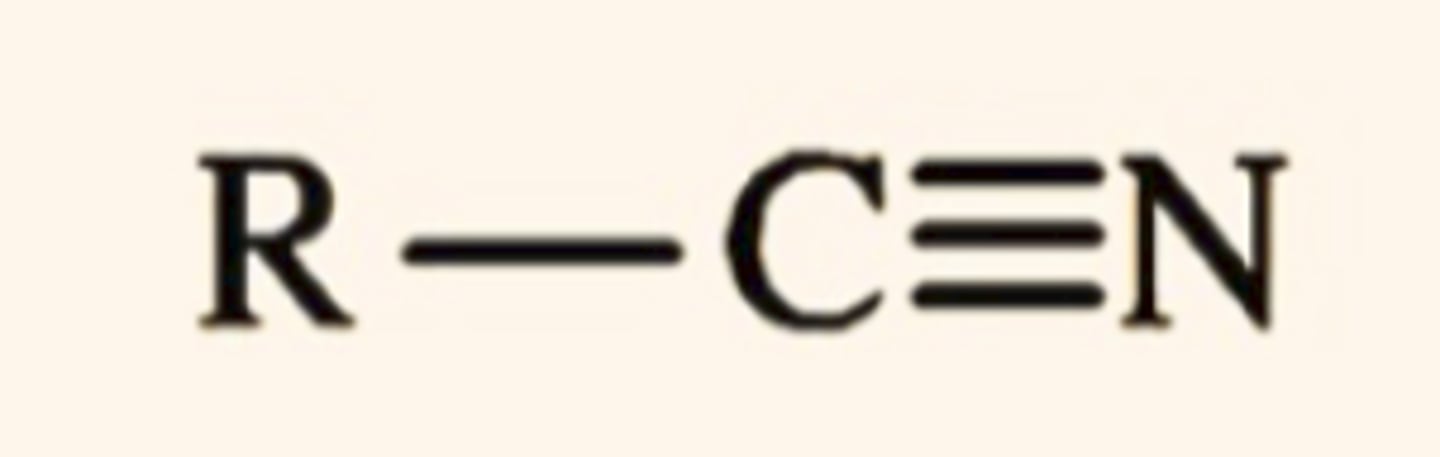Electron Geometry and Functional Groups
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Molecular Geometry: Linear
- electron groups: 2
- bonding groups: 2
- lone pairs: 0
- electron geometry: linear
- approximate bond angles: 180º
- hybridization scheme: sp
Molecular Geometry: Trigonal Planar
- electron groups: 3
- bonding groups: 3
- lone pairs: 0
- electron geometry: trigonal planar
- approximate bond angles: 120º
- hybridization scheme: sp2
Molecular Geometry: Bent (eg 3)
- electron groups: 3
- bonding groups: 2
- lone pairs: 1
- electron geometry: trigonal planar
- approximate bond angles: <120º
- hybridization scheme: sp2
Molecular Geometry: Tetrahedral
- electron groups: 4
- bonding groups: 4
- lone pairs: 0
- electron geometry: tetrahedral
- approximate bond angles: 109.5º
- hybridization scheme: sp3
Molecular Geometry: Trigonal Pyramidal
- electron groups: 4
- bonding groups: 3
- lone pairs: 1
- electron geometry: tetrahedral
- approximate bond angles: <109.5º
- hybridization scheme: sp3
Molecular Geometry: Bent (eg 4)
- electron groups: 4
- bonding groups: 2
- lone pairs: 2
- electron geometry: tetrahedral
- approximate bond angles: <109.5
- hybridization scheme: sp3
Alkyl Halide
- X (7a halogen) + carbon
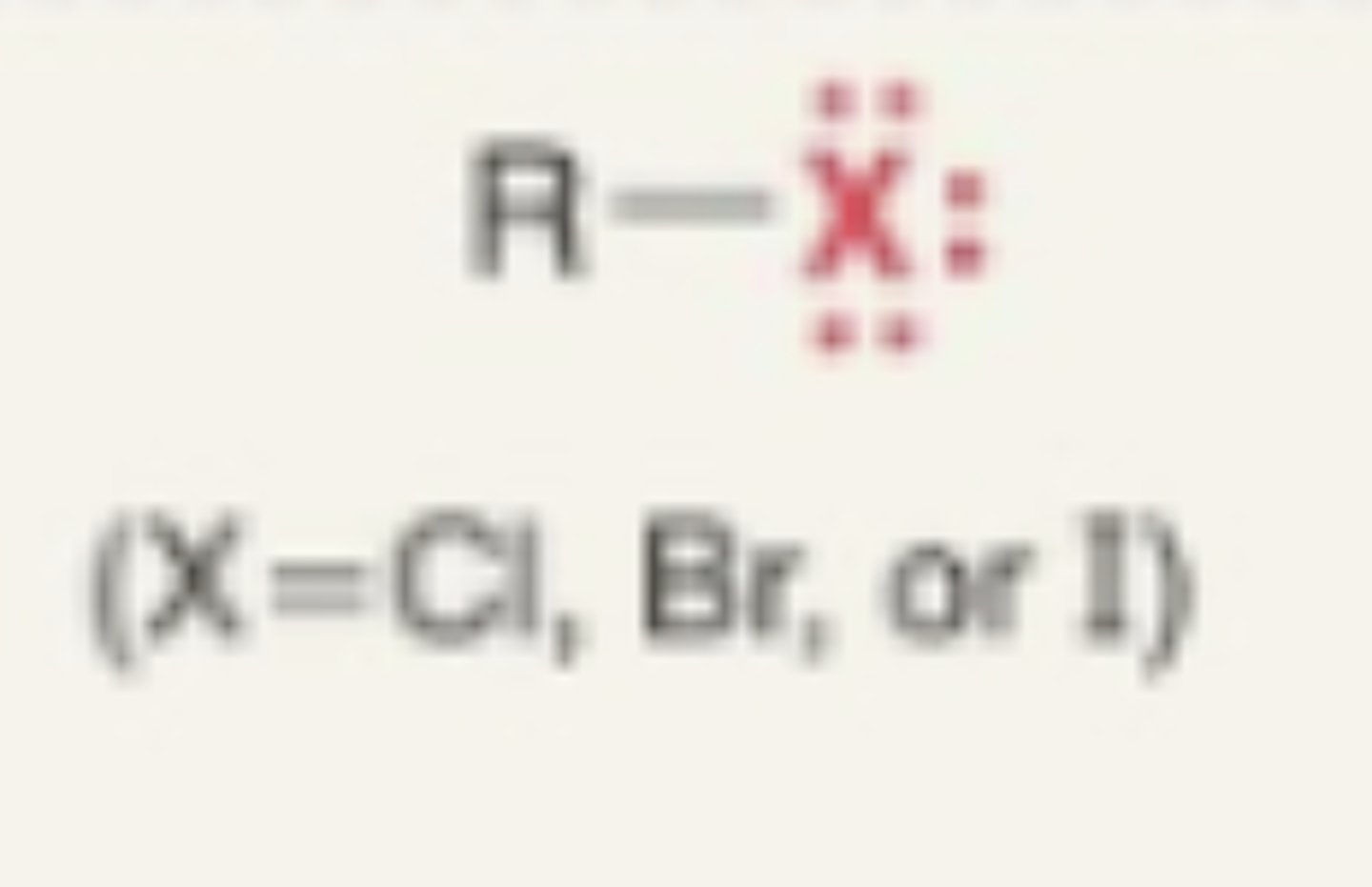
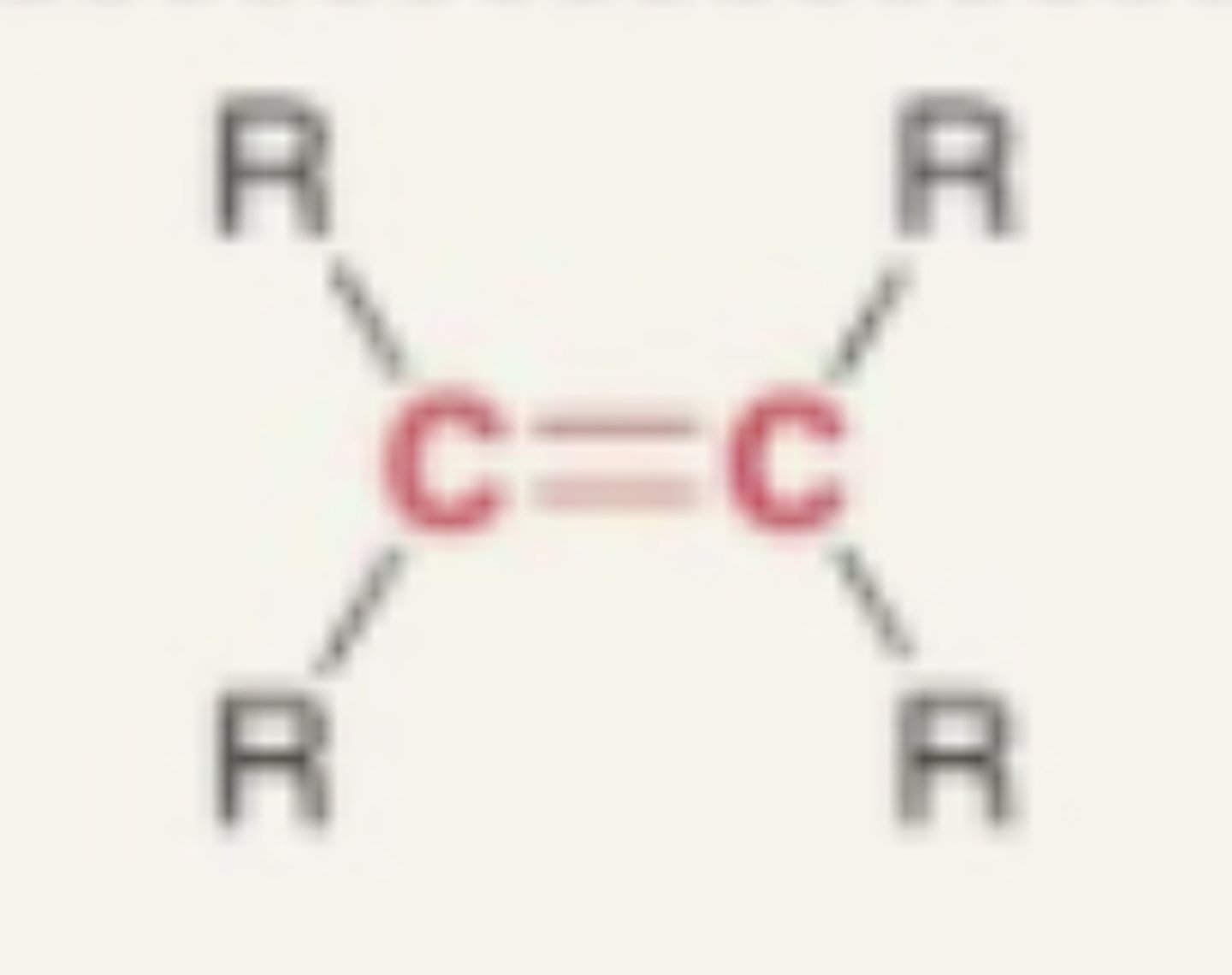
Alkene
- double bonded carbon
Alkyne
- triple bonded carbon

Alcohol
- OH + carbon
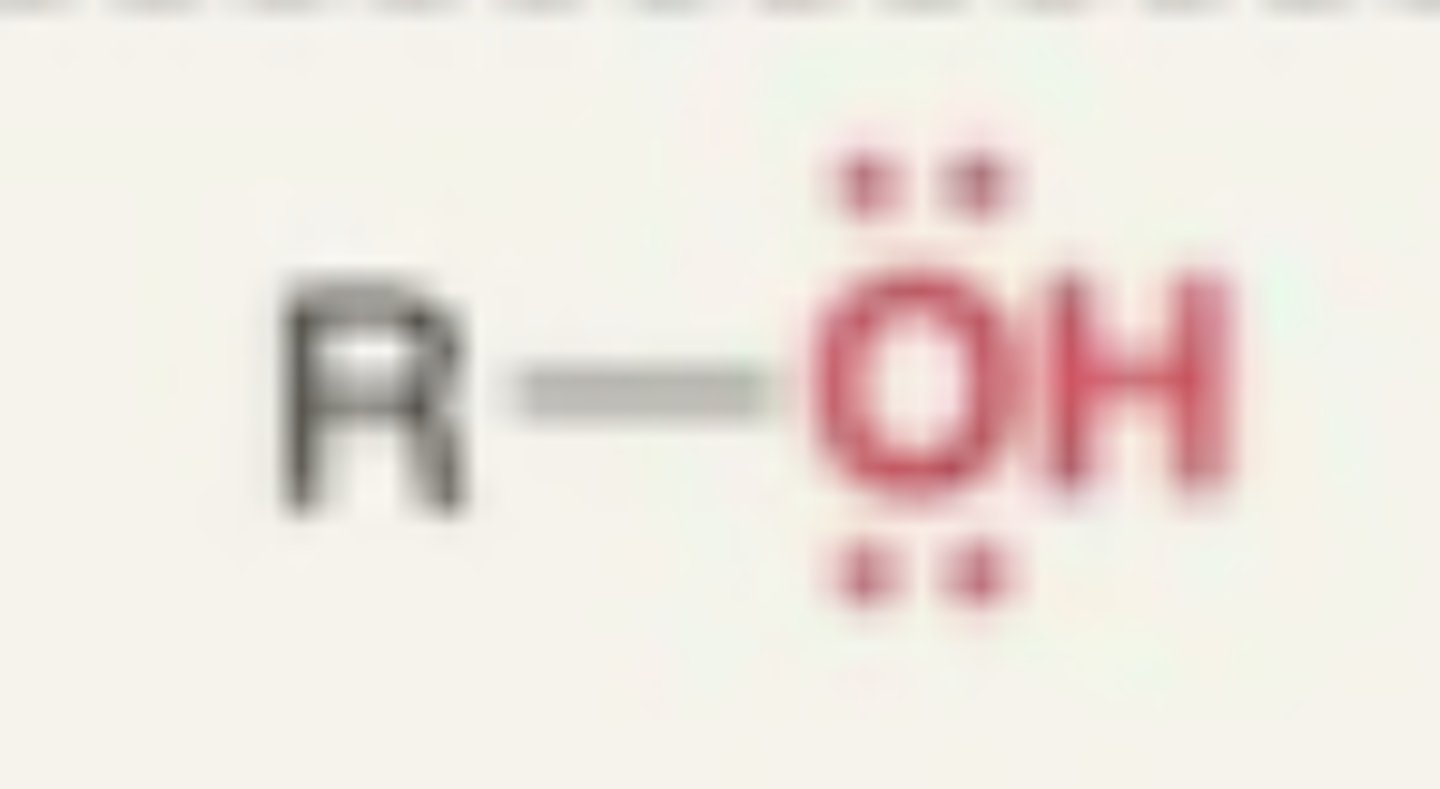
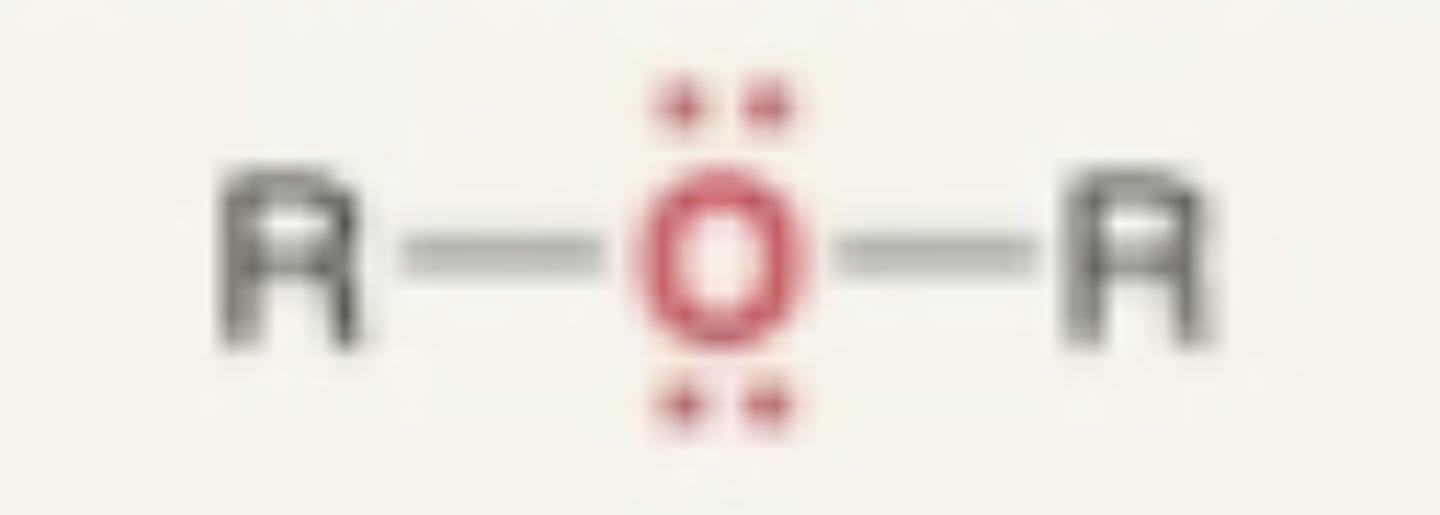
Ether
- O in between 2 carbons
Thiol
- sulfur and hydrogen + carbon
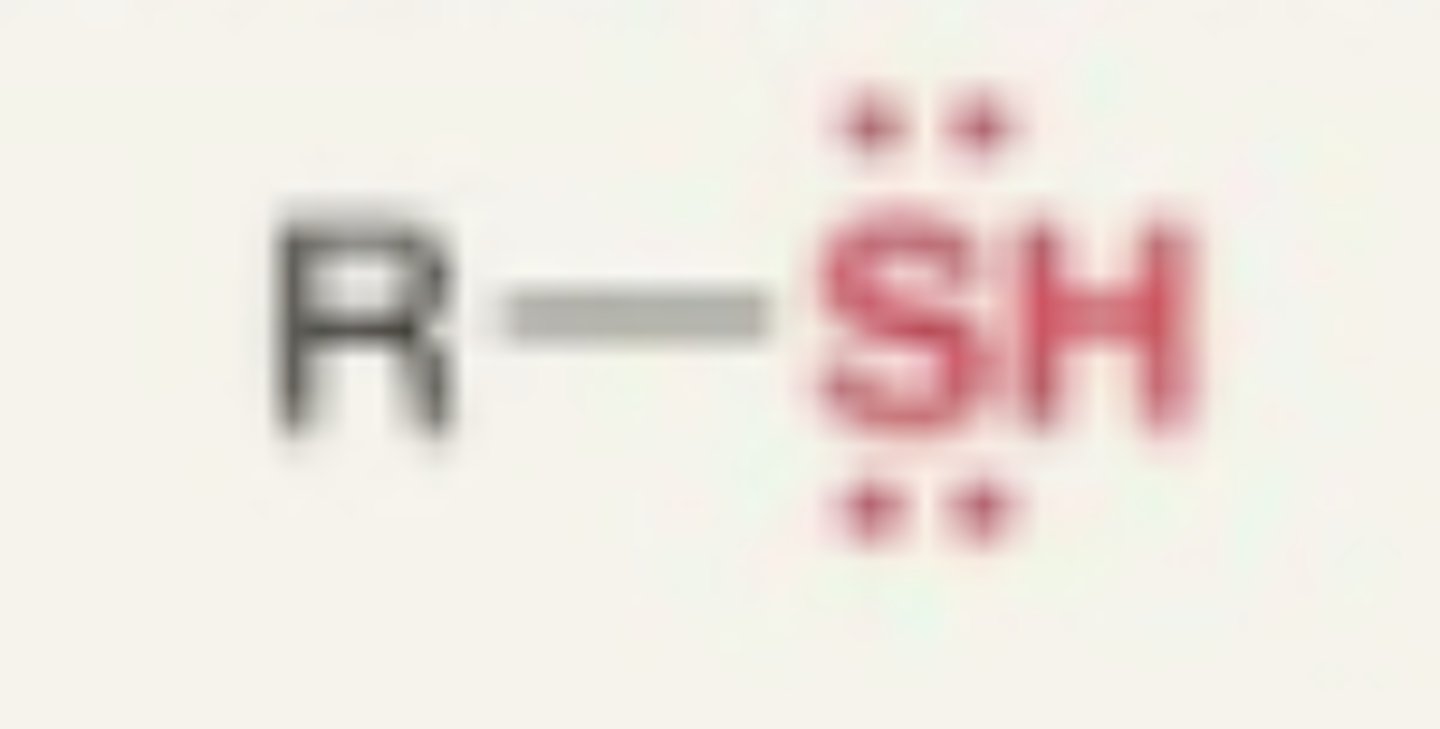
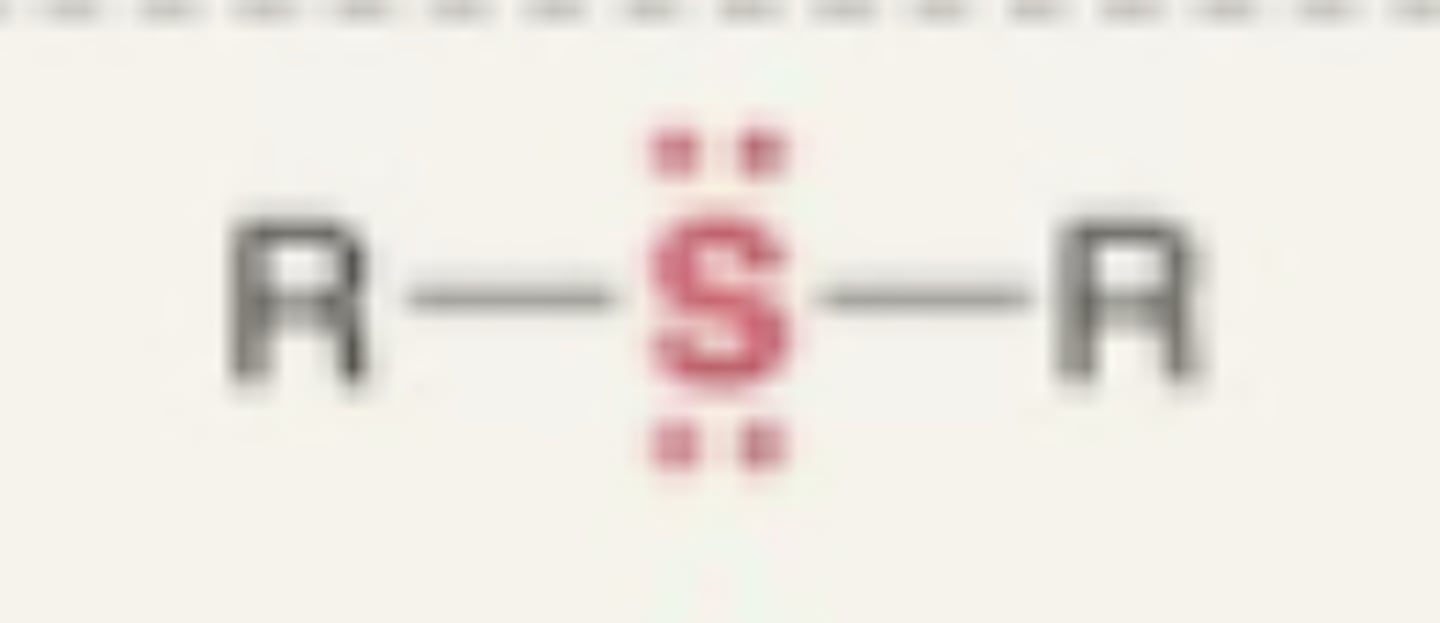
Sulfide
- sulfur between to carbons
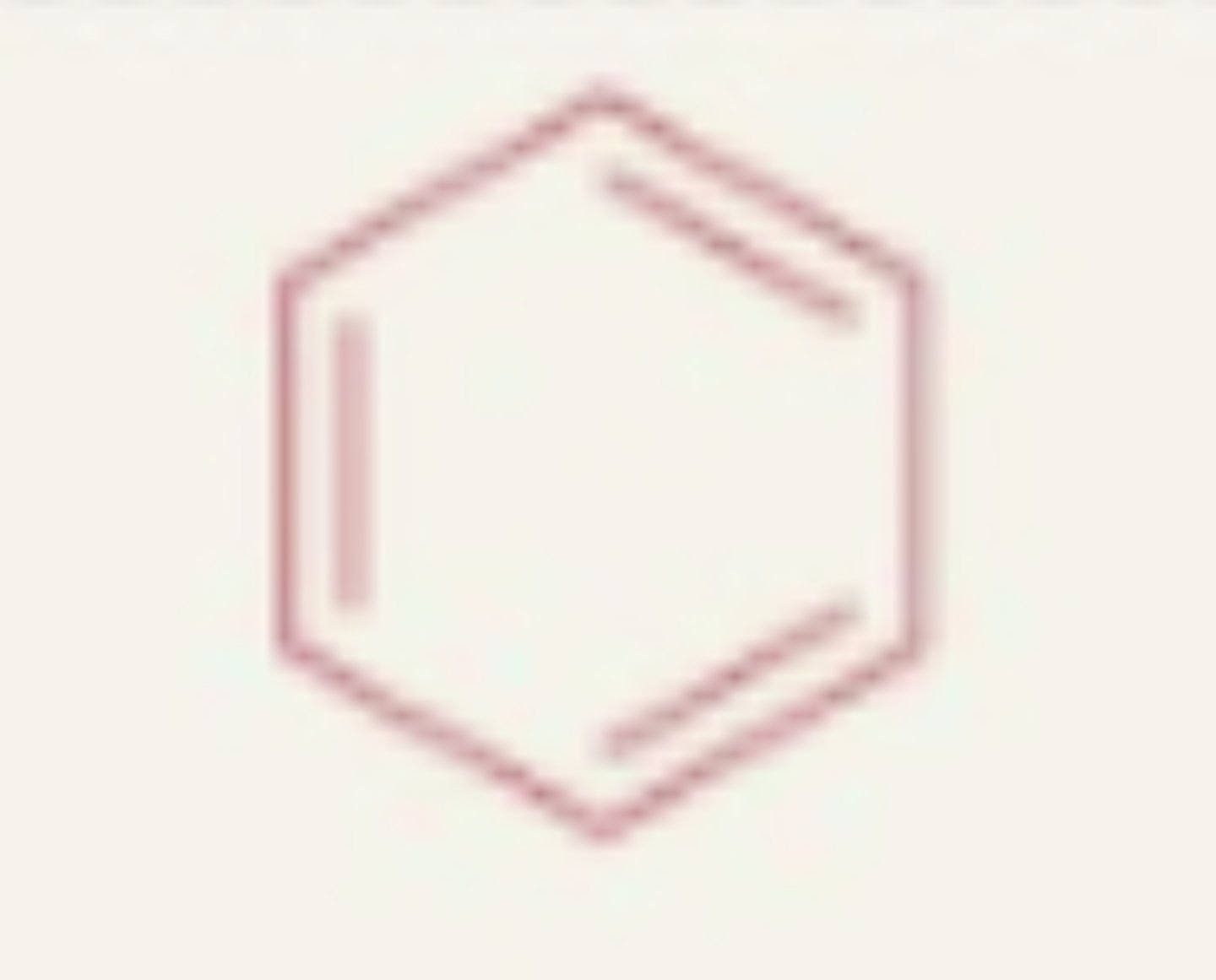
Aromatic (arene)
- ring of carbons
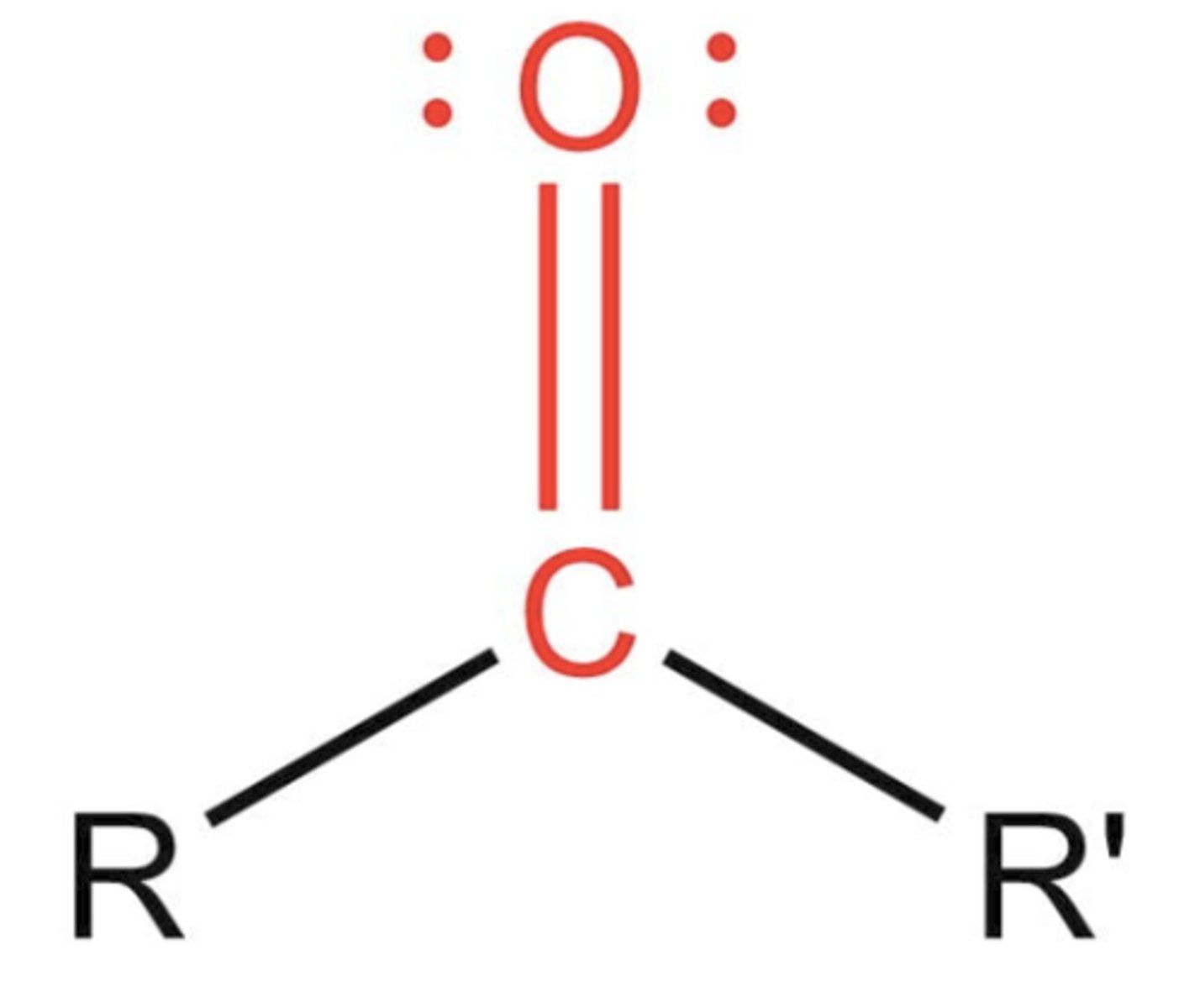
Ketone
- carbonyl group attached to 2 carbons
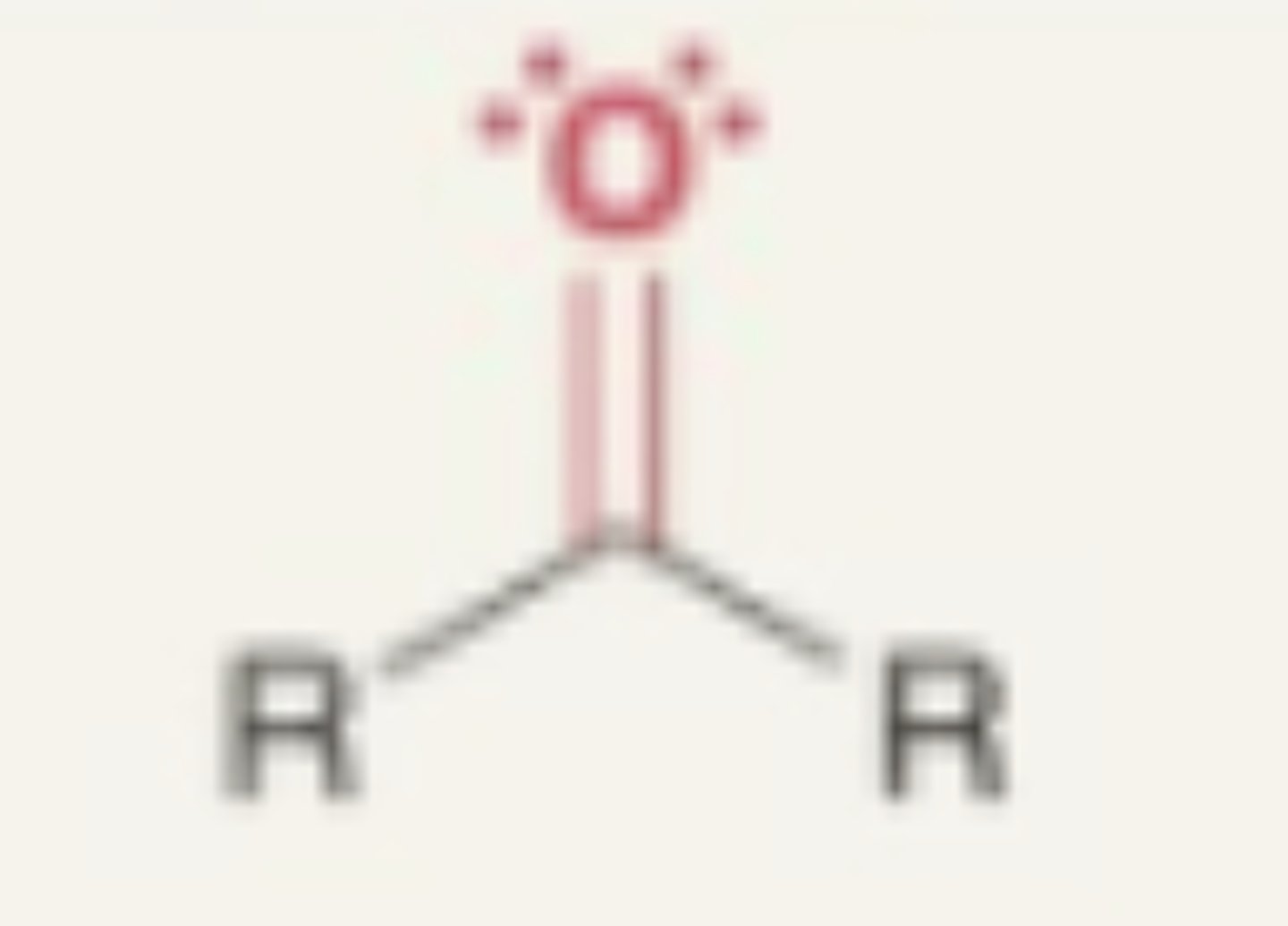
Carbonyl
- not official functional groups
- O double bonded to carbon just that part
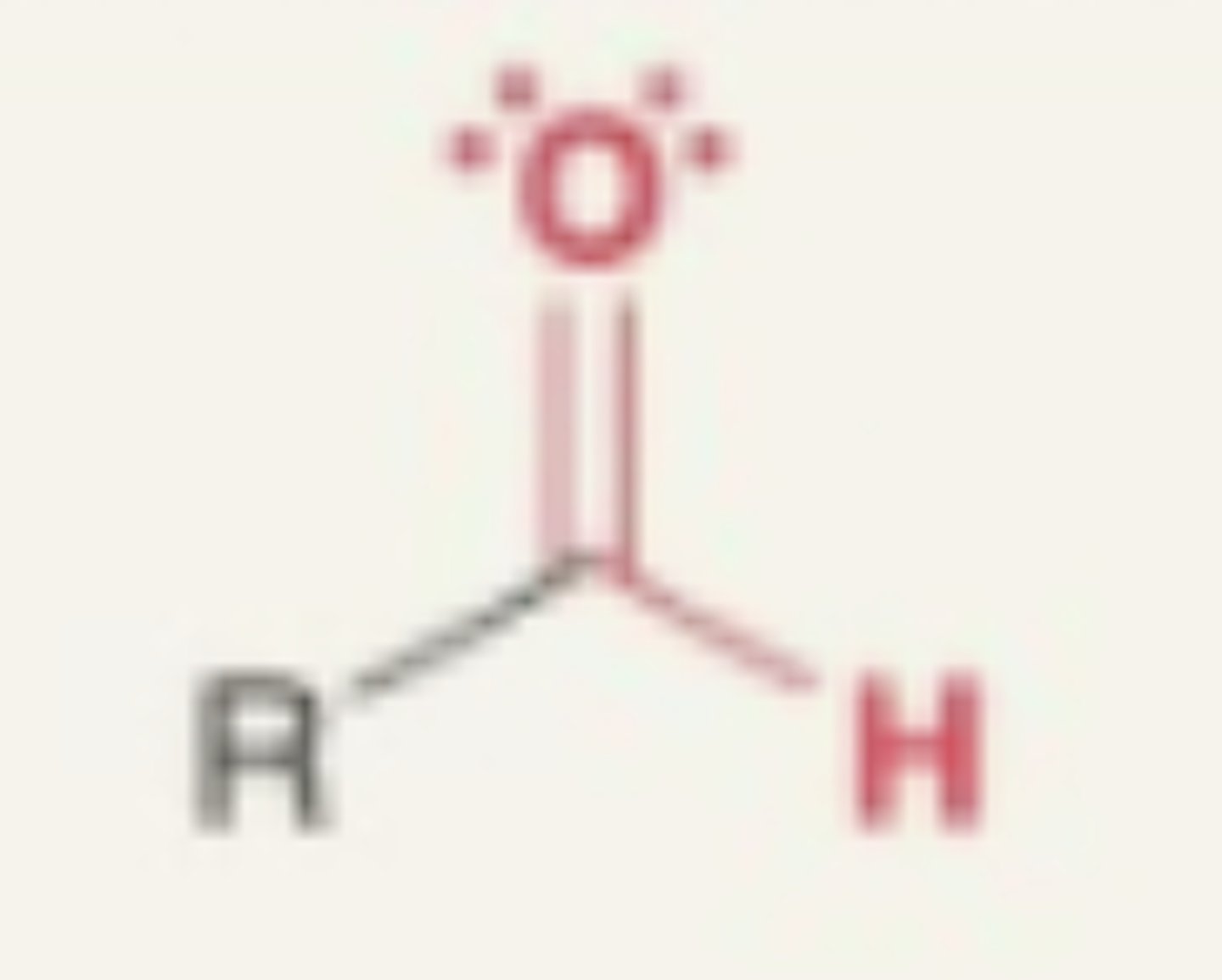
Aldehyde
- carbonyl + hydrogen
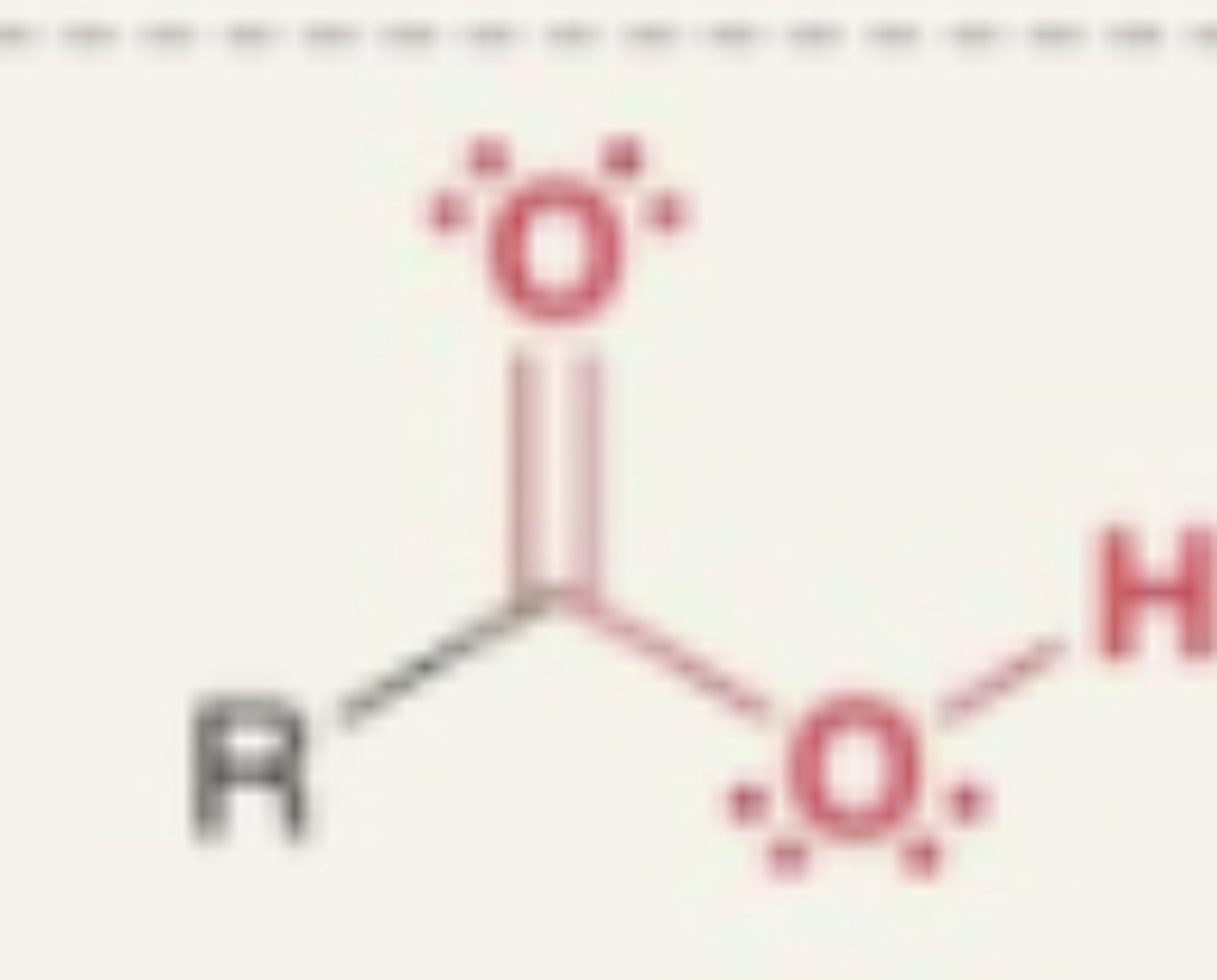
Carboxylic Acid
- carbonyl + OH
- not an alcohol
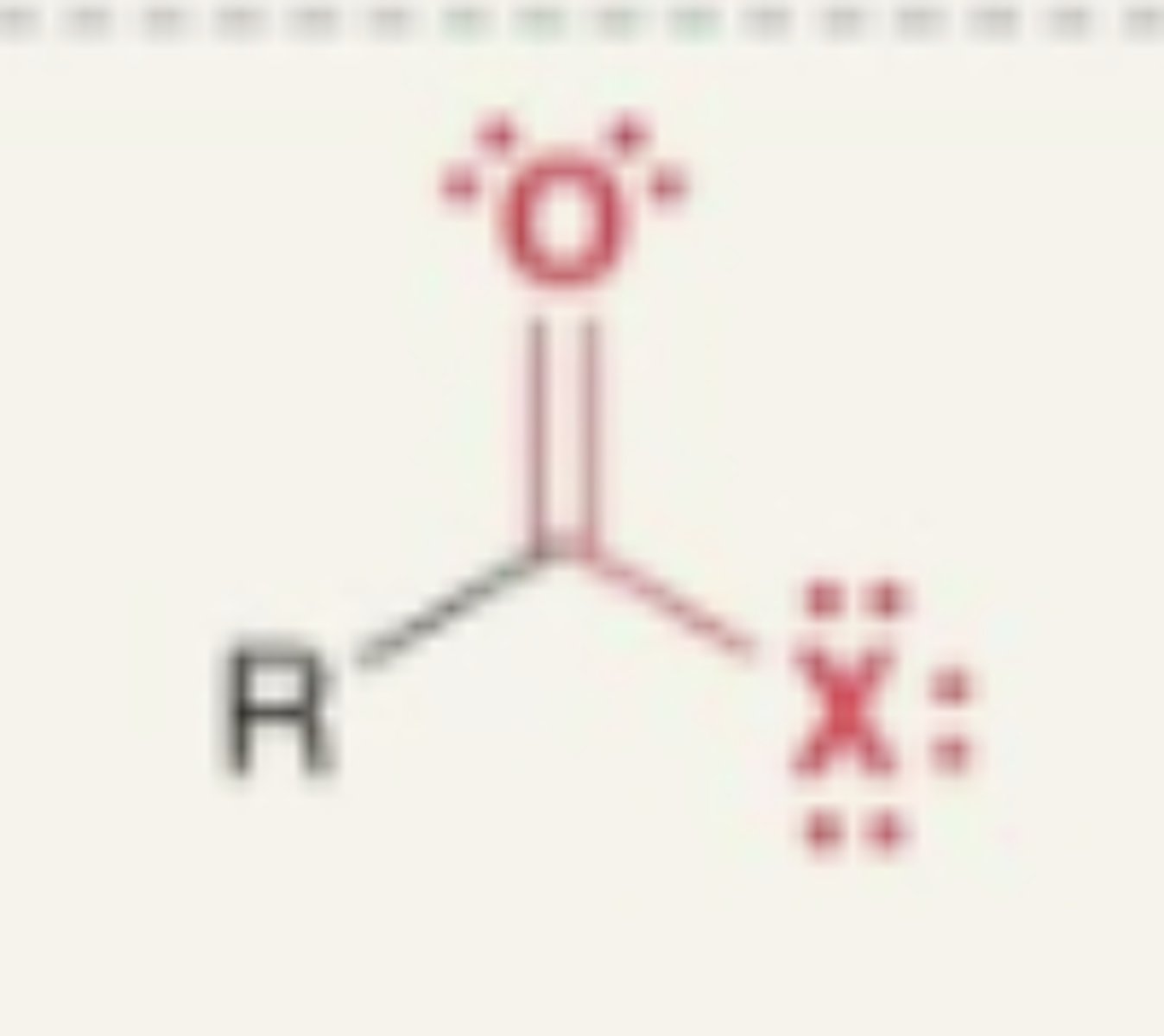
Acyl Halide
- carbonyl + X (7a Halogen)
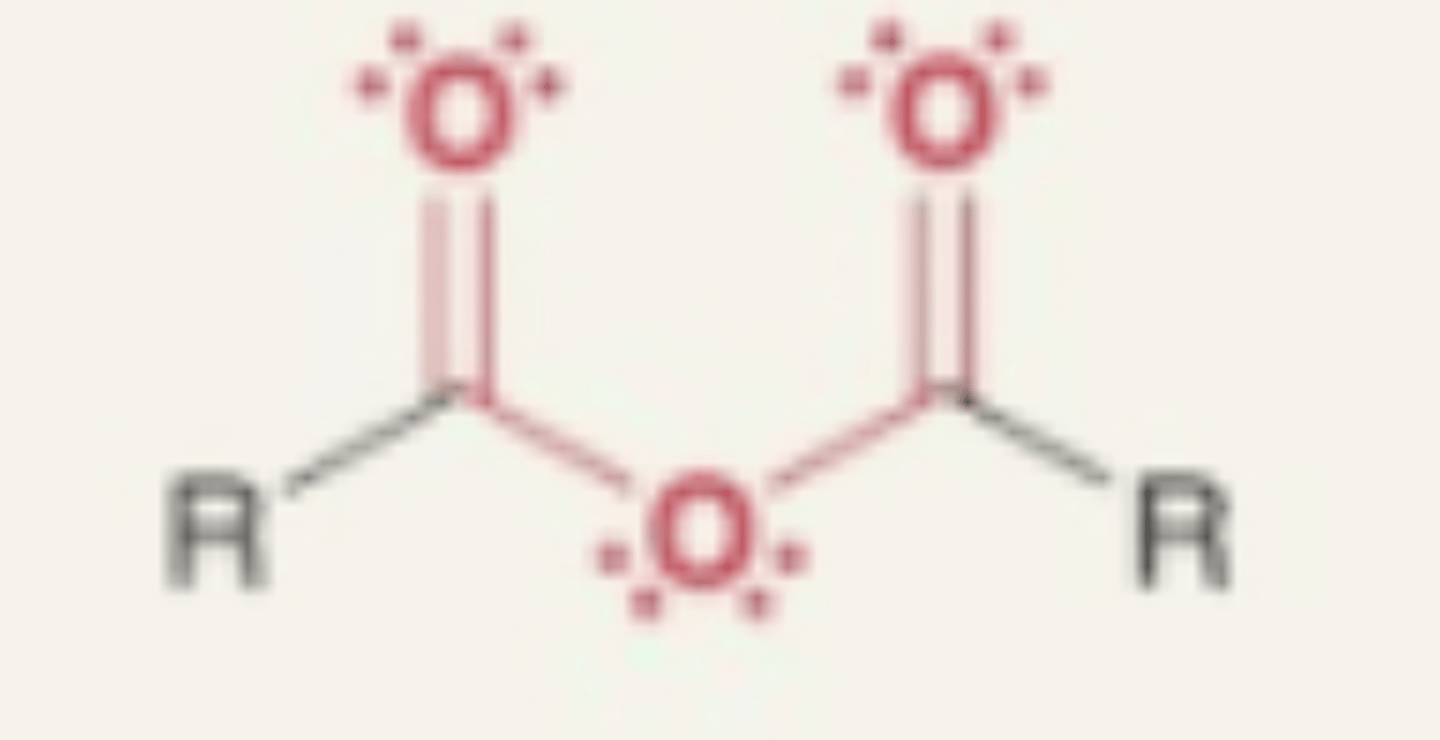
Anhydride
- carbonyl + O + carbonyl
- O between 2 carbonyl
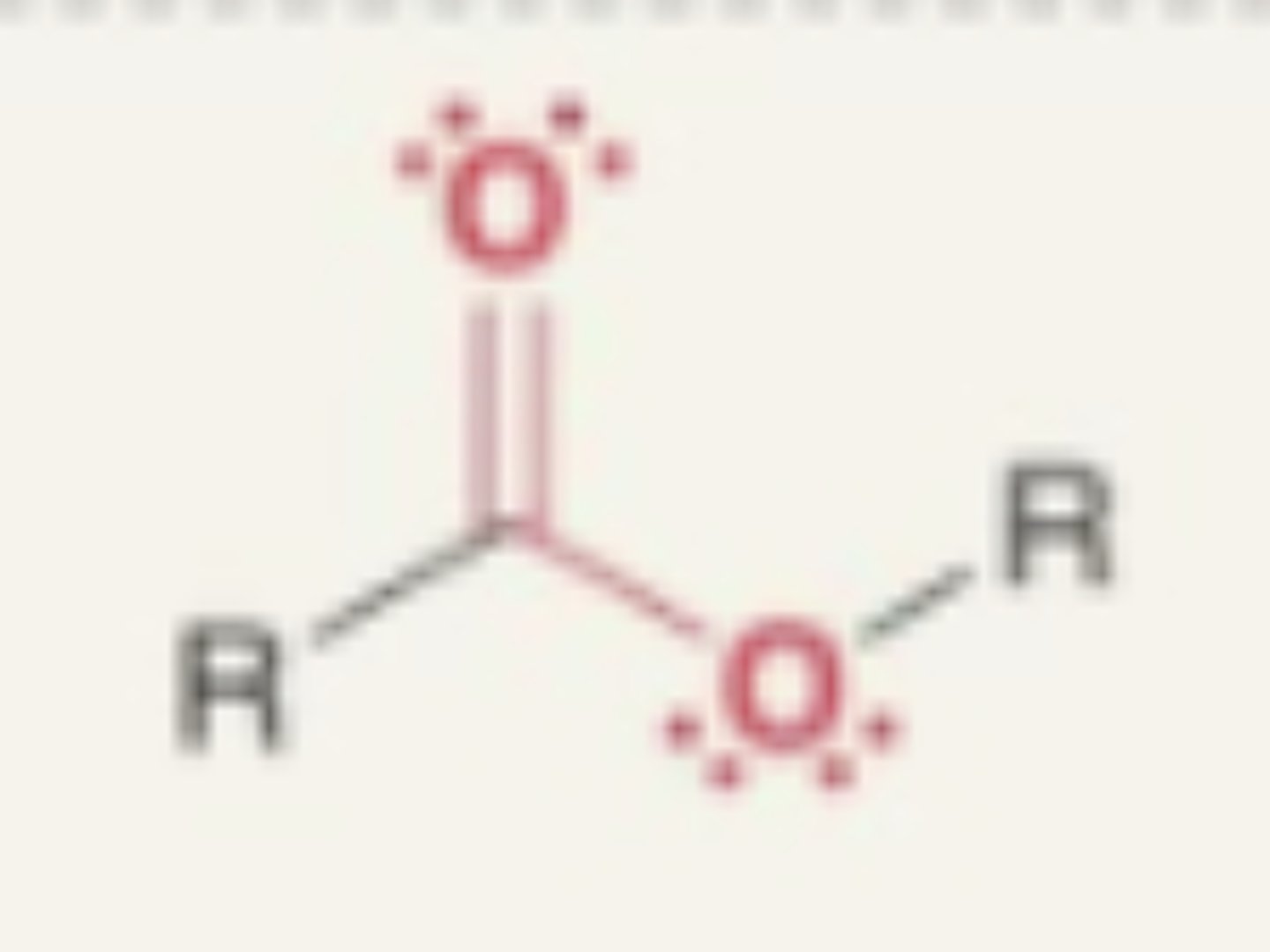
Ester
- carbonyl + O + R
- oxygen between carbon and carbonyl
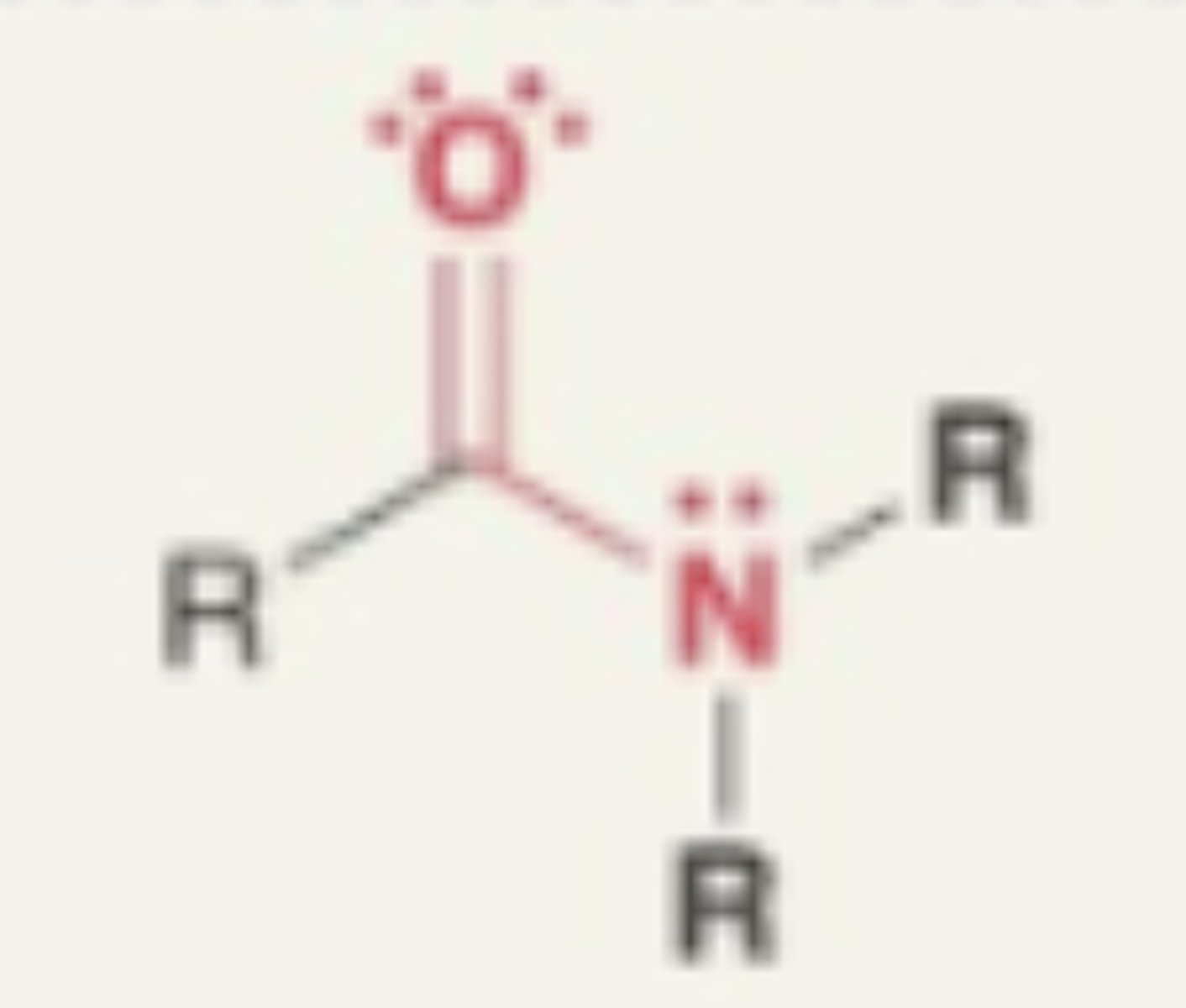
Amide
- carbonyl + N
- needs to be attached to C or H
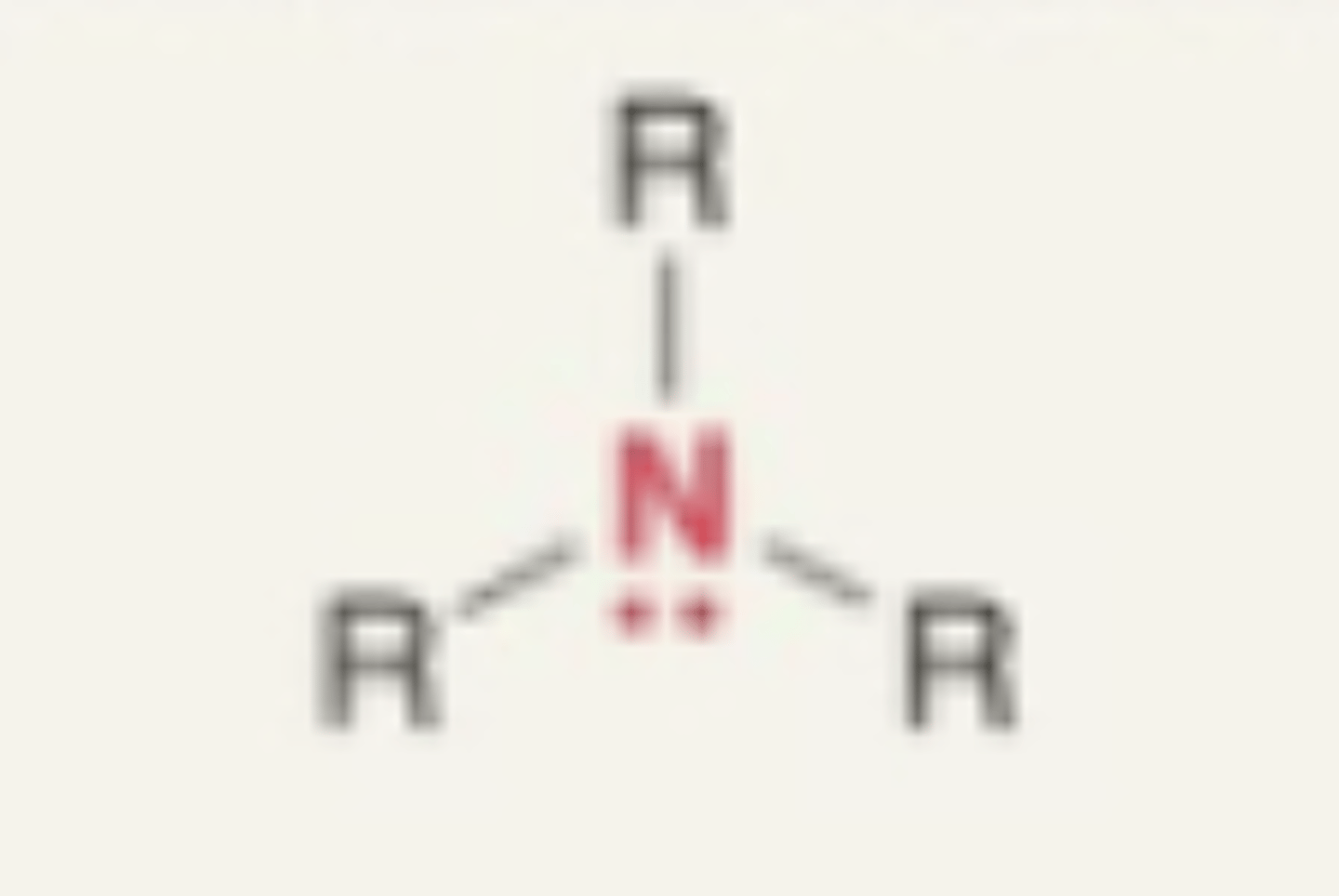
Amine
- N with 3 carbon or hydrogens attached
Nitrile
- carbon triple bonded to nitrogen attached to R