Chemistry - Chapter 2: Atomic Weights
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
isotopes
same elements with different numbers of neutrons and therefore different mass numbers
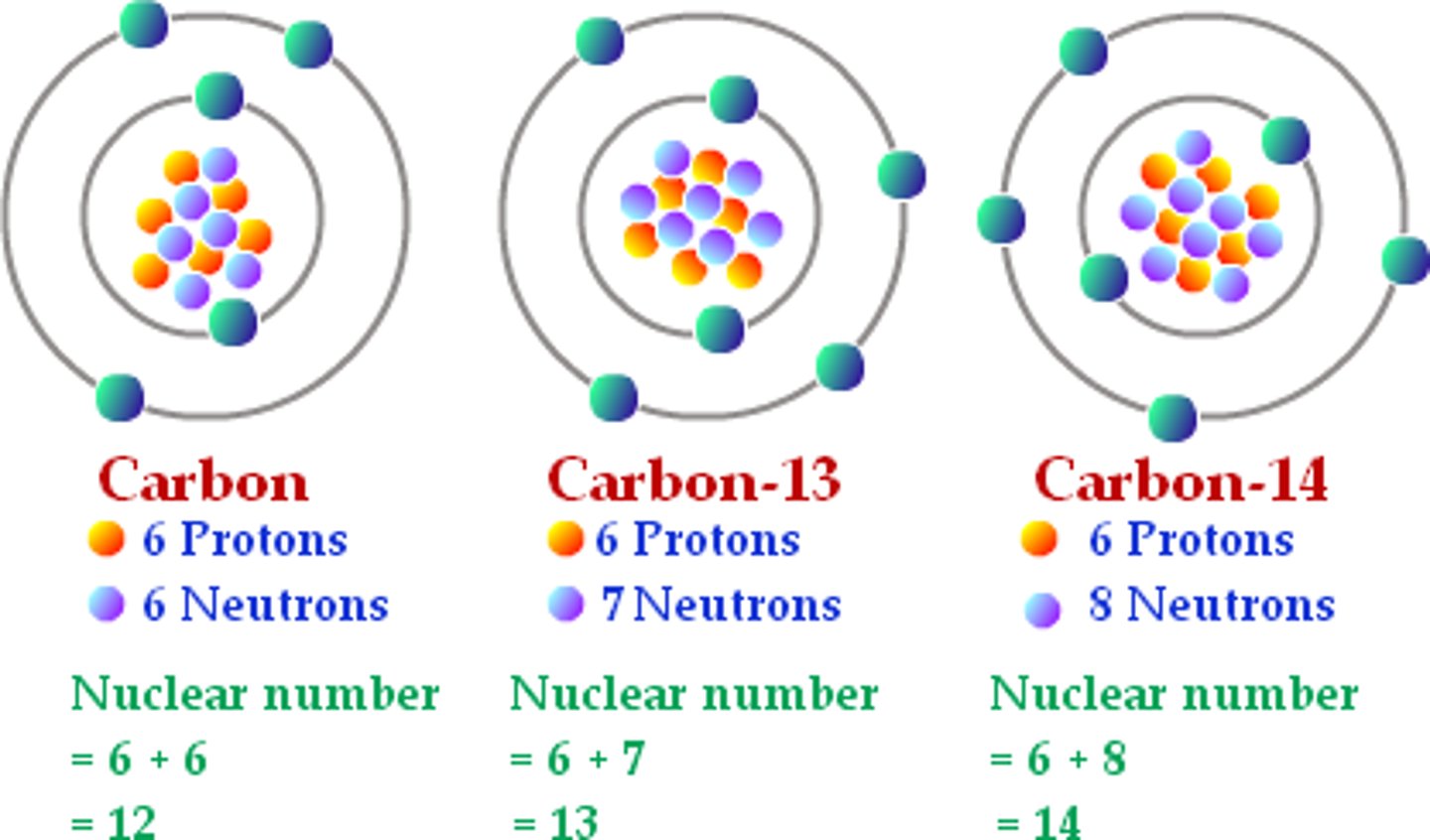
Where are isotopes found?
in nature

What does a mass spectrograph/spectrometer do?
detects the presence and characteristics of isotopes
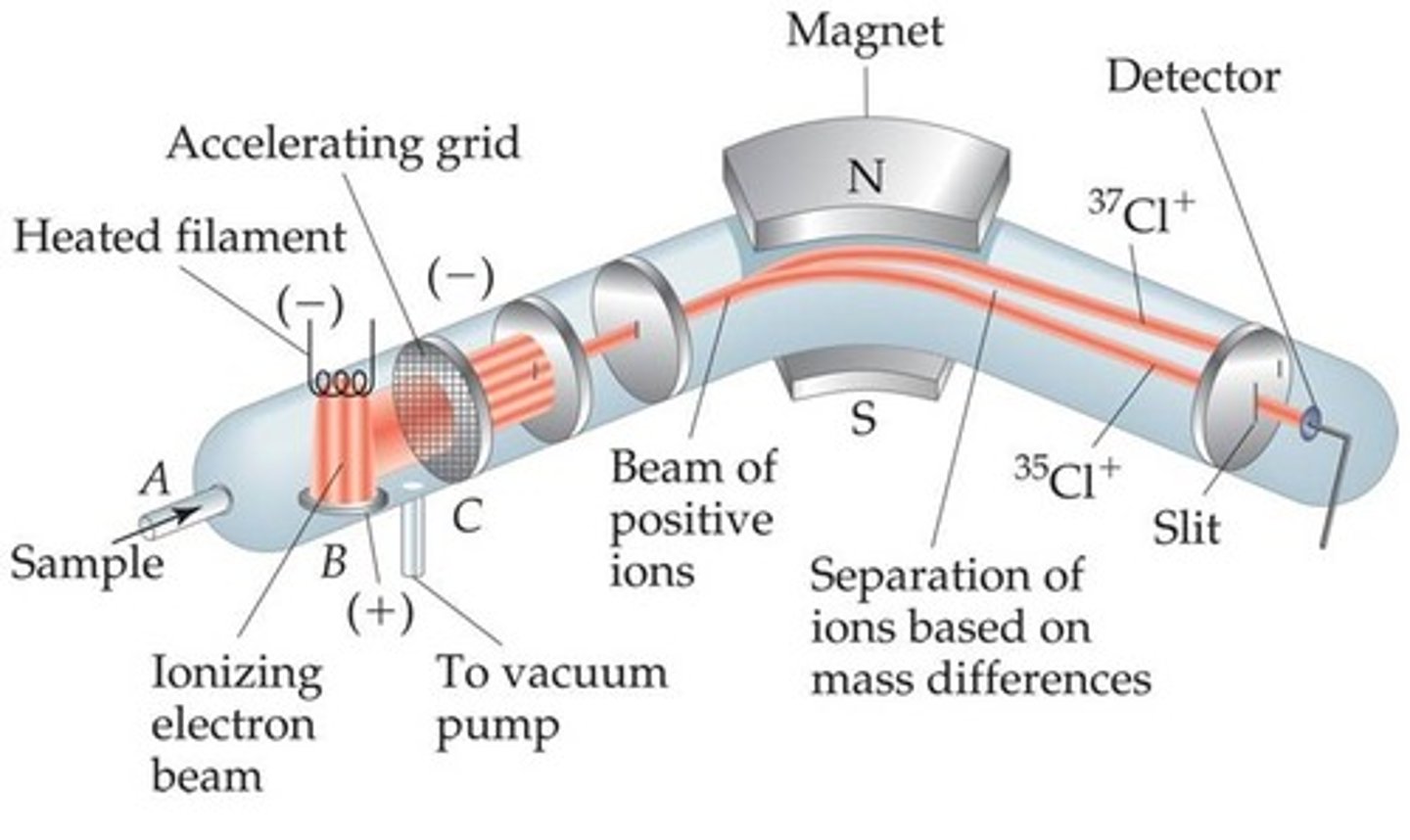
What does a mass spectrograph/spectrometer do?
it charges and accelerates isotopes with an electron beam
What does a mass spectrometer's magnetic field do to the isotopes?
it bends the path of charged atoms (atoms with greater mass have less bent paths than lighter atoms)
atomic weight/mass
average weight of 2+ isotopes
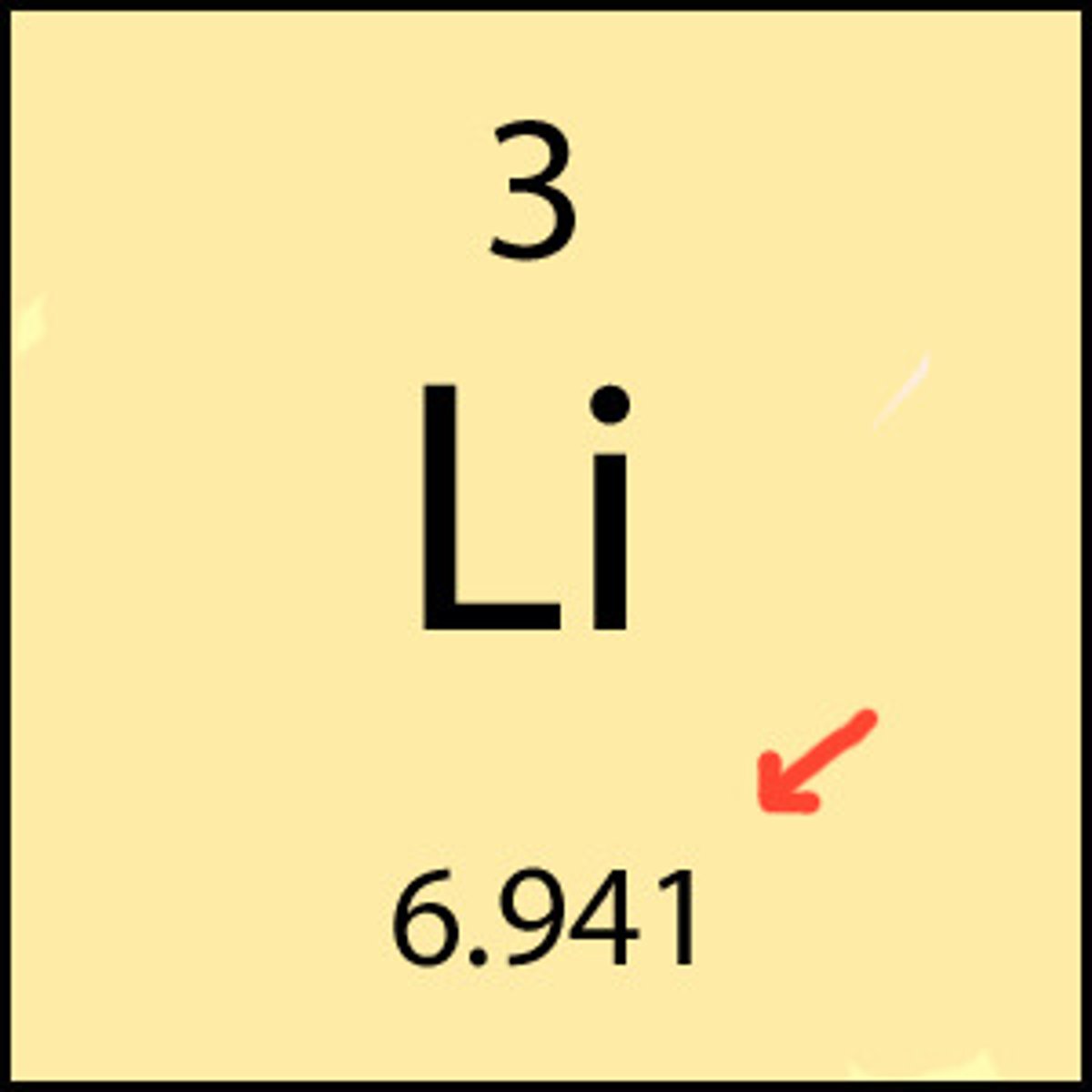
What is atomic weight (amu) based on?
Carbon-12 scale
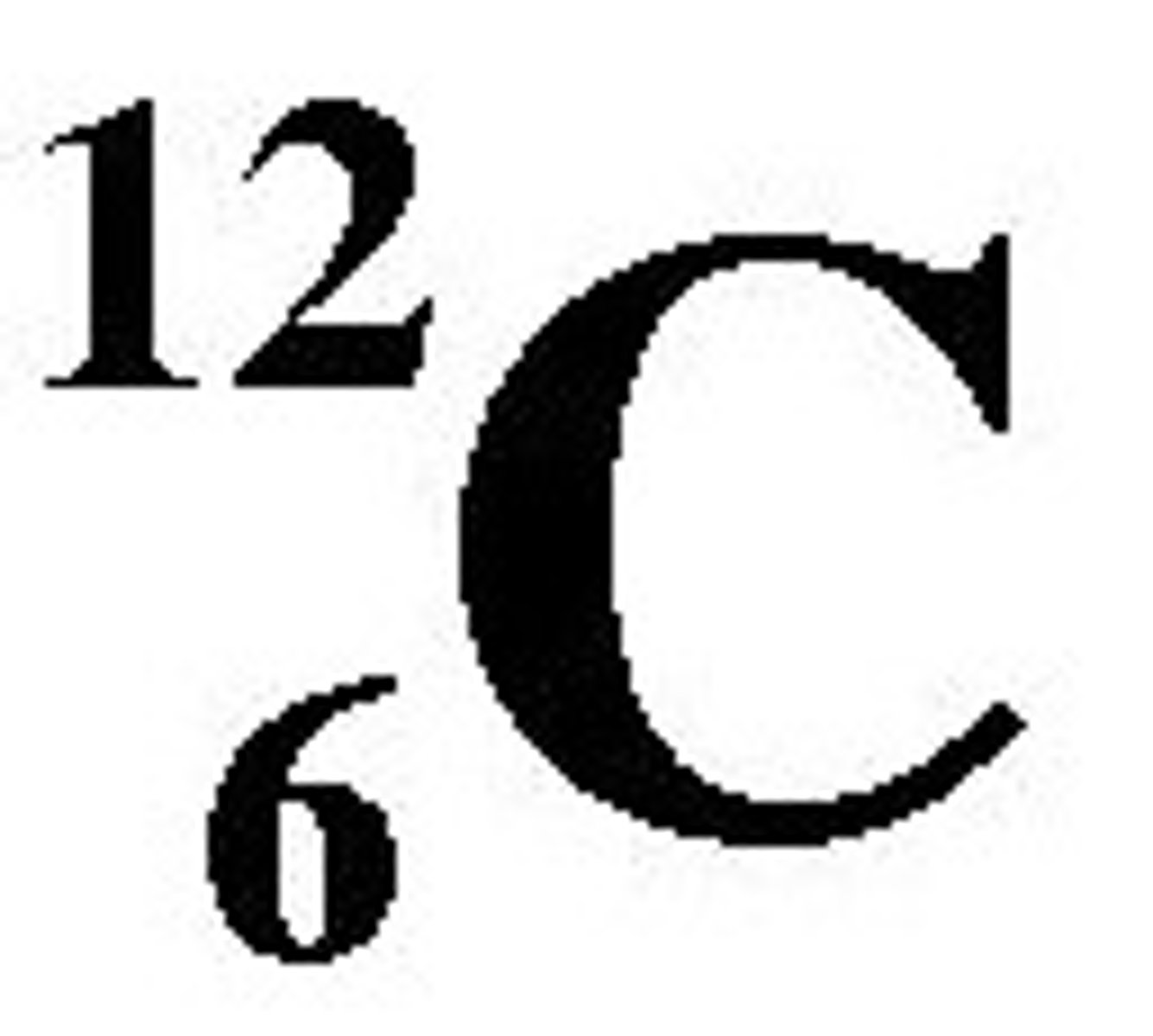
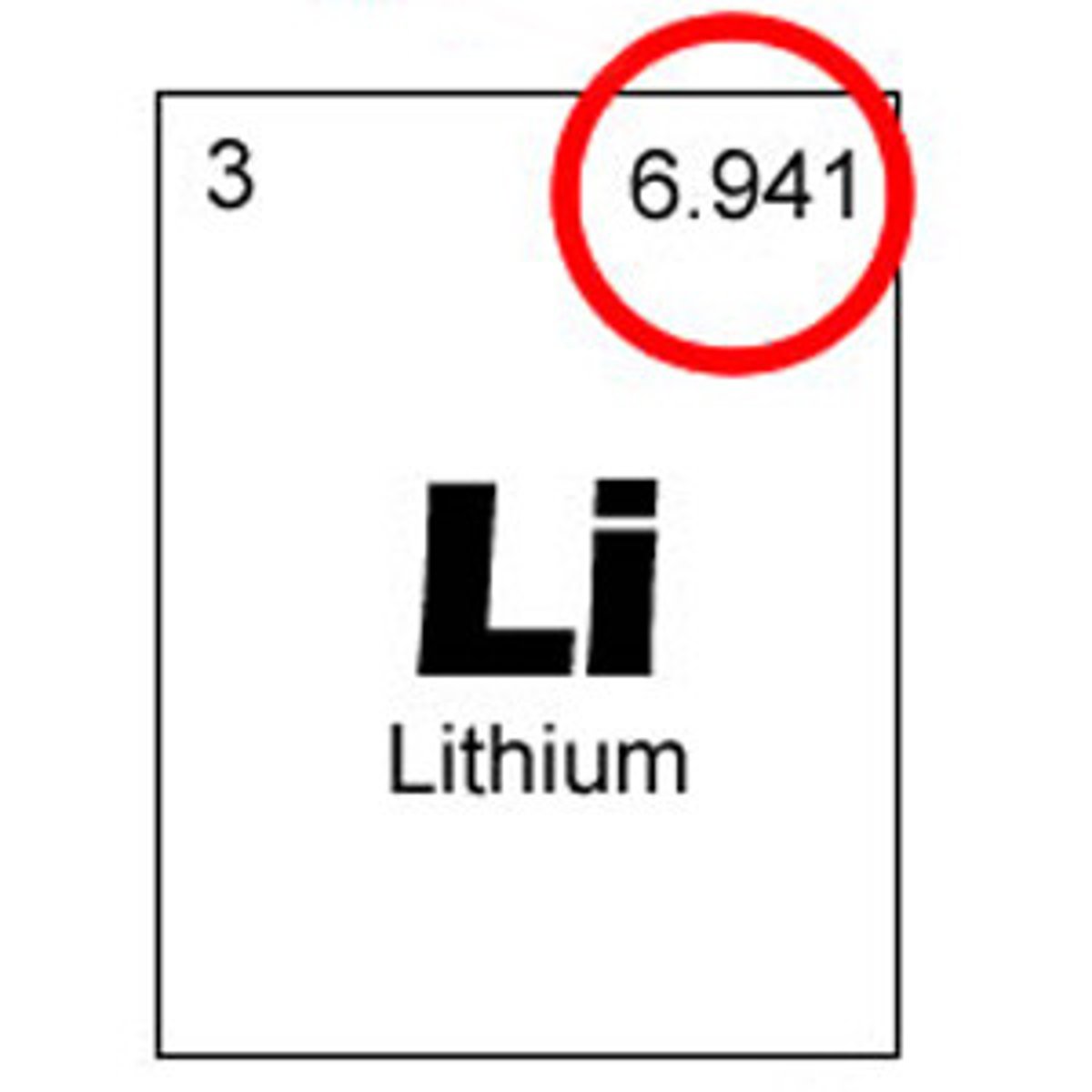
Carbon-12 (12 amu)
most common C isotope, standard unit for measuring atomic weights

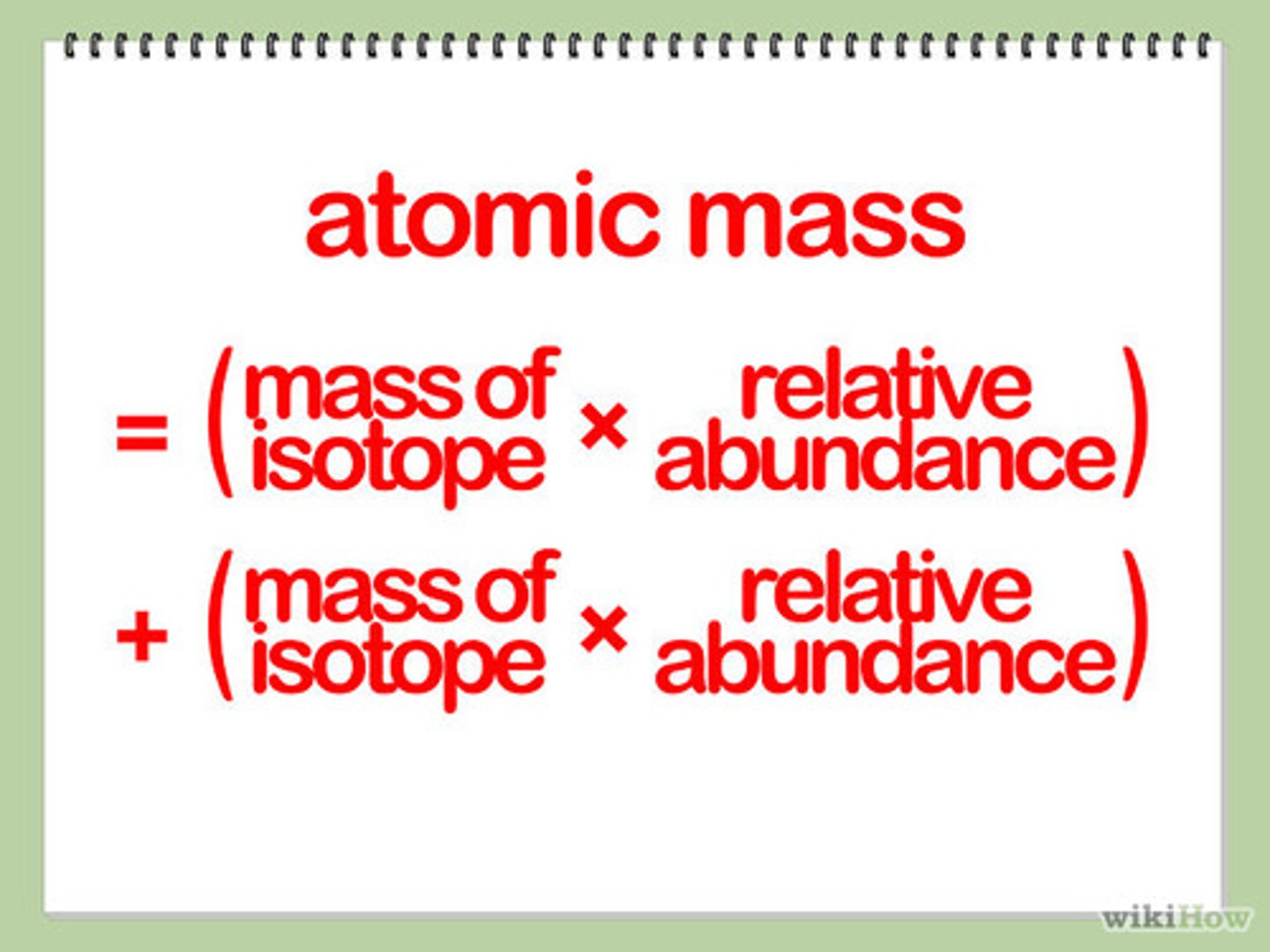
How to find atomic weight
1. Multiply the isotopes' mass by its decimal proportions
2. Add the products
3. Round to the nearest hundredth (unless otherwise stated)
What must the percentage proportion of isotopes add up to?
100%
What must the decimal proportion of isotopes add up to?
1 (A + B = 1)
How to find unknown isotope proportions with known atomic weight?
1. (mass ⋅ A) + (mass ⋅ B) = atomic weight
2. A + B = 1 → B = 1 - A
3. (mass ⋅ A) + (mass [1 - A]) = atomic weight
4. Solve for A
What can atomic weight be measured in?
amu or gram atomic weight/mass
What is gram atomic weight/mass?
atomic weight/mass expressed in grams
1 gram atomic weight =
6.022 × 10^23 atoms (Avogadro's number)
amu to gram atomic weight
1 amu = 1 gram atomic weight
1 gram = ? amu
6.022 × 10^23 amu
1 amu = ? grams
1/6.022 × 10^23 grams = 1.66 × 10^-24 grams
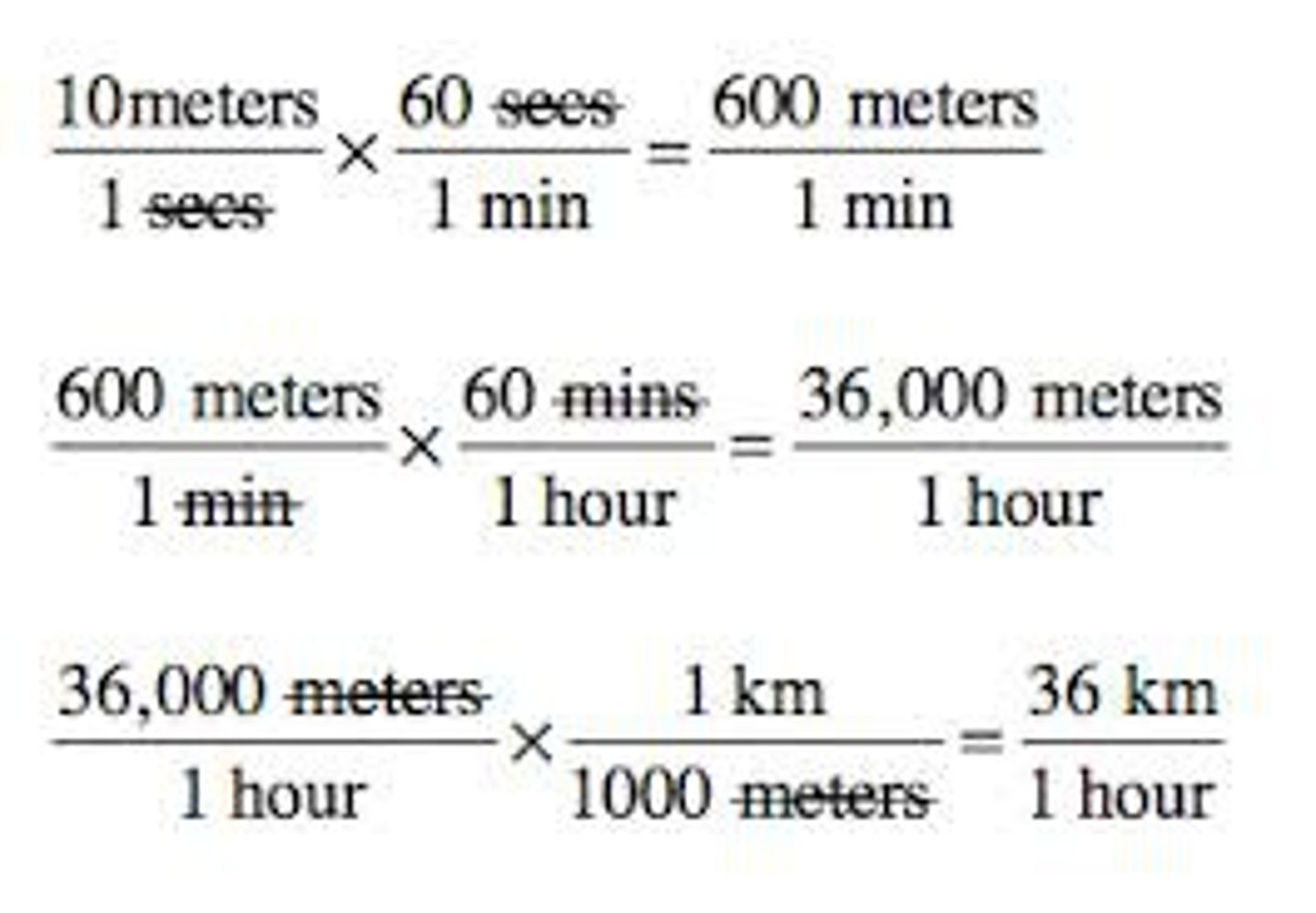
What happens during Unit Factor Analysis/Dimensional Analysis?
units are arranged to cancel out

Dimensional Analysis Set Up
original unit x new unit/original unit (conversion factor) = new unit
Finding grams/atoms dimensional analysis formula
(# of atoms/1) x (1 atomic weight in grams/6.022 × 10^23 atoms) x (mass of element/1 gram atomic weight) = x grams/atoms