Giant Covalent Structures : Allotropes of Carbon
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
What are the three allotropes of carbon?
Diamond, Graphite, and Fullerenes (including nanotubes and buckyballs).
What type of bonds hold carbon atoms together in all three allotropes?
Strong covalent bonds.
How is a diamond structured?
A diamond is a giant molecule of carbon atoms, each atom bonded to four others in a strong, 3D network.
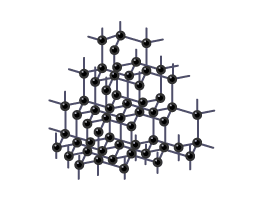
What are some properties of diamond?
Diamond is extremely hard, has a high melting point, is insoluble in water, and does not conduct electricity.
How does the structure of graphite differ from diamond?
Graphite consists of layers of carbon atoms, with each atom bonded to three others in each layers
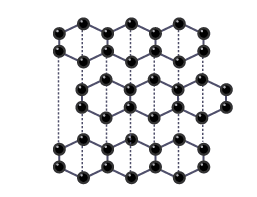
What makes graphite a good conductor of electricity?
Each carbon atom in graphite has a spare electron that forms a delocalised sea of electrons, allowing the flow of electricity.
What is a key property of graphite?
Graphite is slippery, making it useful as a lubricant, and it is also opaque and black.
What are the properties of silicone dioxide (quarts)?
giant covalent structure similar to diamond
found in sand
strong, rigid material
used in sandpaper
What is a nanotechnology?
the use and control of structures called nanoparticles due to their unusual properties
What is graphene?
A sing sheet of carbon atoms where each carbon makes three bonds used for water purification, solar cells etc
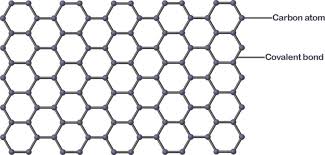
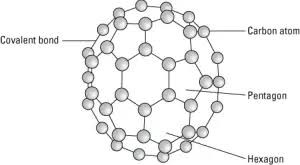
What are Bucky balls (fullerenes)?
Each atom forms 3 covalent bonds in a sphere shape they are used for drug delivery around body, lubricants etc
What is the structure of nanotubes (fullerenes) and what do they do?
Nanotubes are molecular-scale tubes of carbon arranged similarly to the layers in graphite.
Nanotubes can conduct electricity, making them useful in various electronic applications.
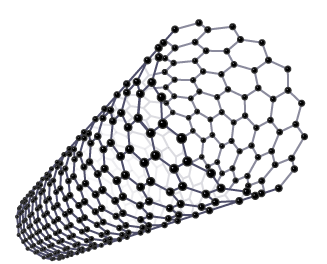
What are the risks of nanoparticles?
so small they may enter the brain
environmental effects (end up in rivers/harm wildlife)
there’s more information about uses than health effects