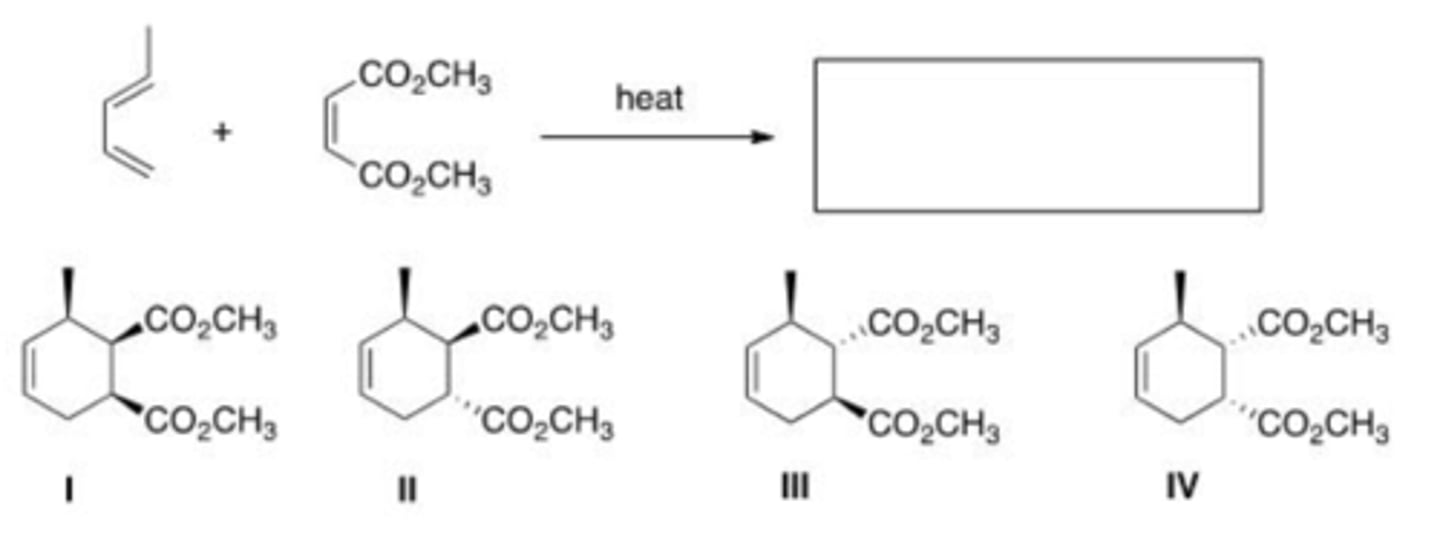
What is (are) the major product(s) of the following reaction?
II
What is (are) the major product(s) of the following reaction?
The placement of P bonds is different
Which of the following statements about resonance structure is true?
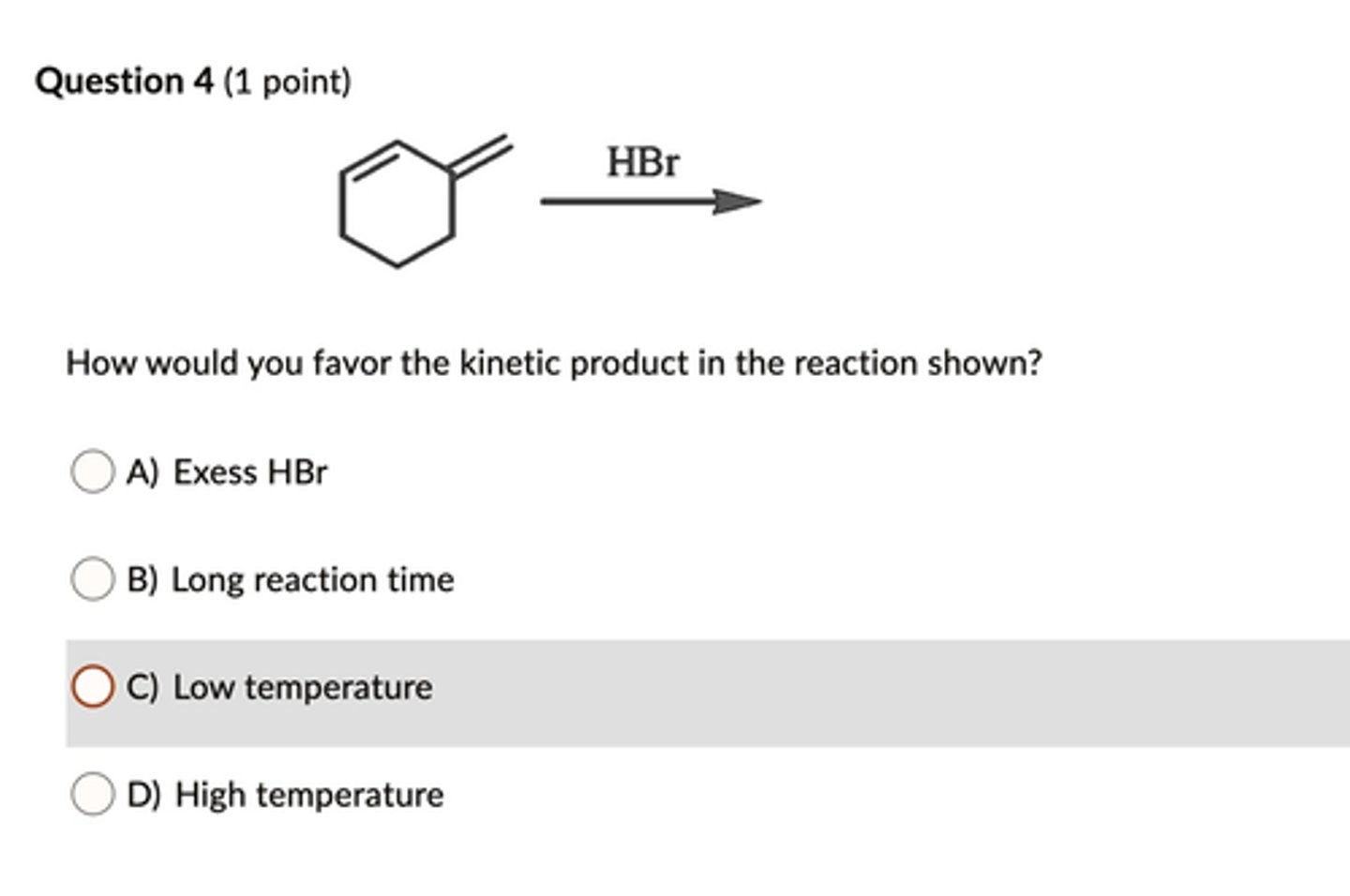
Low Temperature
IV
Which of the following is the least reactive diene in a Diels-Alder reaction?
IV
Which of the following compounds is the least reactive dienophile in a Diels-Alder reaction?
The stereochemistry of the dienophile is retained in the product
Which of the following statements about Diels-Alder is true?
The kinetic product predominates at low temperature
Which of the following statements about kinetic vs. thermodynamic products is true?
Three Pi bonds break: two sigma bonds and one pi bond form
Which of the following statements about the mechanism of the Diels-Alder reaction is true?
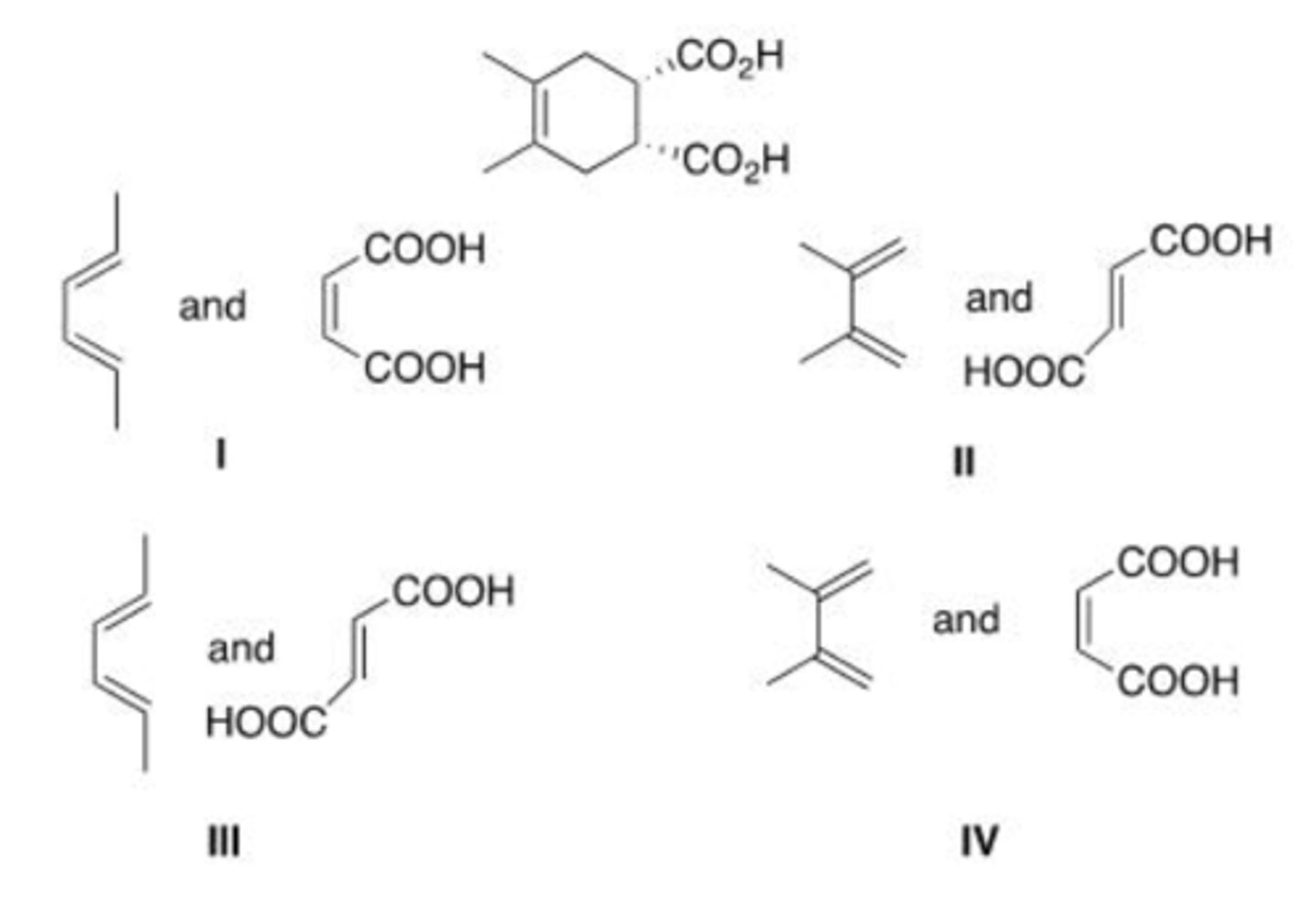
IV
What diene and dienophile are used in a Diels-Alder reaction to prepare the following compound?
II
Which of the following compound absorbs UV light at the longest wavelength?
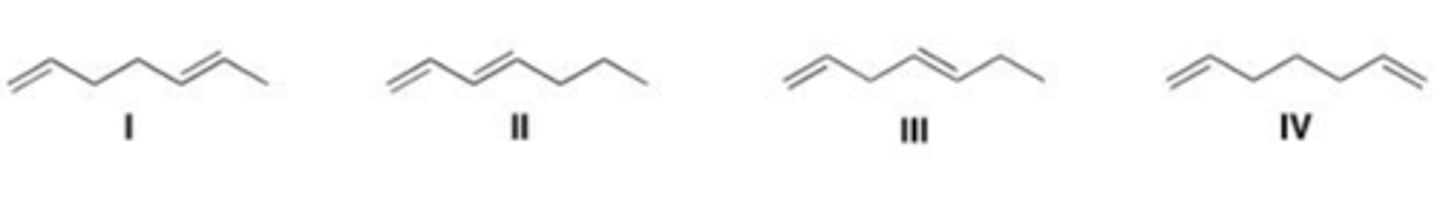
I
What is the major organic product of the following reaction?
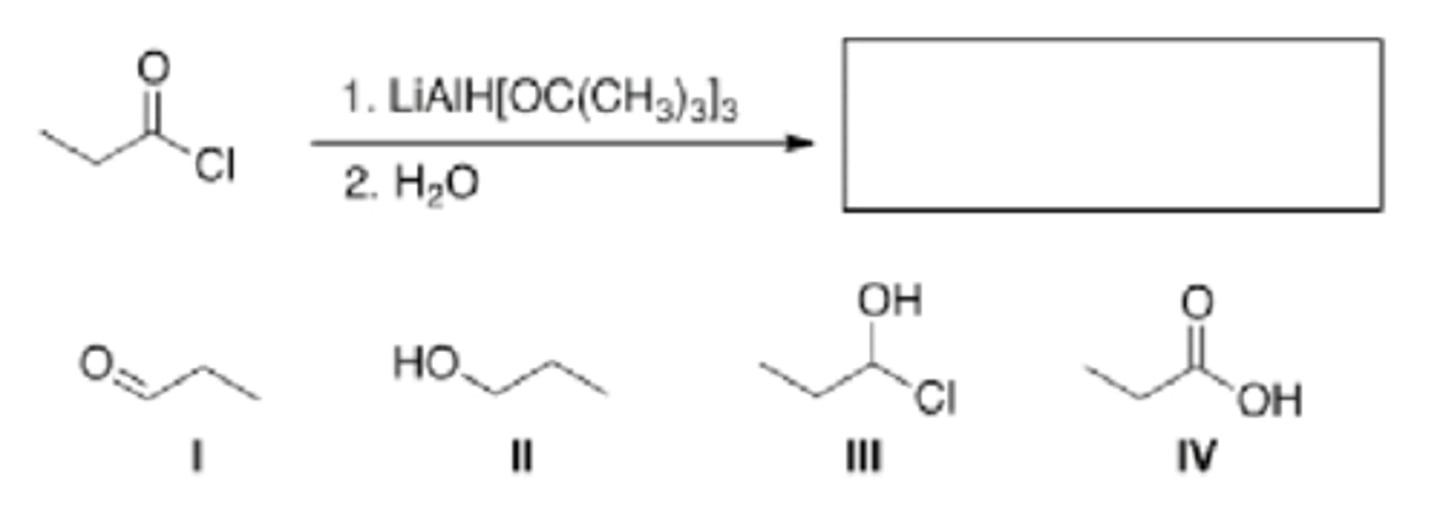
LiAlH4
What reagent would be used to reduce an amide to amine?
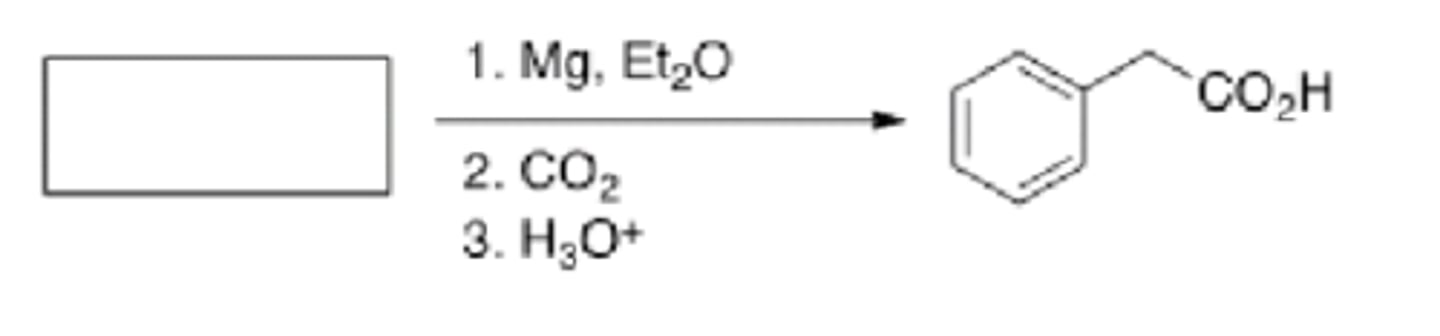
Benzyl bromide
What is the starting material int he reaction below?
Both increased number og C-H bonds and fewer C-Z bonds
If a compound is reduced, what is the result?
Nucleophilic attack followed by protonation
What are the two steps in a nucleophilic addition mechanism?
To protect alcohols from organometallics reagents and other reagents
What is the purpose of Siyl Ether?
Both reagents contain polar metal-hydrogen bonds. The polarity of the B-H bond is less than the polarity of the Al-H bond. So LiAlH4 is the stronger reducing agent
Both LiAlH4 and NaBH4 are reducing agents. Which statement about these reagents is true?
II
What is the product of the following reaction?
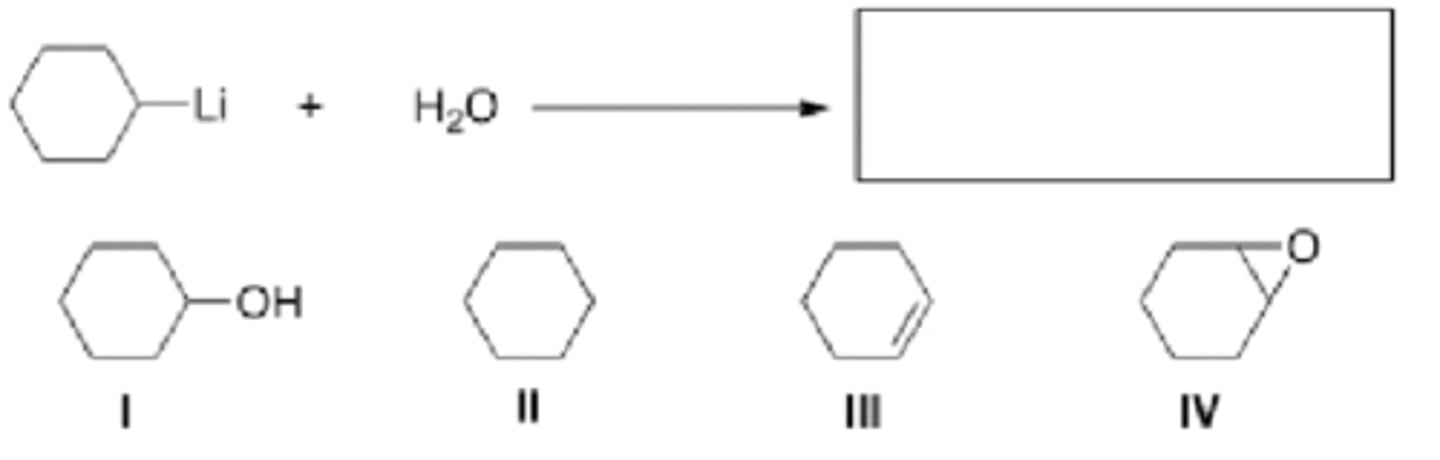
I and II
What is the product of the following reaction?
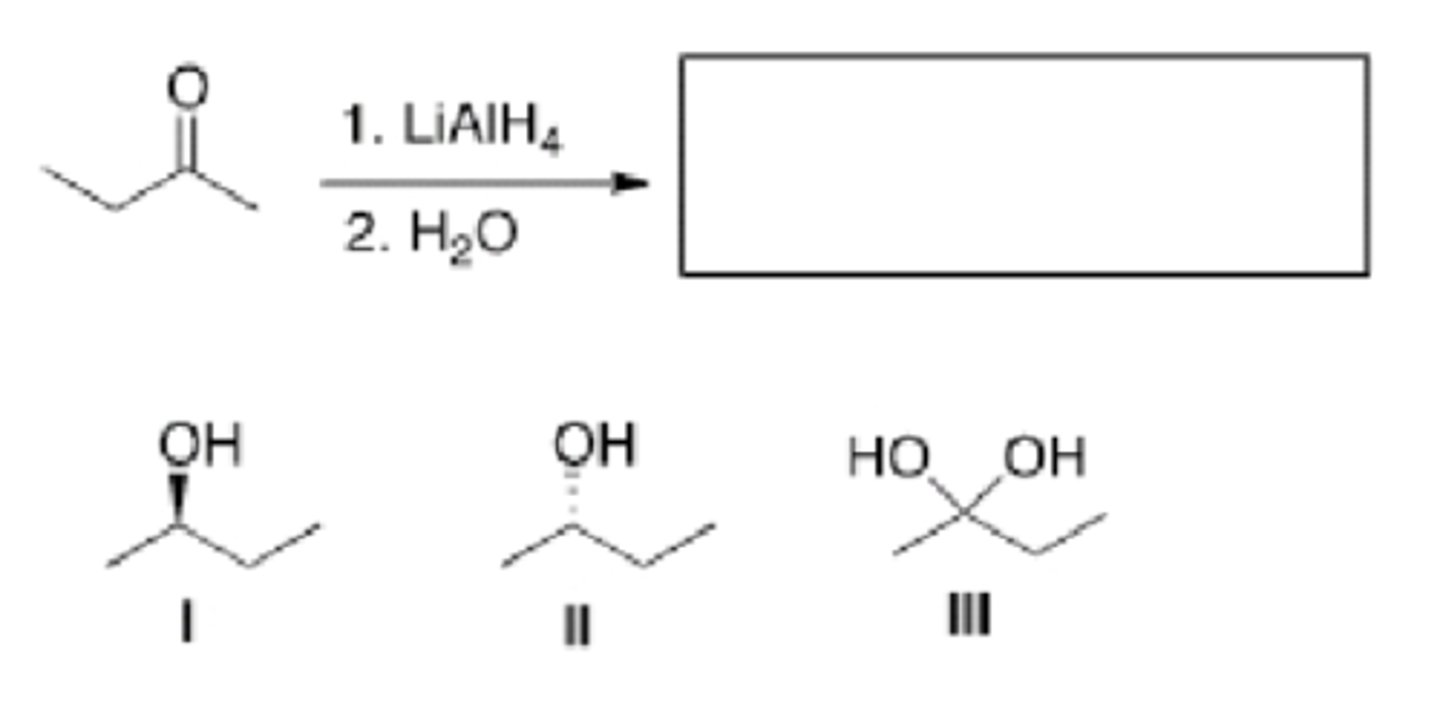
C
What will be the product of the following reaction (before any aqueous work-up)
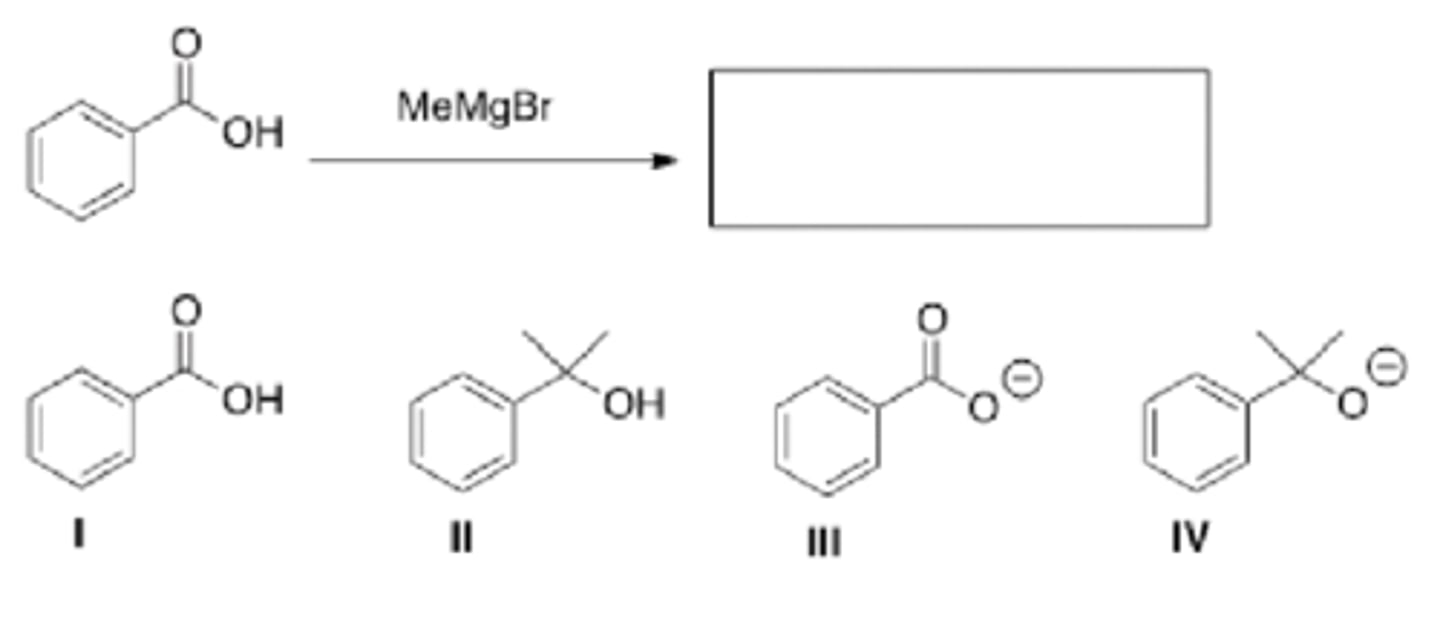
LiAlH4
H20
What is the missing reagent in the reaction below?
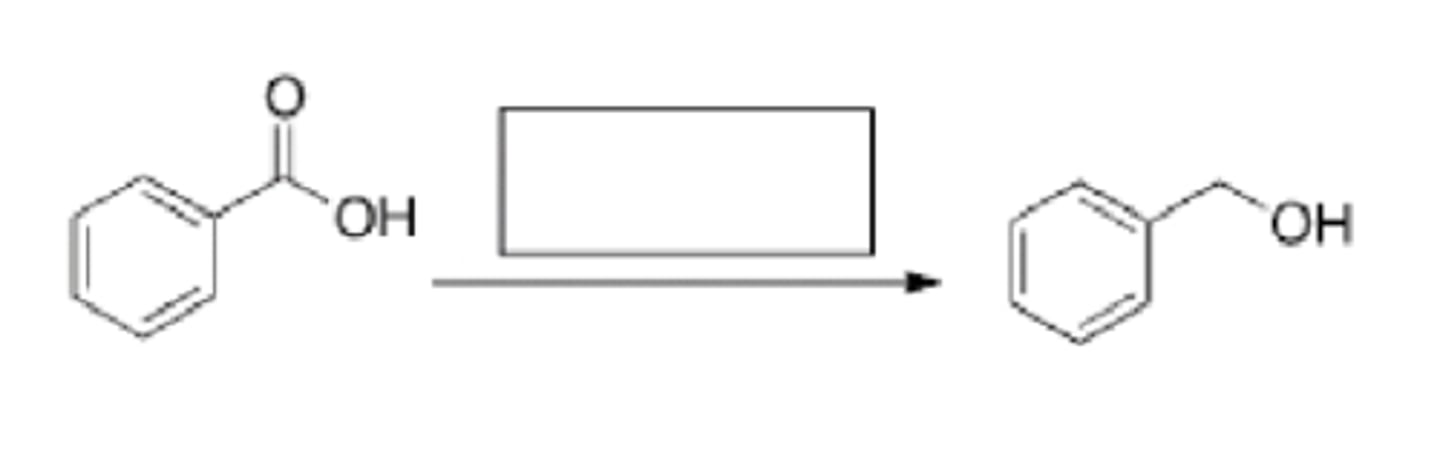
Sterics and electronics
Which of the following terms explain why aldehydes are more reactive than ketones?
Organometallic reagents are strong acids that readily donate proton to water
Which of the following statements about organometallics reagents is NOT true?
IV
What is the major organic product of the following reaction?
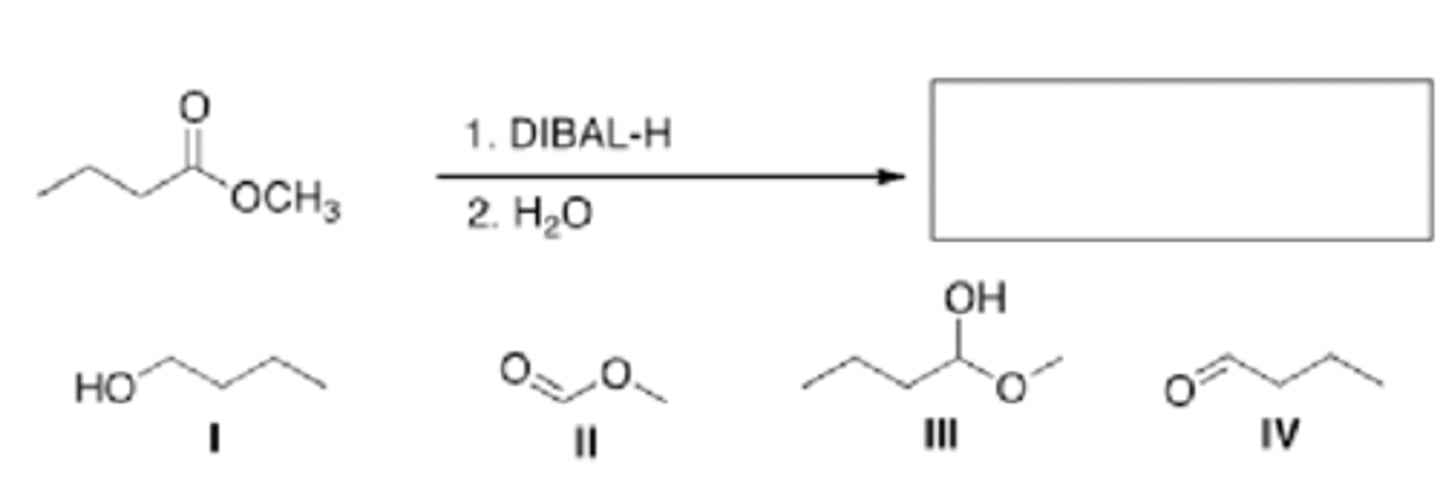
II
Rank the following compounds in order of increasing reactivity in nucleophilic acyl substitution reactions, starting
with the least reactive compound.
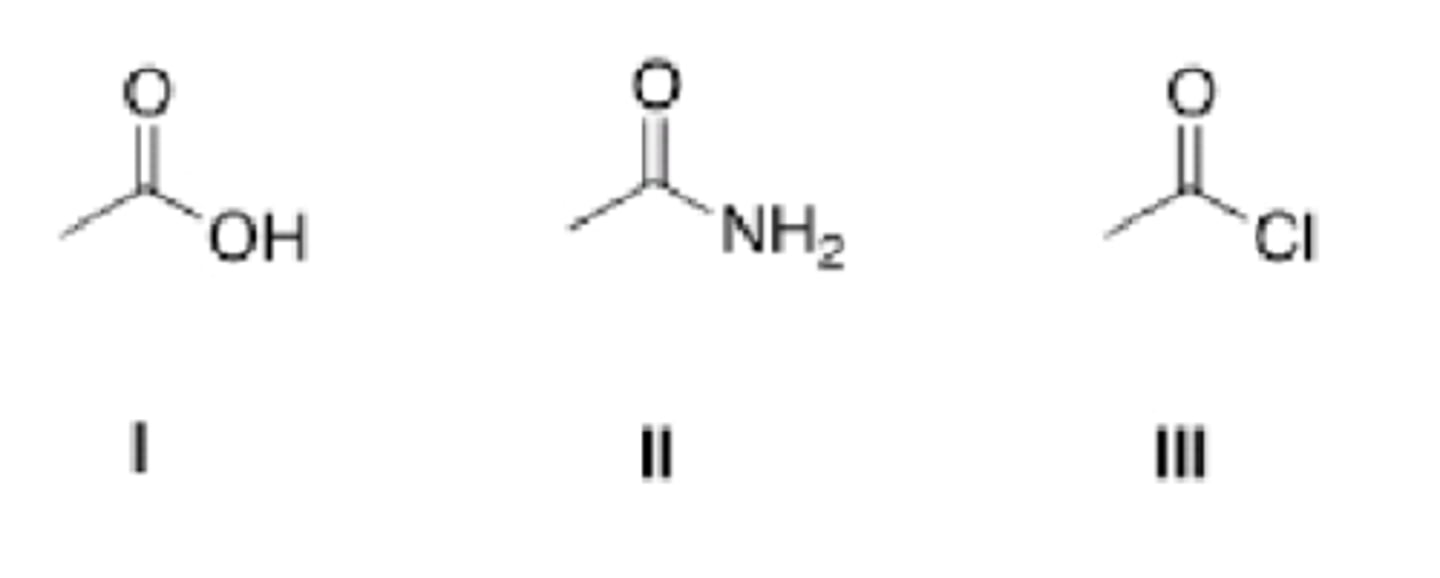
II
What is the major organic product of the following reaction?
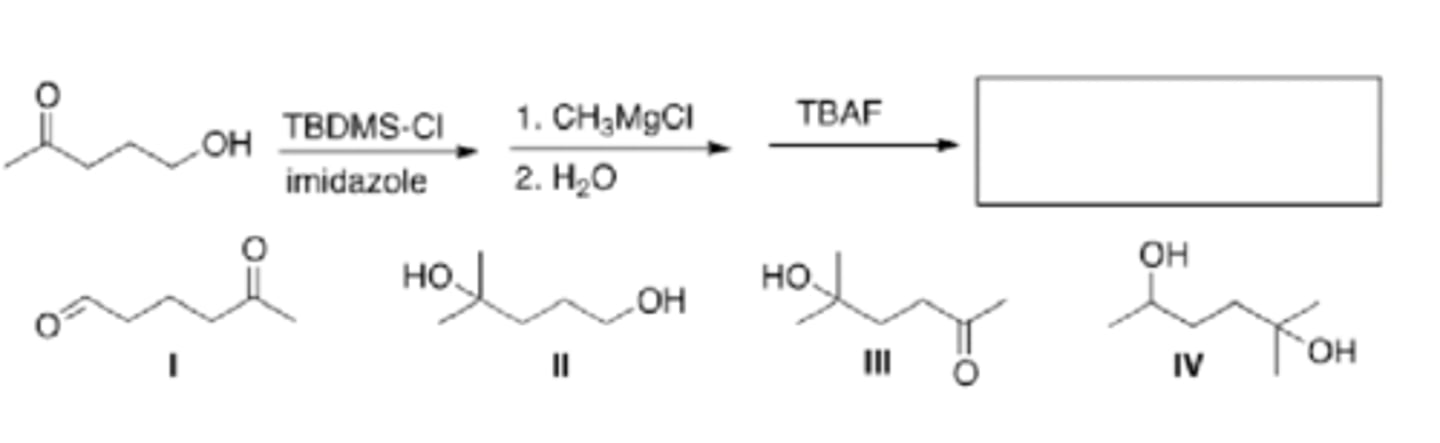
IV
What is the major organic product of the following reaction?
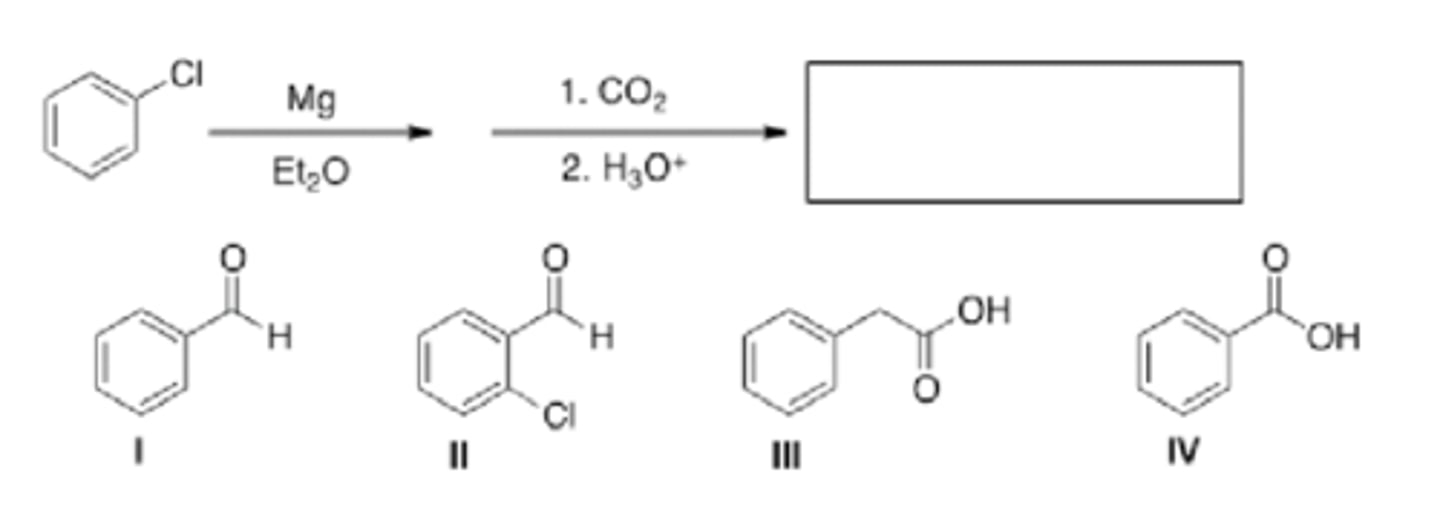
I
What is the major organic product of the following reaction?
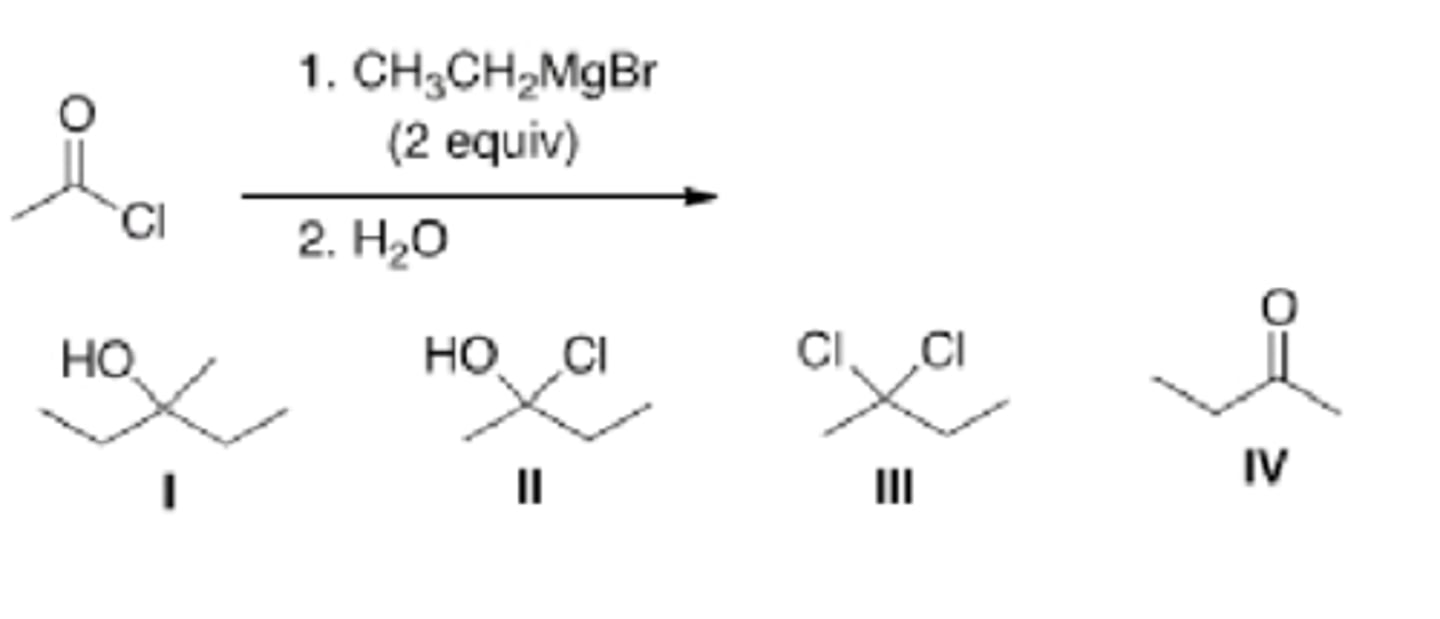
I
What is the major organic product of the following reaction?
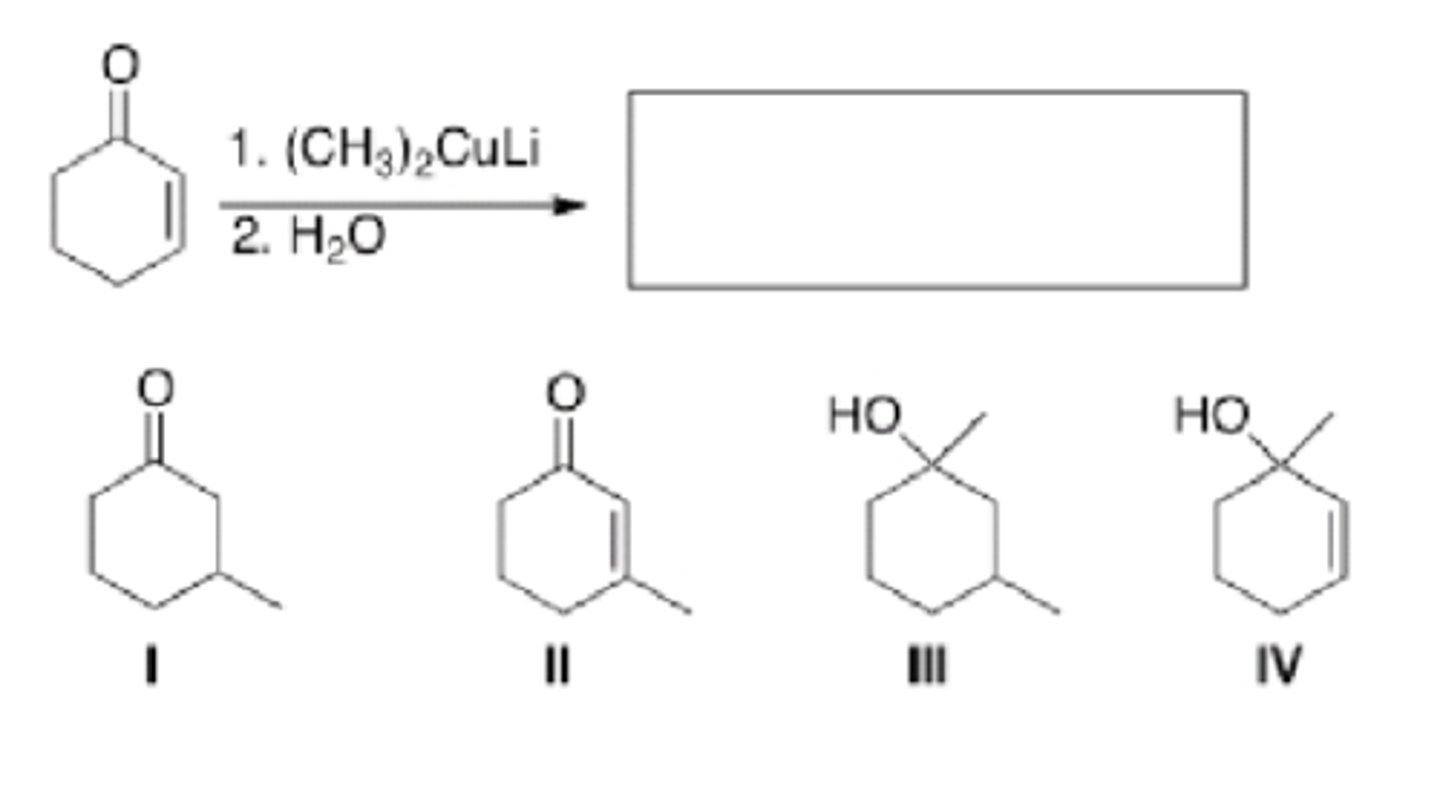
II
What is the major organic product of the following reaction?
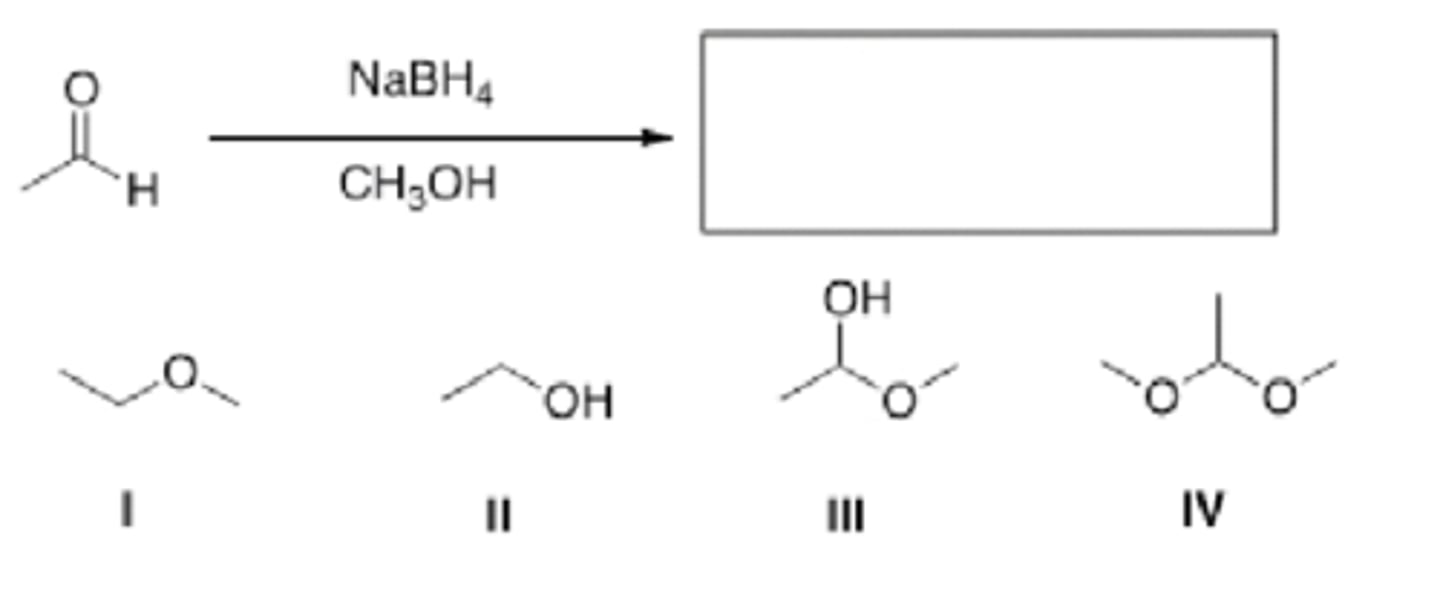
-5 kcal/mol
for step [1], 58 kcal/mol
chain propagation step, only 2 and 3
This reaction could concievably occur by the following chain mechanism [1], [2], and [3]. Determine DH for step [2].
![<p>This reaction could concievably occur by the following chain mechanism [1], [2], and [3]. Determine DH for step [2].</p>](https://knowt-user-attachments.s3.amazonaws.com/2a0ab7b6-0602-4b48-a2c6-7a09f7ef1ab3.jpg)
2
How many products, including stereoisomers, are formed when R-2,4-dimethylpent-2-ene is treated with HBr in presence of peroxides?
I and II
2
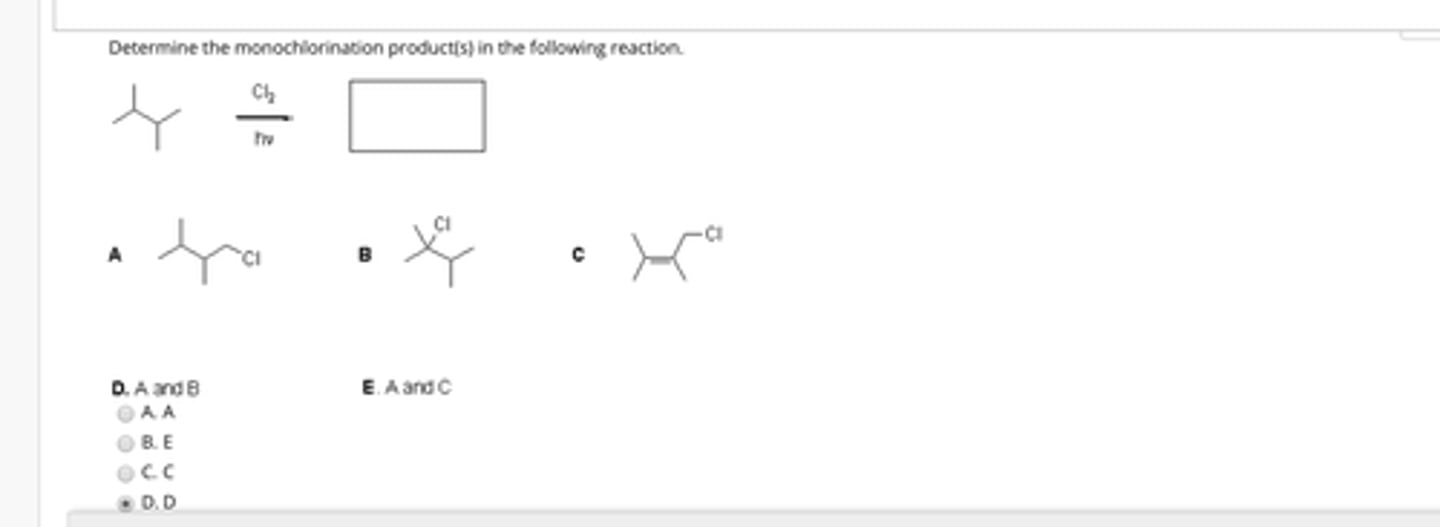
How many monochlorination products can be formed from the reaction of (ch3)3Ch with Cl2 and Hv?
4
How many monocholrination products (constitutional isomers and stereoisomers) are formed from the reaction of pentane with Cl2 and hv?
radicals rearrange
What of the following statements about the radical reactions is NOT true?
IV

III
Which of the indicated hydrogens is most readily abstracted in a free radical halogenation reaction?
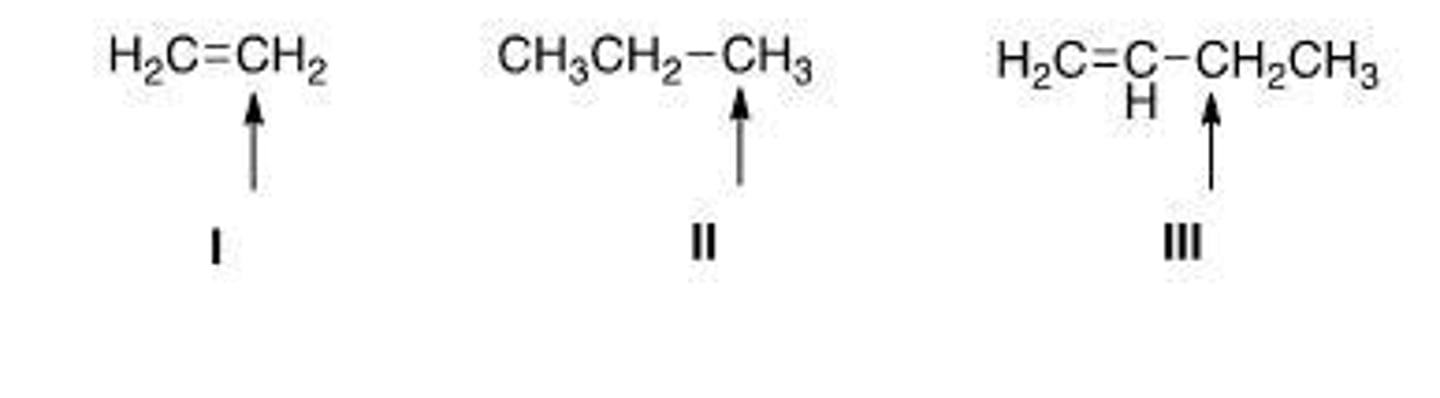
propagation
Which steps are the rate-determining steps in the mechanism of radical halogenation?
IV
What is the product of the following reaction?
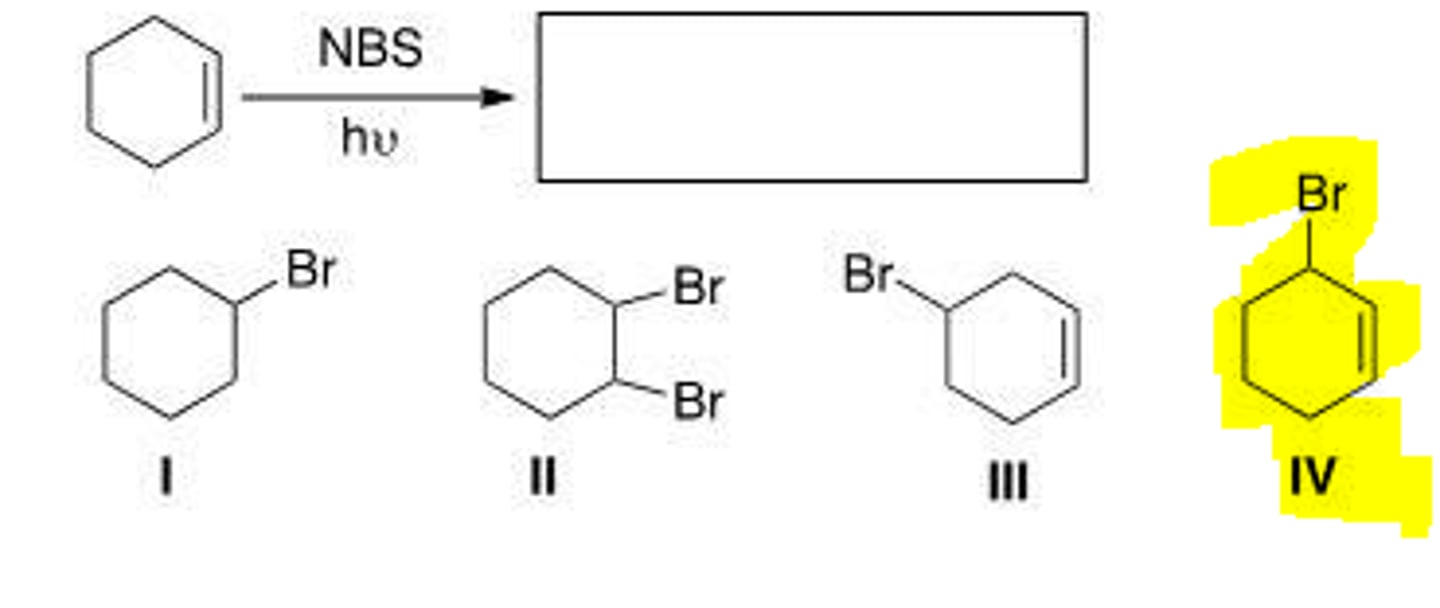
The first chain-propagating steps is rate-determining.
The reaction proceeds by way of a flat sp2 hybridized free radical
Which of the following statements is TRUE about free radical halogenation of alkenes?
Chlorination is faster than Bromination
Which of the following statements about Chlorination and Bromination is TRUE?
An achiral starting material always gives either an achiral or a racemic product
Which of the following statements about the stereochemistry of halogenation reactions is TRUE?
4
How many allylic halides can be formed when 3-methylcyclohexane undergoes allylic halogenation with one equivalent of NBS and light?
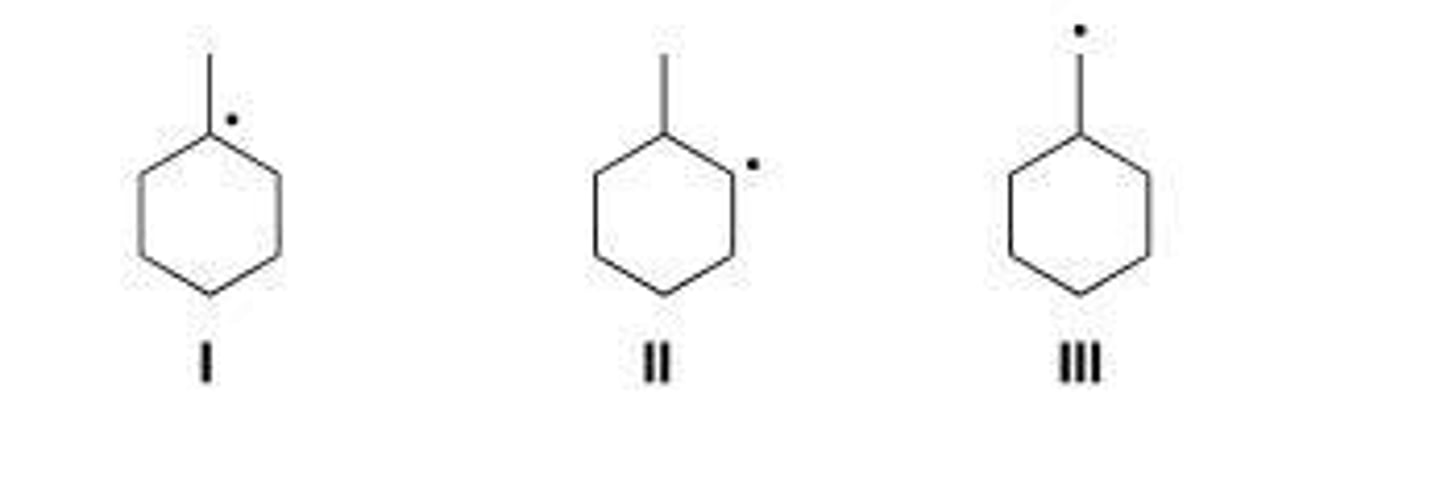
III
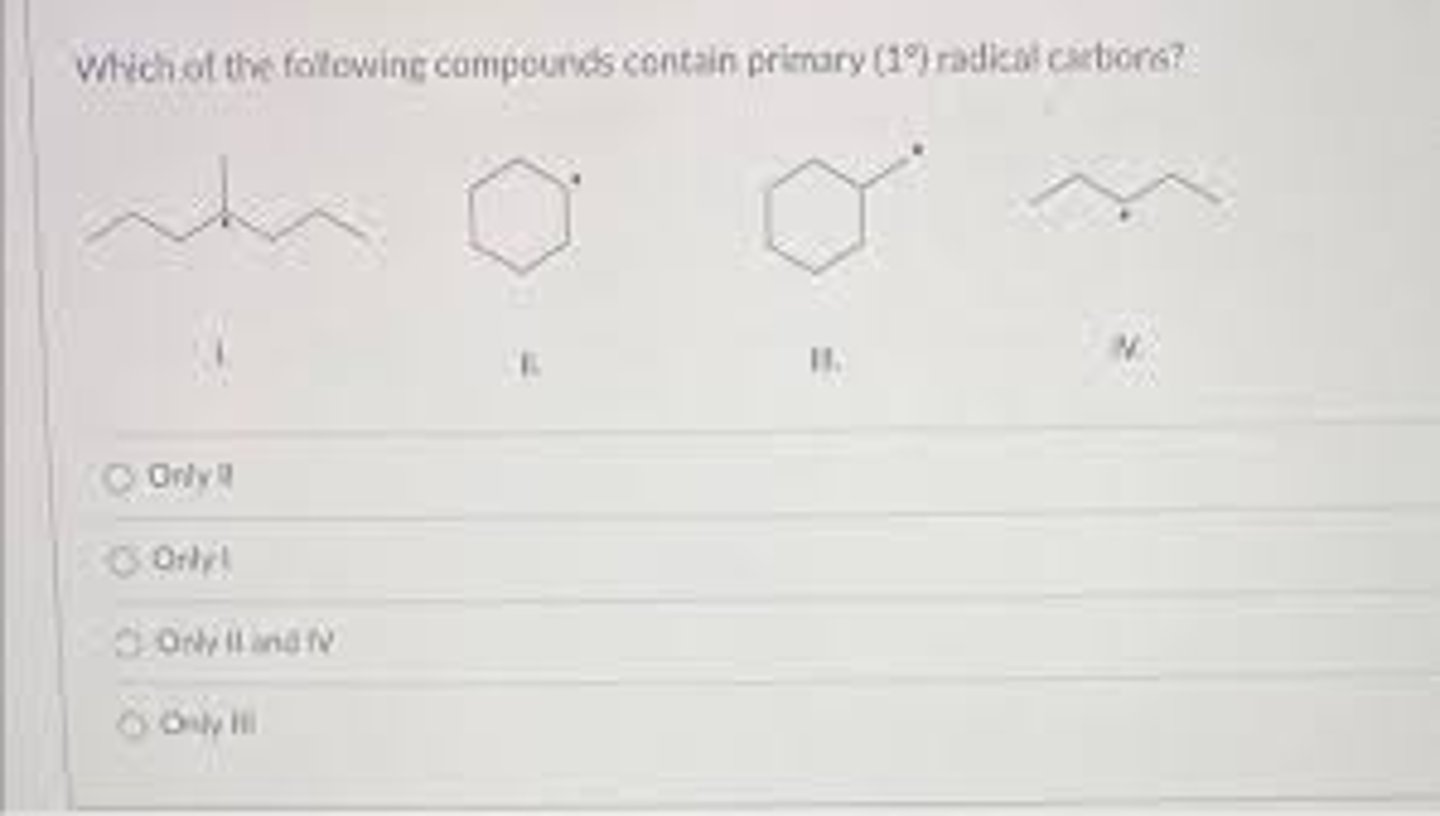
secondary radical carbon, IV
tertiary radical carbon, I
O2
Which of the following is a radical scavenger?
less stable radicals generally do not rearrange to more stable radicals
Which of the following statements about radicals is TRUE?
II
What is the product in the following sequence reation?
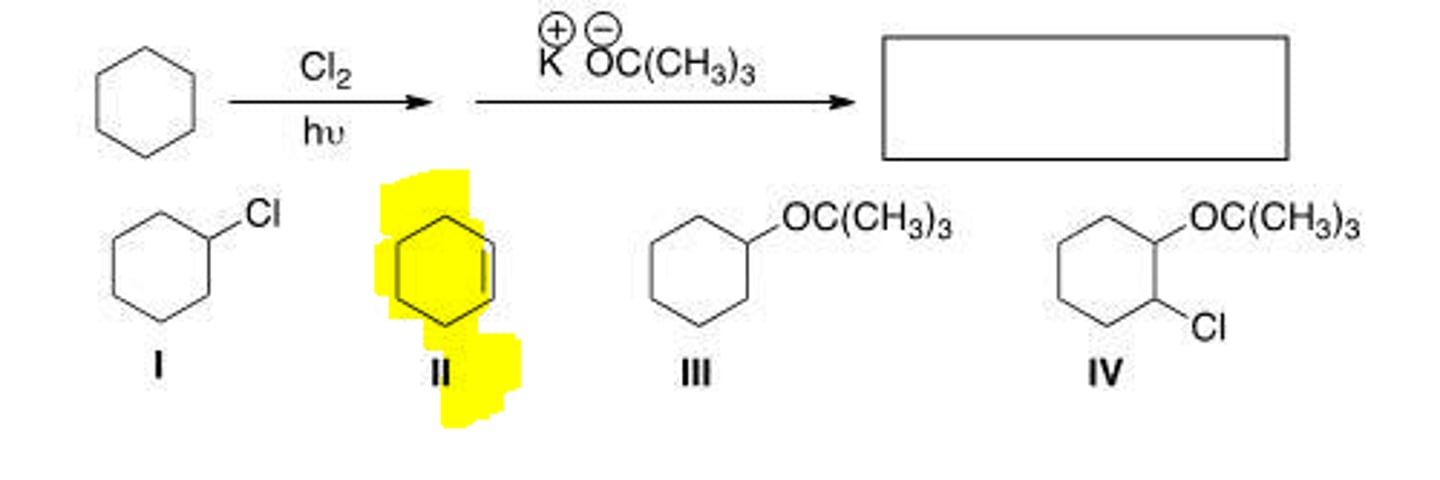
I
What is the product of the following reaction?
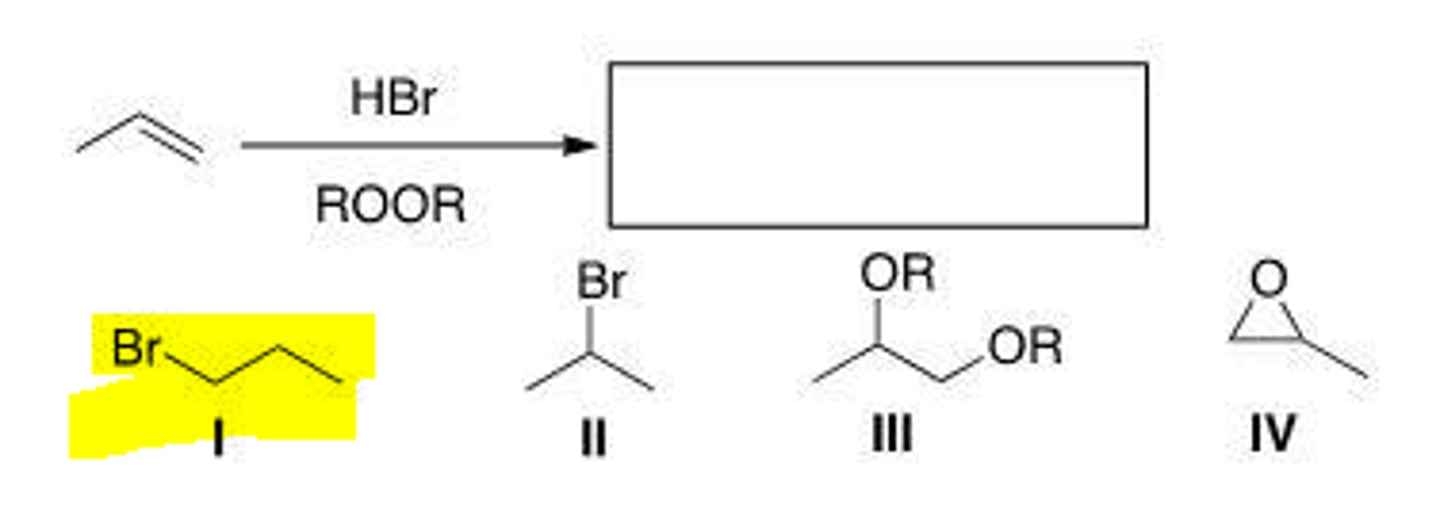
III
Rank the following radicals in order of increasing stability, putting the least stable first.