AP Bio Fall Final Review
1/132
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
133 Terms
evolution
changes in populations, species, or groups of species; the process by which the frequency of heritable traits in a population changes from one generation to the next
natural selection
“survival of the fittest”
paleontology
provides fossils that reveal the prehistoric existence of extinct species , resulting in studies in changes in species and the formation of new species
biogeography
uses geography to describe the distribution of species, which has revealed unrelated species in different regions of the world look alike when found in similar environments
embryology
reveals similar stages in development (ontogeny) among related species, and the similarities help establish evolutionary relationship (phylogeny)
homologous structures (homologies)
body parts that resemble one another in different species because they have evolved from a common ancestor
vestigal
when homologous structures no longer serve any function
analogous structures (analogies)
body parts that resemble one another in different species, not because they have evolved from a common ancestor, but because they evolved independently as adaptations to their environment
molecular biology
examines the nucleotide and amino acid sequence of DNA and proteins from different species
adaptations
an inherited trait (physical, physiological, or behavioral) that increases an organism's chances of survival and reproduction in its specific environment, becoming more common in a population through natural selection over generations
fitness
relative ability to survive and leave offspring
darwin’s theory for evolution by natural selection
populations possess an enormous reproductive potential
population sizes remain stable
resources are limited
individuals compete for survival
variation among individuals in a population
much variation is heritable
only the most fit individuals survive
evolution occurs as favorable traits accumulate in the populations
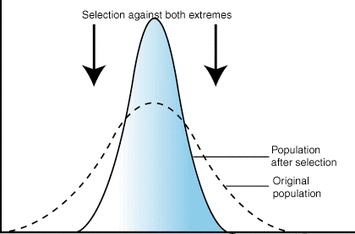
stabilizing selection
favoring of intermediate trait
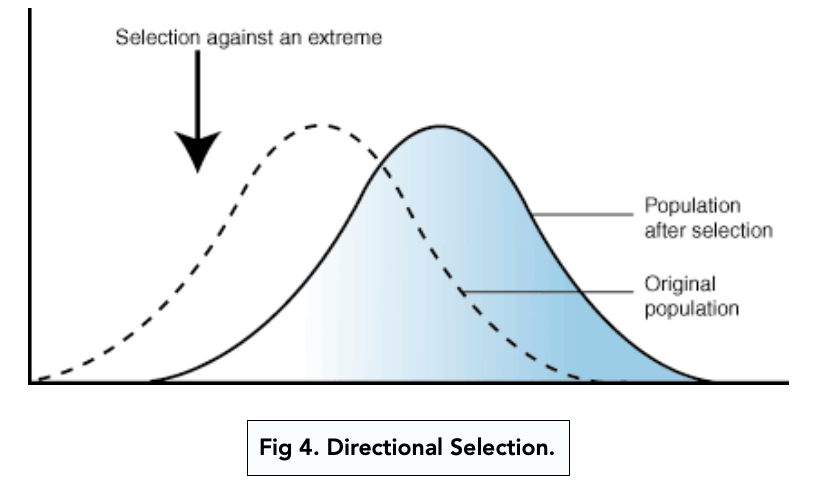
directional selection
favoring of one extreme phenotype
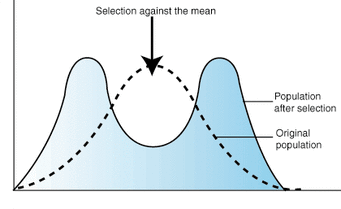
disruptive selection
favors extreme phenotypes and selects against common ones
sexual selection
favoring of favorable traits for mating
sexual dimorphism
differences in the appearance of males and females
artificial selection
form of directional selection carried out by humans when they breed animals that possess desirable traits
mutations
change in the genetic material of an organism that provide the raw material for new variation
sexual reproduction
creates individuals with new combinations of alleles that originate from:
crossing over: exchange of DNA between nonsister chromatids of homologous chromosomes
independent assortment of homologues: creates daughter cells with random combinations of maternal and paternal chromosomes
random joining of gametes: contributes to the diversity of gene combinations in the zygote during fertilization
diploidy
presence of two copies of each chromosomes in a cell
outbreeding (mating with unrelated partners)
increases the possibility of mixing different alleles and creating new allele combinations
balanced polymorphism
maintenance of different phenotypes in a population
heterozygote advantage
occurs when the heterozygous condition bears a greater selective advantage than either homozygous condition
hybrid vigor (heterosis)
describes the superior quality of offspring resulting from crosses between two different inbred strains of plants
frequency-dependent selection (minority advantage)
occurs when the least common phenotypes have a selective advantage
gene flow
the movement of individuals between populations resulting in the removal of alleles from a population when they leave or the introduction of alleles when they enter
genetic drift
random increase or decrease of alleles
founder effect
a type of genetic drift that occurs when allele frequencies in a group of migrating individuals are, by chance, not the same as that of their population of origin, and the new population will only resemble the individual found in the smaller population
bottleneck effect
a type of genetic drift that occurs when a population undergoes a dramatic decrease in size and can leave a random assortment of survivors
nonrandom mating
occurs when individuals choose mates based upon their particular traits
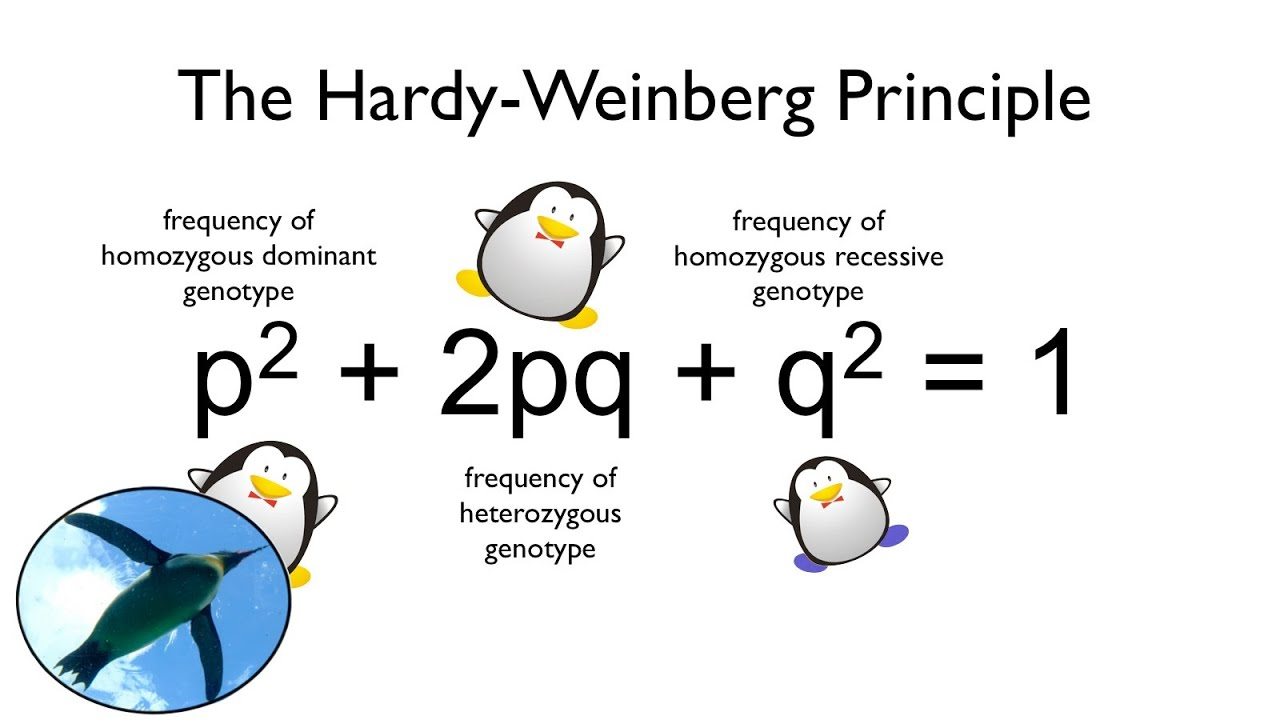
hardy weinberg equiliibrium
at genetic equilibrium, there is no evolution; however, the following conditions must be true:
all traits are selectively neutral (or natural selection
mutations don’t occur
the population must be isolated from other populations (no gene flow)
population is large (no genetic drift)
mating is random
allopatric speciation
begins when a population is divided by a geographic barrier so that interbreeding between the two resulting populations is prevented
sympatric speciation
formation of new species without the presence of a geographic barrier that can happen due to:
balanced polymorphism
polyploidy: the possession of more than the normal set of chromosomes found in diploid cells
hybridization: occurs when two distinctly different forms of a species (or closely related species that are normally reproductively isolated) mate and produce progeny along a geographic boundary called a hybrid zone
adaptive radiation
relatively rapid evolution of many species from a single ancestor
prezygotic isolating mechanisms
mechanisms that prevent fertilization:
habitat isolation: species don’t encounter one another
temporal isolation: species mate during different seasons or at different times of day
behavioral isolation: species does not recognize another species as a mating partner because it doesn’t perform the correct courtship rituals, display the proper visual signs, sing the correct mating songs, or release the proper chemicals
mechanical isolation: male and female genitalia are structurally incompatible
gametic isolation: male gametes don’t survive in the environment of the female gamete of when female gametes don’t recognize male gametes
postzygotic isolating mechanisms
consists of mechanisms that prevent the formation of fertile progeny:
hybrid inviability: when the zygote fails to develop properly and aborts or dies before reaching reproductive maturity
hybrid sterility: hybrids become functional adults, but are reproductively sterile
hybrid breakdown: hybrids produce offspring that have reduced viability or fertility
divergent evolution
two or more species that originate from a common ancestor and become increasingly different over time that can happen as a result of allopatric or sympatric speciation or adaptive radiation
convergent evolution
two unrelated species that share similar traits that arise because each species has independently adapted to similar ecological conditions or lifestyles
parallel evolution
two related species or two related lineages that have made similar evolutionary changes after their divergence from a common ancestor
coevolution
the reciprocal evolutionary change where two or more species influence each other's adaptations through close interactions
microevolution
the details of how populations of organisms change from generation to generation
macroevolution
general patterns of change in groups of related species that have occurred over broad periods of geologic time
punctuated equilibrium
evolutionary history consists of geologically long periods of stasis with little or no evolution, interrupted by geologically short periods of rapid evolution
ionic bond
form between 2 atoms when one or more electrons are transferred from one atom to the other; the atom that gains electrons has an overall negative charge, and the atom that loses electrons has an overall positive charge, meaning these atoms are ions
covalent bonds
form when electrons between atoms are shared, meaning that neither atom completely retains possession of the electrons
nonpolar covalent bonds
form when electrons are shared equally
polar covalent bonds
form when electrons are shared unequally
hydrogen bonds
weak bonds between molecules that form when a positively charged hydrogen atom in one covalently bonded molecule is attracted to a negatively charged area of another covalently bonded molecule
solvent
the substance (usually a liquid, like water) that dissolves another substance
hydrophilic
“water loving”
hydrophobic
“water fearing”
solute
substance that dissolves in a solvent
aqueous
a solution in which water is the solvent
specific heat capacity
the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius
heat of fusion
energy required to change water from a solid to a liquid
heat of vaporization
energy required to change water from liquid to a gas
cohesion
attraction between like substances that occurs in water cause of the hydrogen bonding between water molecules
surface tension
strong cohesion between water molecules produces a high surface tension, creating a water surface that is firm and allows it to resist an external force, behaving like a stretched elastic membrane
adhesion
attraction of unlike substances resulting from the attraction of the poles of water molecules to other polar substances
capillary action
the upward movement of a liquid (like water) through narrow spaces (capillaries in plants, tubes) against gravity, driven by the combined forces of adhesion (water sticking to tube walls) and cohesion (water molecules sticking to each other), along with surface tension, crucial for plant water transport from roots to leaves
monosaccharide
simplest kind of carbohydrate that consists of a single sugar molecule
disaccharide
2 sugar molecules joined by a glycosidic linkage
dehydration synthesis
a chemical reaction that joins two molecules together by removing a water molecule, forming a new covalent bond and creating a larger molecule called a polymer. In this process, the hydroxyl group of one monomer combines with a hydrogen atom from another, releasing a water molecule and linking the monomers
polysaccharide
a series of connected monosaccharides, making it a polymer
saturated fatty acid
a long hydrocarbon chain with only single bonds between its carbon atoms, meaning it's "saturated" with the maximum number of hydrogen atoms, giving it a straight, rigid shape that packs tightly, making fats solid at room temperature
unsaturated fatty acid
a lipid with one or more carbon-carbon double bonds in its hydrocarbon tail, causing kinks that prevent tight packing, keeping them liquid at room temperature (like oils) and crucial for membrane fluidity, especially in varying temperatures
phospholipid
an amphipathic lipid (hydrophilic "head" + hydrophobic "tails") that forms the cell membrane's bilayer, consisting of a phosphate group, glycerol, and two fatty acids, creating a selectively permeable barrier for the cell
transport proteins
specialized membrane proteins that move ions, small molecules, or larger substances across the cell membrane
peptide bonds
bonds between amino acids
primary structure
describes the order of amino acids
secondary structure
3D shape that results from hydrogen bonding between the amino and carboxyl groups of nearby amino acids, producing a spiral (alpha helix) or a folded plane that is pleated (beta pleated sheet)
tertiary structure
includes addition 3D shaping with the following contributing to the structure:
hydrogen bonding between R groups of amino acids
ionic bonding between R groups of amino acids
hydrophobic effect that occurs when hydrophobic R groups move to the center of the protein (away from water)
formation of disulfide bonds that help maintain the folds of the amino acid chain
quaternary structure
a protein that is assembled from 2 or more separate peptide chains
DNA nucleotide
consists of a nitrogen base, five-carbon sugar (deoxyribose), and a phosphate group
DNA nucleotides
adenine (purine)
thymine (pyrimidine)
cytosine (pyrimidine)
guanine (purine)
RNA vs. DNA
sugar in the nucleotides that make an RNA molecule is ribose, not deoxyribose as it is in DNA
the thymine nucleotide doesn’t occur in RNA and is replaced by uracil that pairs with adenine
RNA is usually single-stranded and doesn’t form a double helix as it does with DNA
activation energy
the minimal energy needed to kickstart a chemical reaction
enzymes
proteins that act as catalysts for metabolic reactions
characteristics:
enzymes are substrate specific
an enzyme is unchanged as a result of a reaction an can perform its enzymatic function repeatedly
an enzyme catalyzes a reaction in both forward and reverse directions
the efficiency of an enzyme is affected by temperature and pH; if they are not optimal, the enzyme will denature
the induced fit model describes how enzymes work
induced fit model
explains enzyme-substrate interaction as a dynamic process where the enzyme's active site changes shape slightly upon substrate binding, creating a tighter, more precise fit
substrate
the substance or substances upon which the enzyme acts
hydrolysis
when water and energy from ATP break the last phosphate bond of the ATP molecule to form ADP and an inorganic phosphate group
phosphorylation
new ATP molecules are assembled by phosphorylation when ADP combines with a phosphate group using energy obtained from energy-rich molecules
allosteric activator
a molecule that binds to an enzyme at a site other than the active site (an allosteric site) to change the enzyme's shape, which increases its activity and affinity for its substrate, speeding up the reaction
allosteric site
a specific region on an enzyme where a regulatory molecule binds, causing a change in the enzyme's activity.
allosteric inhibitor
a molecule that binds to an enzyme at a site other than the active site (an allosteric site), causing a conformational (shape) change that reduces the enzyme's activity, often by preventing the substrate from binding effectively to the active site, thereby regulating metabolic pathways through negative feedback.
feedback inhibition
the end product of a metabolic pathway inhibits an earlier enzyme in the same pathway, slowing down or stopping its own production
competitive inhibition
a substance that mimics the substrate inhibits an enzyme by occupying the active site, preventing the enzyme from catalyzing the substrate
noncompetitive inhibition
a substance inhibits the action of an enzyme by binding to the enzyme at a location other than the active site and changes the shape of the enzyme, disabling enzyme activity
selectively permeable membrane
only small, uncharged, polar molecules and hydrophobic molecules freely pass across the membrane
channel proteins
provide open passageways through the membrane for certain hydrophilic substances such as polar and charged molecules
aquaporins
channel proteins that increase the passage rate of water molecules
ion channels
allow the passage of ions across the membrane
carrier proteins
bind to specific molecules, which are then transferred across the membrane after the carrier protein undergoes a change in shape
transport protein
uses energy to transport materials across the membrane; type of active transport
recognition proteins
give each cell type a unique identification, providing a distinction between cell types
receptor proteins
provide binding sites for hormones or other trigger molecules, causing a specific cell response to be activated
cholesterol
distributed throughout the phospholipid bilayer to provide some stability to the plasma membranes of animal cells; at high temperature, it helps maintain firmness, and at cool temperatures, it keeps the membrane flexible
ribosomes
synthesize proteins by translating messenger RNA (mRNA) into polypeptide chains
rough er
when ribosomes are present, it creates glycoproteins and its primary role is the synthesis, folding, modification, and transport of proteins destined for secretion, insertion into membranes, or delivery to other organelles
smooth er
responsible for synthesis of lipids and steroid hormones and breaking down toxins, drugs, and toxic by products in the liver