PPFA 9- Equilibrium 2 - Isotonicity
1/30
Earn XP
Description and Tags
explore th ethermodynamics of transfer of water through membranes , why must IV fluids be isotonic .Basic calculation of osmolority and identify risks in real pharmacy cases .
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
What is chemical potential?
it’s the effective free energy per mole of a component in a mixture
note - the molar gibbs free energy
What is the equation for Chemical potential ?
At constant temperature and pressure -
G= gibbs free energy
n= number of moles
µ=G/n
units are gibbs/moles
Examples for n ?
For a 2 component system ( n1 +n2):
G= µ1 x n1 + µ2 x n2
Therefore the chemical potential is lower in mixtures than in pure substances
component = salt ,solute,solution…
What are key properties of Chemical potential?
it represents each components contribution to total free energy ( G)
chemical potential ( µ) drives diffusion - due to differences in concentration ie.moves from high µ to low µ
chemical potential application - transport of drug into the bloodstream , transport across biological membrane
Why does diffusion stop at equilibrium ?
the µ of the absorption site = µ of blood site
µa=µb, therefore no concentration gradient

Describe the transport across Semi-permeable Membranes ?
For semipermeable membranes (only liquid moves, solute cannot)
If solute can’t cross → The system balances chemical potential by moving the solvent (water)
Water flows from low solute side( dilute) → high solute side ( concentrated)→ Because the side with more solute has lower water chemical potential.
The osmotic pressure di>erence equals the driving force for water movement
What is Colligative properties and give the most important one ?
when we add solute to a liquid it changes the liquids properties ( such as vapour pressure,freezig point and boiling point)
these changes depend on how many solute particles are present ,not how heavy they are = colligative properties
In Pharmacy -
The most important on is osmotic pressure , because it controls how water moves in and out of cells and drug solutions
Explain Osmotic properties in a simple osmosis experiment
In the experiment 🇦 :
a solution is separated from the pure solvent by a semipermeable membrane
pure solvent passes through the membrane ,and the solution rises in the inner tube
the net flow ceases when the pressure exerted by the column of liquid is equal to the osmotic pressure of the solution
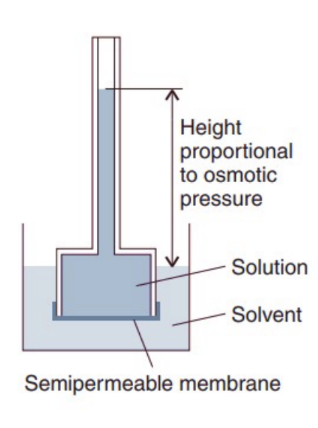
What is osmotic pressure?
-osmotic pressure (Π) is the pressure required to stop the movement of solvent across the semipermeable membrane Because it will balance chemical potential
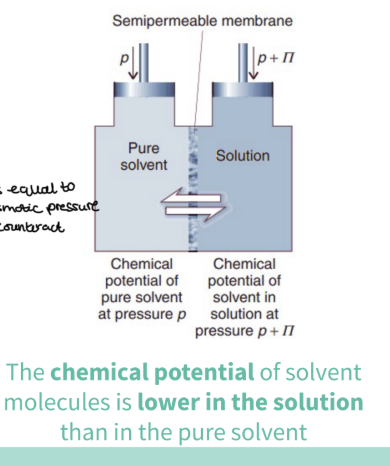
How do we stop this net flow ?
we apply extra pressure - which is equal to the osmotic pressure- to counteract
at this point the chemical potential of water in solution ( at p+π)=the pure solvent (p)
EQUILIBRIUM IS REACHED
What is Molarity ?
Molarity (M):
Number of moles of solute per litre of solution. Unit: mol/L.
Only counts molecules (not particles after dissociation).
Example:
1 M NaCl = 1 mole NaCl in 1 L solution.
What is Osmolarity?
Osmolarity (Osm/L):
Number of osmoles of solute particles per litre of solution.
Takes dissociation into account.
Formula:
where i = van’t hoff factor (number of particles produced per formula unit).
Examples of osmolarity calculations?
Glucose (non-electrolyte):
1 M glucose → does not dissociate → i = 1
Osmolarity = Osm/L 1 × 1 = 1 osm/l
NaCl (electrolyte):
1 M NaCl → dissociates into Na and Cl → i = 2 + −
Osmolarity = Osm/L 1 × 2 = 2 osm/l
CaCl2:
1 M CaCl2 → dissociates into Ca2 + 2Cl → i = 3 + −
Osmolarity = Osm/L 1×3= 3 osm/l
Why is osmolarity required in pharmaceutical labelling required ?
It indicates how much solute exerts the same osmotic pressure as 1 mole of an ideal, non-ionised substance in 1 litre.
Used to ensure IV fluids are safe and compatible with body fluids.
drugs need to have the same op in the body and in the drug
Clinical relevance - What is tonicity ?
Tonicity refers to the effective osmotic pressure of a solution relative to body fluids, especially blood serum.
Why does isotonicity matter ?
They have the same osmotic pressure so the total amount of water is the same for RBC , they will stay the same size
What happens to red blood cells if the osmotic pressure changes ?
Hypertonic solution- higher conc op outside ⟶ Cells shrink (water leaves)
Hypotonic solution- lower op outside ⟶ Cells swell and may burst (lysis)
What is the difference between osmotic pressure and isotonicity?
osmotic pressure- general movement in the membrane
isotonic - related to the body , specific
What is an Isotonic solution ?
Isotonic solution = a solution that has the same osmotic pressure (same solute concentration) as inside the cell.
No net water movement across the membrane The cell keeps its normal shape — no swelling or shrinking
Isotonic solutions are ideal for injections and use in sensitive tissues (e.g., eyes).
How do you know if a solution is safe ?
Infusion safety: The normal osmotic pressure of human plasma is equivalent to a plasma osmolality which is in the range 275-295 mOsm/kg
Solutions in the 300–500 mosmol/kg range may be used cautiously.
Solutions >550 mosmol/kg should not be rapidly infused due to risk of vein damage
NOTE- Central line patients tolerate higher tonicities better due to slow infusion and rapid dilution in central circulation
Example 1 - A patient is prescribed IV antibiotics reconstituted in 5% dextrose. What happens if we
instead use plain water for injection?”
Leads to osmotic imbalance, water enters the Red blood cells haemolysis ( burst /swell)
Why is osmolarity important in oral medications for premature infants ?
Checking the osmolarity of these preparations is crucial to minimize gastrointestinal stress and reduce the risk of conditions like pneumatosis intestinalis
Recommended Osmolarity Limit: Oral preparations for premature infants should have an osmolarity less than 400–500 mosmol/L
High Osmolarity in Common Medications: Many drugs administered to preterm infants have osmolarities greatly exceeding this limit. For Example, paracetamol (acetaminophen) solutions can have osmolarities ranging from 10,000 to 16,000
mosmol/L
How can you reduce osmolarity of medications?
you can mix it with infant formula
this is called the mitigation strategy
the final osmolarity (OM) of the mixture can be calculated using the following equation:
OM= [(OD X VD) + (OF X VF)] / ( VD + VF)
where :
OD= Osmolarity of the drug solution
VD= Volume of the drug solution
OF = Osmolarity of the infant formula
VF= Volume of the infant formula
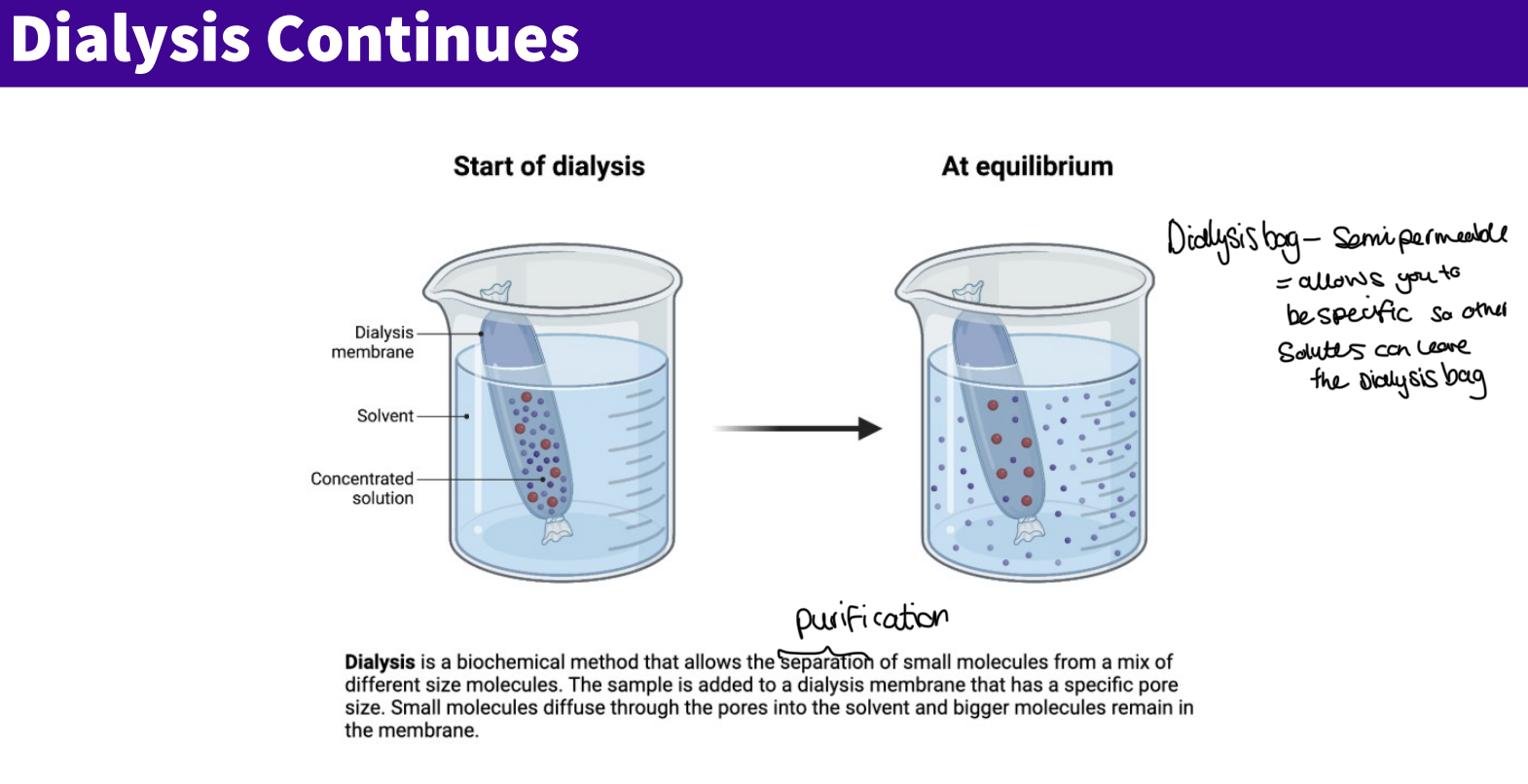
What is dialysis?
Uses osmosis + diffusion through a semi permeable membrane. ‐
Macromolecule + impurity mix placed inside a dialysis bag.
Small impurities (ions, small proteins) pass out, large macromolecules stay in.
Bag immersed in solvent; membrane lets small molecules escape.
Multiple solvent changes remove the majority of impurities.
More dialysis
Dialysis is a biochemical method that allows the separation of small molecules from a mix of different size molecules . the sample is added to dialysis membrane that has a specific pore size, small molecules diffuse through the pores into the solvent and bigger molecules remain in the membranes
What is haemodialysis?
Haemodialysis: where blood is drawn out of the body, passed through a machine containing a filter (dialyser) to remove waste and excess fluid, and then returned to the body.
What is peritoneal dialysis ?
Peritoneal dialysis: In peritoneal dialysis, a cleansing fluid (dialysate) is introduced into the peritoneal cavity (the space in the abdomen). This fluid absorbs waste products and excess fluid from the blood vessels lining the abdominal cavity.
How osmosis affects the performance of solutions used in oral rehydration therapy
( ORT) ?
Normal Gut Function: Fluids move via Cl secretion and −
Na /glucose absorption. +
Diarrhoea Effect: Pathogens disrupt this balance, causing
dehydration.
ORT Mechanism: Glucose enables Na (and water) uptake through +
co-transport.
Glucose Risk: Too much raises osmolarity, worsening dehydration.
Starches/Proteins Benefit: Slowly release glucose/amino acids
without high osmolarity.
Food-Based ORT: Cereal- or rice-based solutions are cheap, safe,
and effective for home use.- beneficial slow releasing glucose
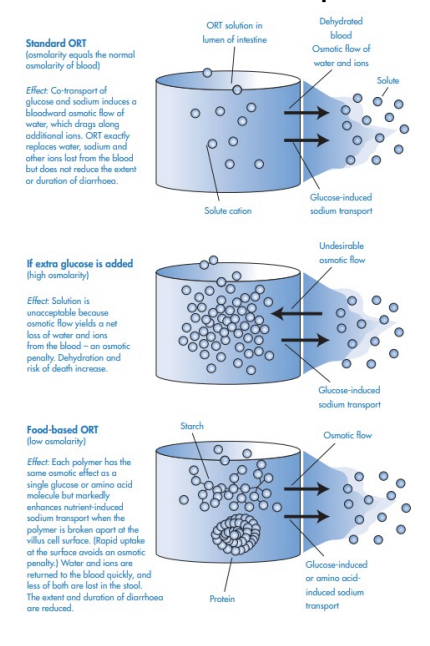
Example 2- A community pharmacist in a low-resource setting advises families to use rice water instead of plain sugar water for rehydration. Why is this safer?
Glucose helps sodium absorption via co-transport in the gut. Water follows
osmotically → rehydrates.
High glucose raises osmolarity above plasma → pulls water into the gut instead of
bloodstream → worsens dehydration.
Starch breaks down slowly → glucose released gradually → keeps osmolarity low but still supports Na+ and water uptake.
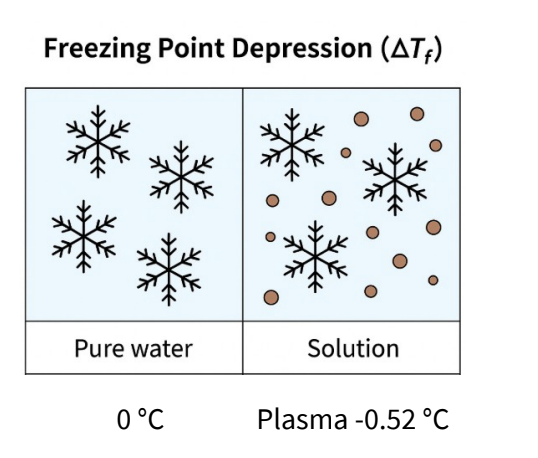
What is freezing point depression ?
Freezing point depression (ΔTf) is the lowering of the
freezing point of a solvent when a solute is dissolved
in it.
It is a colligative property, meaning it depends only
on the number of solute particles, not their type.
• Pure water freezes at 0°C.
• Adding solutes lowers the freezing point (solution
freezes below 0°C).
• Blood plasma has ΔTf ≈ 0.52°C
Solutions with the same ΔTf depression are isotonic.
note- use this property to adjust the isotonicity of drugs
How do you prepare an isotonic solutions?
Isotonic solutions match the osmotic pressure of blood (ΔTf = 0.52°C).
This can be calculated using freezing-point depression (ΔTf), a colligative property.
Drug solutions that aren’t isotonic can be adjusted using substances like NaCl
Use the formula:
w = (0.52 - a) / b
where:
a = drug concentration × ΔTf of 1% drug
b = ΔTf of 1% adjusting substance
w = grams of adjus3ng substance per 100 mL
Adjusted solutions help prevent cell damage like lysis.