Chemistry (Atomic models+ periodic propreties)
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Dalton's Atomic Theory (1808)
- Each element is made up of tiny particles called atoms
- The atoms of a given element are identical
- Chemical compounds are formed when atoms of different elements combine (chemical reaction)
- Chemical reactions involve reorganization of the atoms
Thomson's Atomic Theory: Plum Pudding Model (1904)
- Atoms are made of a positively charged cloud (negative electrons are embedded)
- Atoms are spheres of positively charged "dough" or fluid with negatively charged electrons (the "plums" or "raisins") scattered throughout, creating a neutral atom overall
- The total positive charge of the sphere balanced the total negative charge of the scattered electrons, making the atom electrically neutral.
Rutherford Atomic Theory: The Nuclear Atom (1911)
- Atoms have a dense, positively charged nucleus at its center containing most of the atom's mass, surrounded by a large amount of empty space where negatively charged electrons orbit.
- Nucleus: A tiny, dense, positively charged center of the atom that contains protons and almost all the atom's mass.
- Electrons: Light, negatively charged particles that revolve around the nucleus at some distance.
- Empty Space: The vast majority of the atom's volume consists of empty space.
The Modern View
- Composition of an atom:
- Protons
- Found in the nucleus
- Positive charge
- Electrons
- Found outside the nucleus
- Negative charge
- Neutrons
- Found in the nucleus
- No charge
- Describes an atom as having a central, dense nucleus containing positively charged protons and neutral neutrons, surrounded by a diffuse electron cloud of negatively charged electrons
Atom
Smallest particle of an element
Molecule
Two or more atoms joined together acting as a unit
Ions
positively (cation) and negatively (anion) charged atoms
Isotopes
Atoms with the same number of protons but different numbers of neutrons
Atomic Radius
The measurement of an atom's size, defined as the distance from its nucleus to its outermost electron shell
- Decreases as it goes across a period from left to right (Electrons are drawn closer to the nucleus --> size decreases)
- Increases when going down in a group (Orbital size increases in a group going from top to bottom)
Natural Law
- A fundamental chemical law
- An observation of mass that applies to many different systems
Law of Conservation of Mass
- Stated by Antonio Lavoisier
- Mass is neither created or destroyed
Law of definite Proportion
- Stated by Joseph Proust
- A given compound always contains the same proportion of element by mass
Ionization of Energy
The amount of energy required to remove an electron from an atom/gaseous state
- While going across a period from left to right, the ionization energy increases
- While going down a group from top to bottom, the energy decreases
Electron Affinity
the energy change associated with the addition of an electron to a gaseous atom or ion
- he process can be represented as: X(g) + e⁻ → X⁻ + energy
The Atomic Model of Bohr (Orbit)
The electron in a hydrogen atom moves around the nucleus in a certain allowed circular orbits.
Ground state: The most stable configuration of the Orbit (Orbit 1 closes to the nucleus)
Drawbacks:
- The model can only be applied to hydrogen atoms
- The electrons do not move around the nucleus in circular orbits
Constants:
- Planck's Constant: 6.63 x 10^-34 j/s
- Speed of light: 3x10^8 m/s
Quantum Mechanical Model
The region of the atom where the probability to find an electron is higher.
S, p, d, f
Quantum Numbers
They express the properties of the orbital
Principal Quantum Number (N)
- Related to the size and energy of the orbital
- 1, 2, 3, 4, 5...
Angular Quantum Number (L)
- Related to the shape
- 0, 1, 2, 3, 4
Magnetic Quantum Number (Ml)
- Related to the orientation
- 1, 0, -1...
Electron spin quantum number (Ms)
- Electrons can spin in either of two possible directions
- +1/2 or -1/2
Heisenberg uncertainty principle
If we know the speed of an electron, we cannot know the position and vice versa.
Pauli Exclusion Principle
In a given atom, no two electrons can have the same set of quantum numbers.
C (speed of light)
3×10^8 m/s
h (Planck’s Constant)
6.63×10^-34 js
Formula for wavelength
λ = hc/E
Formula required to excite the hydrogen electron
E= -2.17×10^-18(Z²/n²) —> ΔE = E_final - E_initial (z= nuclear charge nitrogen is 1 so z is always 1—-n= Orbit *the orbit closet to the nucleus in 1 and everything after that goes in numerical order)
Formula for frequency
f = c/λ
Polyelectronic Atoms: Aufbau Principle
Atoms with more than one electron
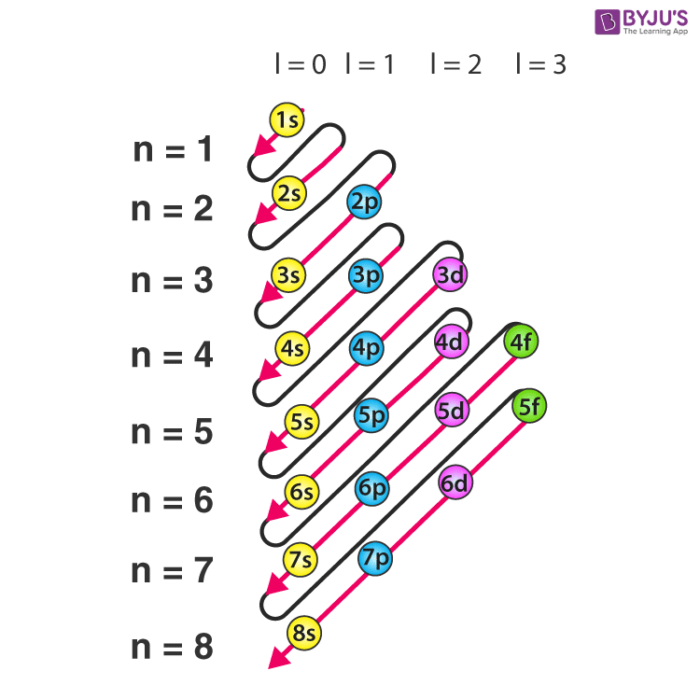
Electronic configuration
Orbitals: Quantum Number Values
s —> 2e- L= 0 s= 1
p —> 6e- L= 1 p= 3
d —> 10e- L= 2 d= 5
f —> 14e- L= 3 f= 7