MCAT MilesDown General Chemistry
1/263
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
264 Terms
A (top) Z (bottom) X (element)
A= mass number = protons + neutrons
Z= atomic number = number of protons
note: atomic weight = weighted average
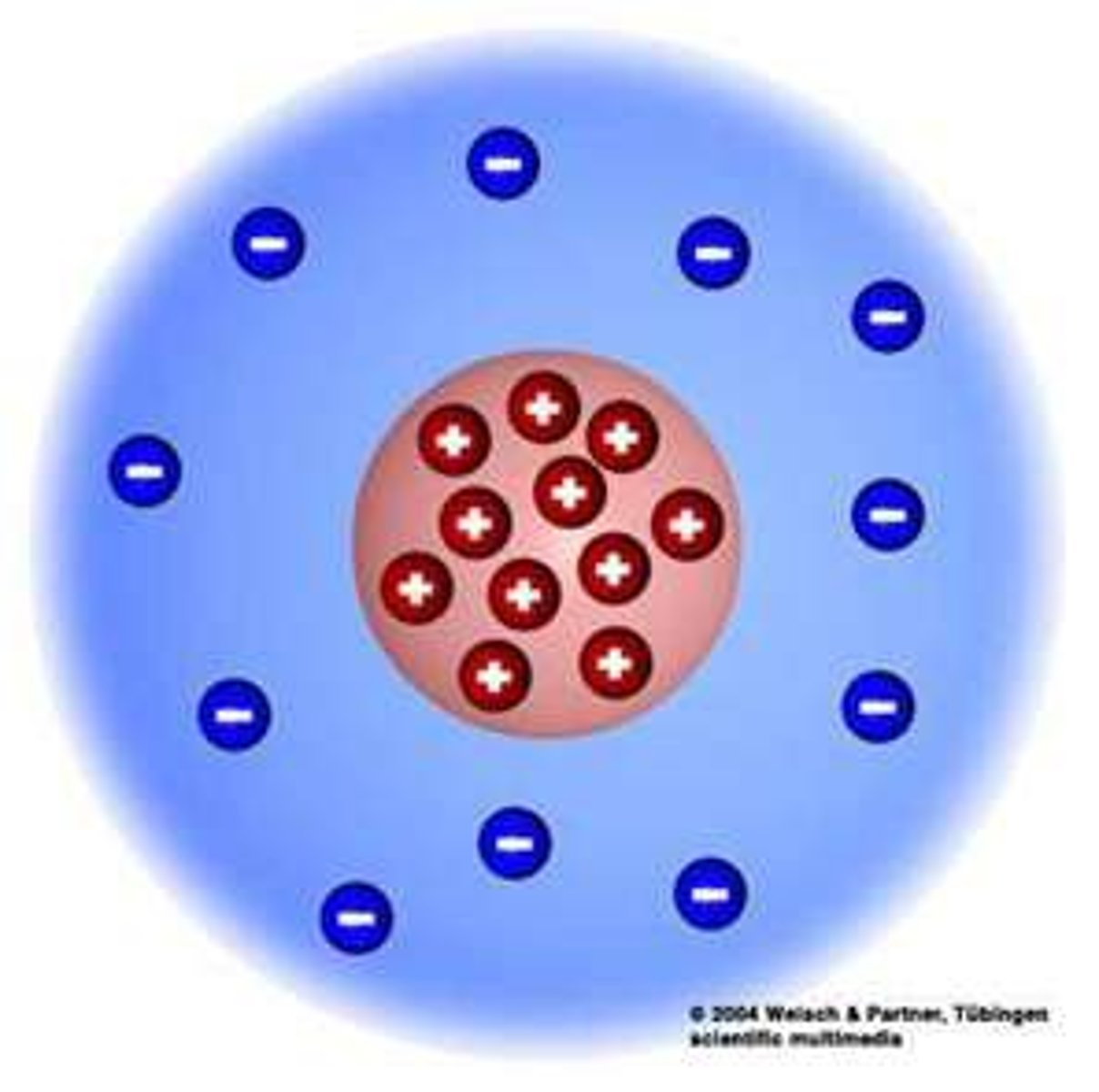
Rutherford model
-1911
-electrons surround a nucleus
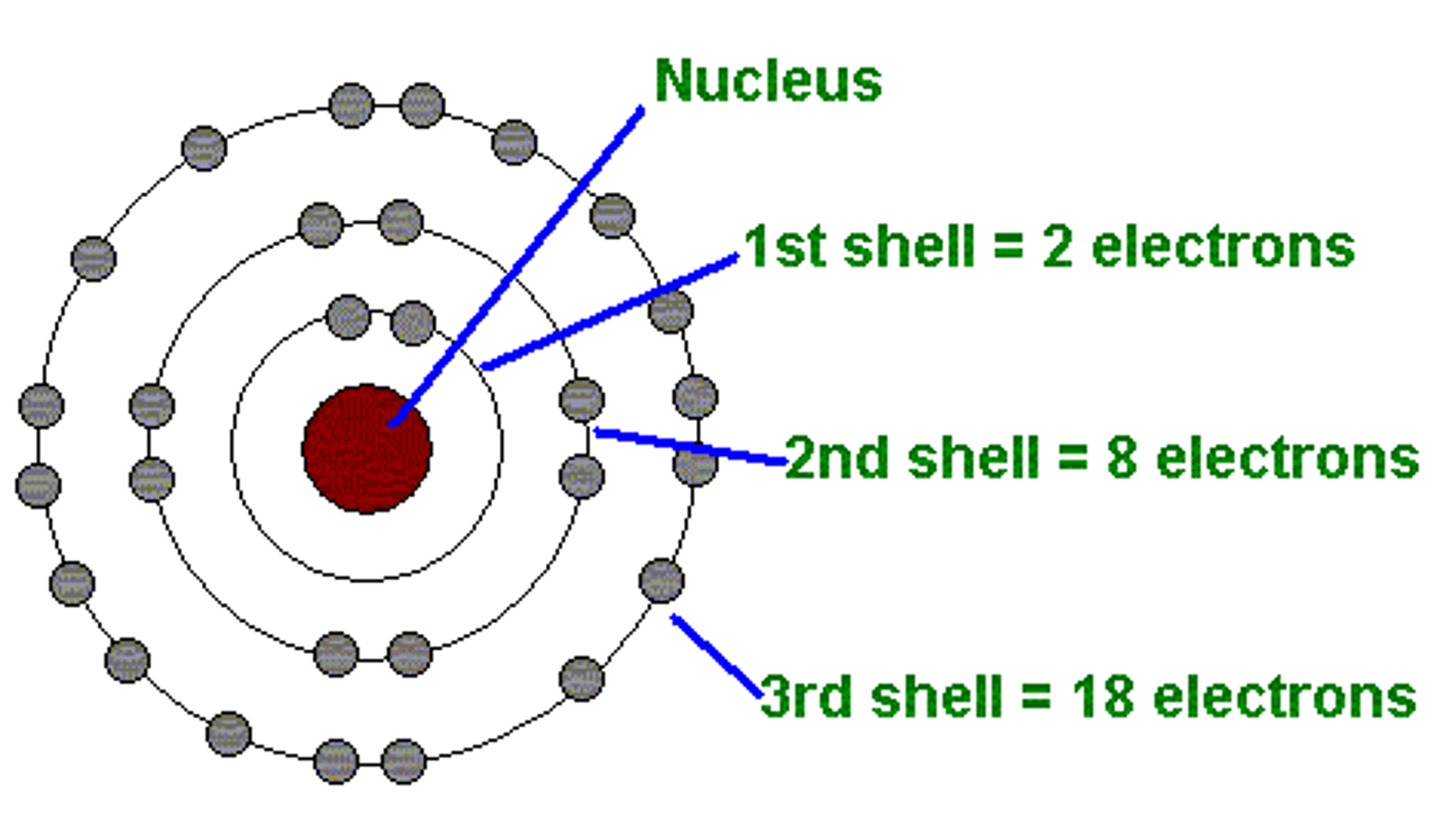
Bohr model
-1913
-described orbits in more detail
-farther orbits= more energy
-photon emitted when n goes lower, absorbed when n goes higher (n indicates level)
AHED mnemonic
Absorb light
Higher potential
Excited
Distant (from the nucleus)
Heisenberg Uncertainty
-it is impossible to know the momentum and position simultaneously
Hund's Rule
e- only double up in orbitals if all orbitals first have 1 electron
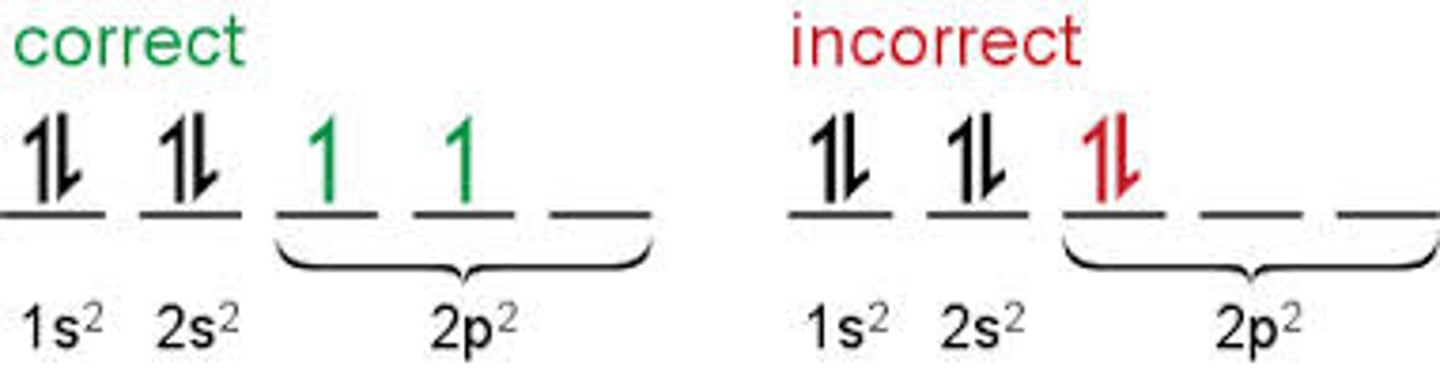
Pauli Exclusion Principle
paired e- must be +1/2 or -1/2
-spins have to be opposite directions
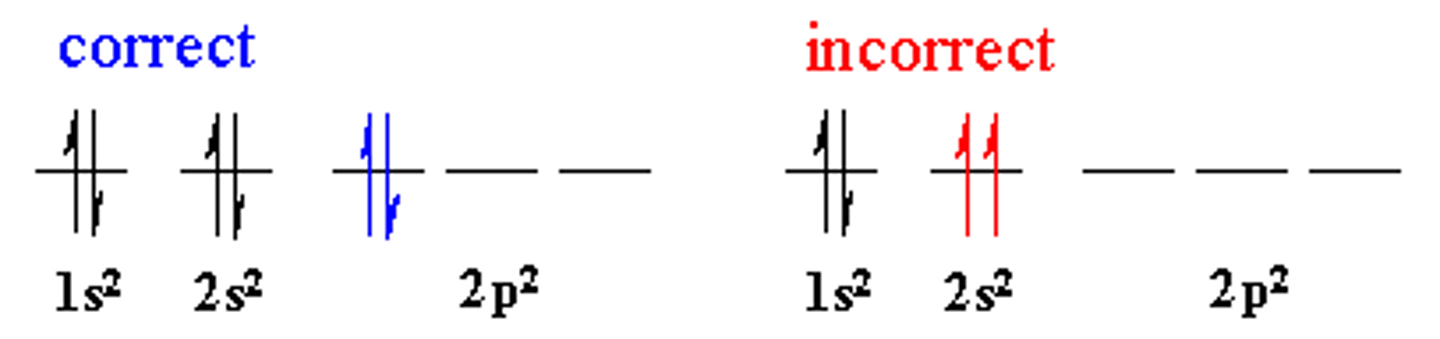
Avogadro's number
6.022 x 10^23 = 1 mol
Planck's (h) constant
6.626 x 10^-34 J*s
speed of light (c)
3.0 x 10^8 m/s
light energy equations
E= (hc)/gamma
E= hf
f= frequency
h= Planck's constant
c= speed of light
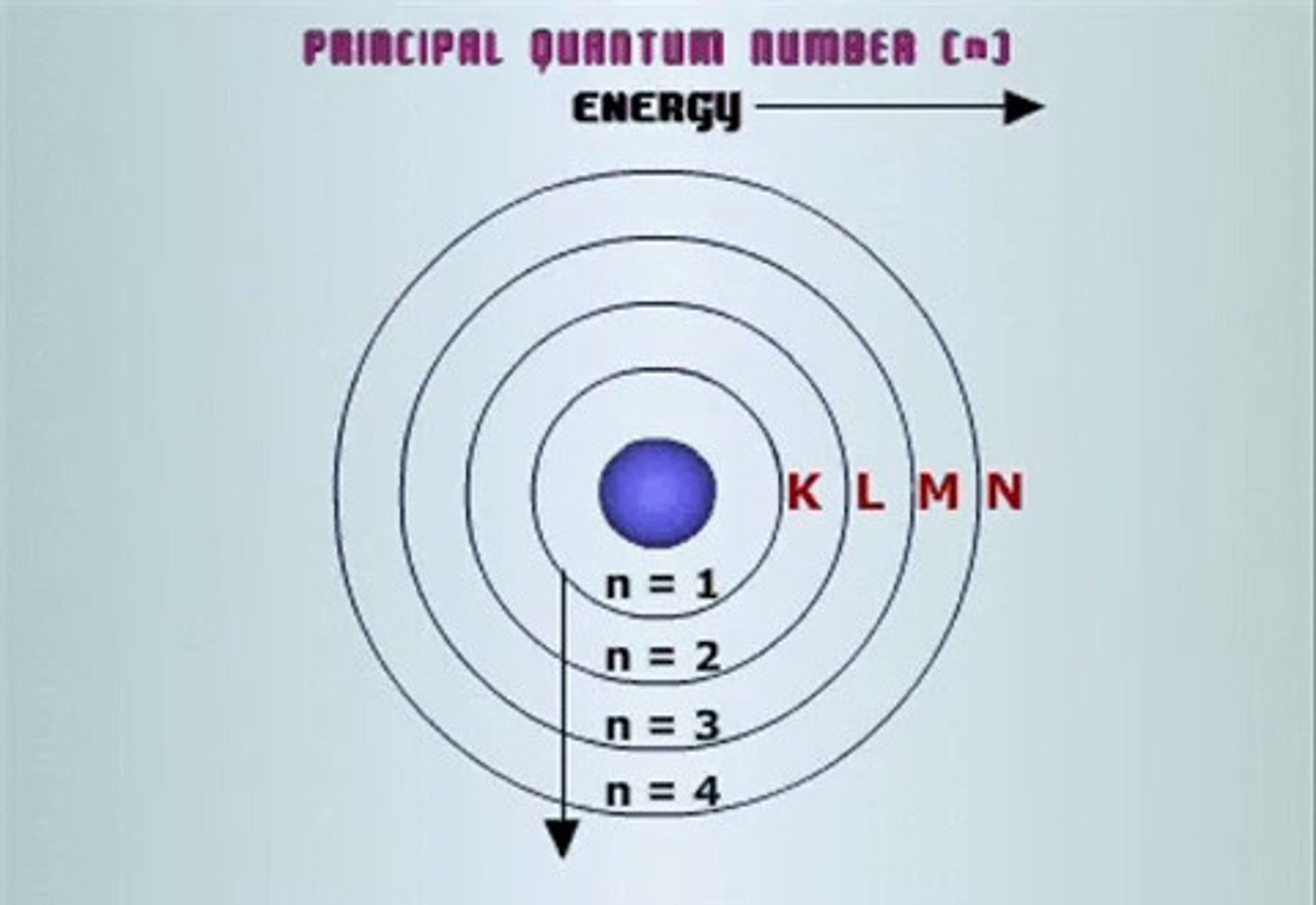
quantum number: n
name: principal
what it labels: e- energy level or shell number
possible values: 1,2,3...
(basically tells distance from nucleus)
notes: except for d- and f-orbitals, the shell number matches the row of the periodic table
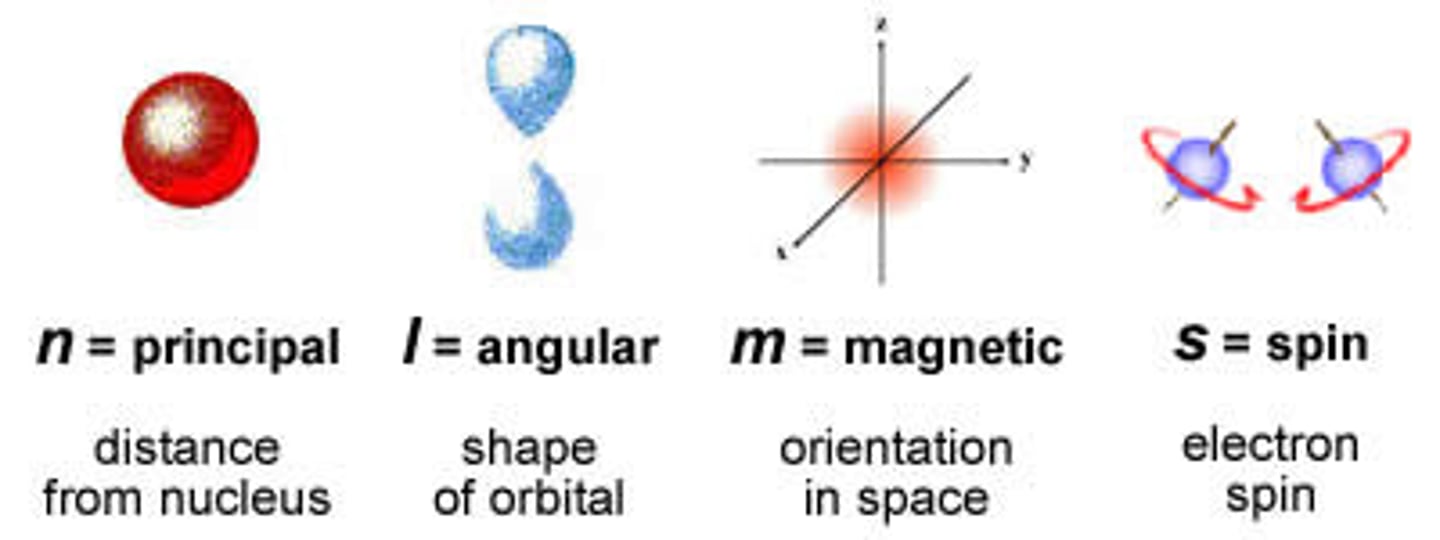
quantum number: l
name: azimuthal
what it labels: 3D shape of the orbital
possible numbers: 0,1,2,...,n-1
notes:
0= s orbital
1= p orbital
2= d orbital
3= f orbital
4= g orbital
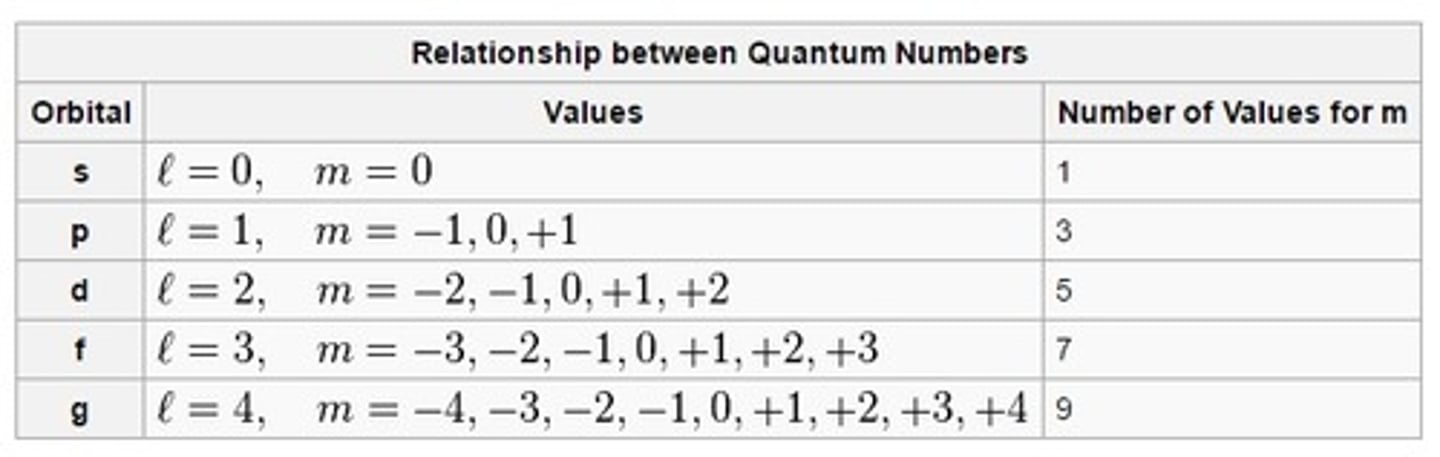
quantum number: mi
name: magnetic
what it labels: orbital sub-type
possible values: -n -> +n
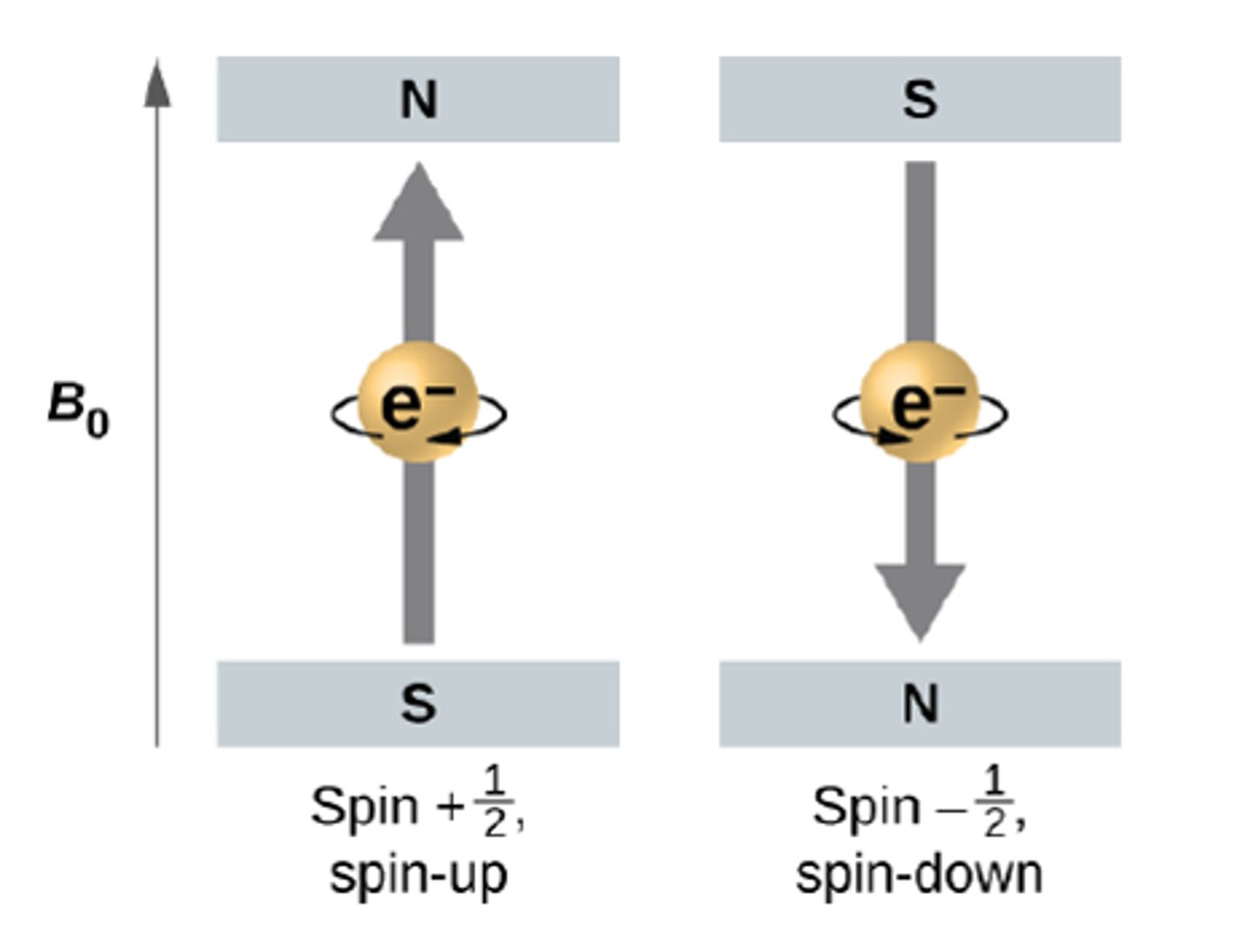
quantum number: ms
name: spin
what it labels: electron spin
possible values: +1/2, -1/2
maximum electrons in terms of n
2n^2
maximum electrons in sub-shell
2(2l +1)
free radical
an atom or molecule with an unpaired electron
shape of s orbital
sphere
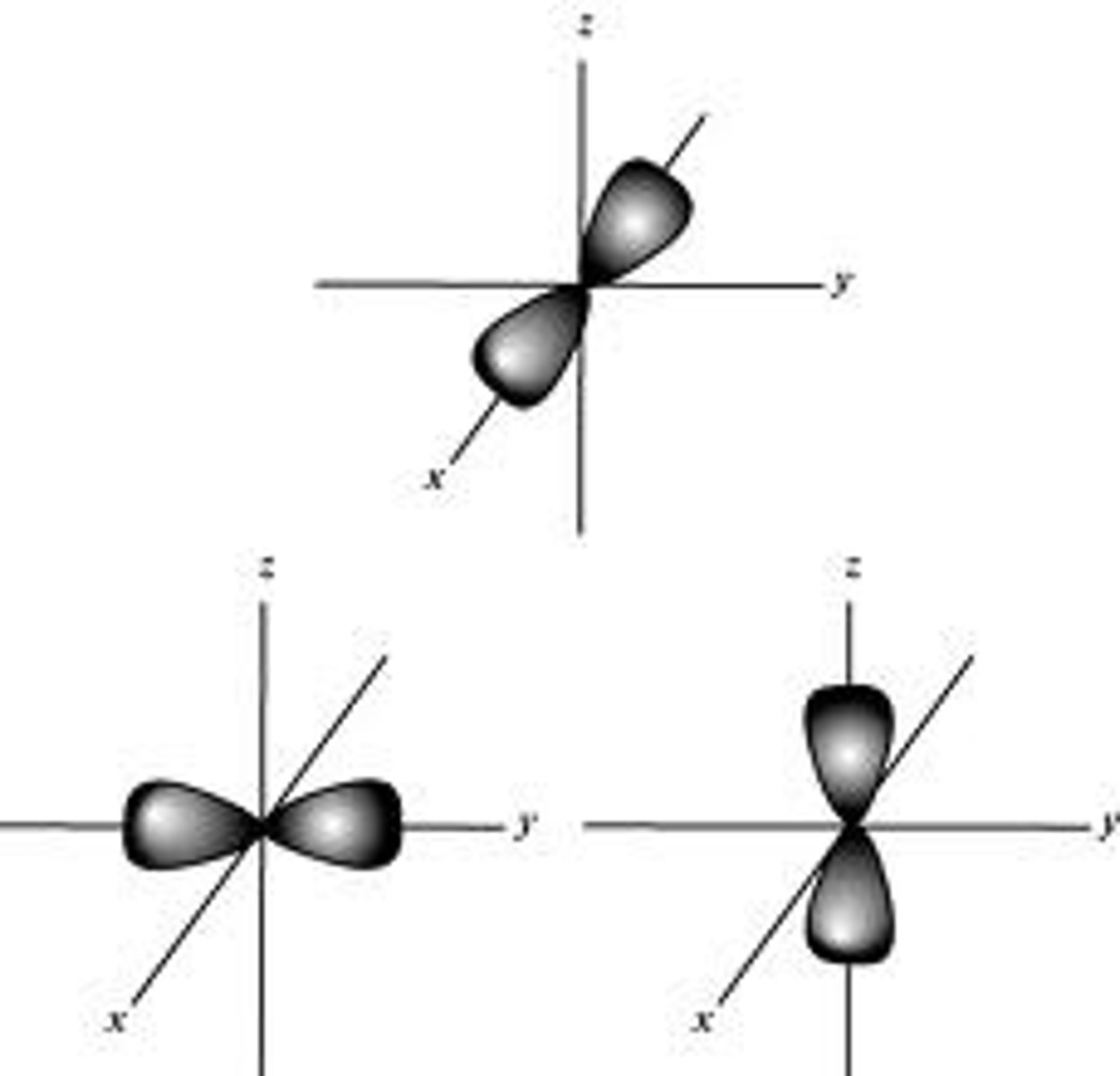
shape of p orbital
dumbbell
shape of d orbital
clover leaf
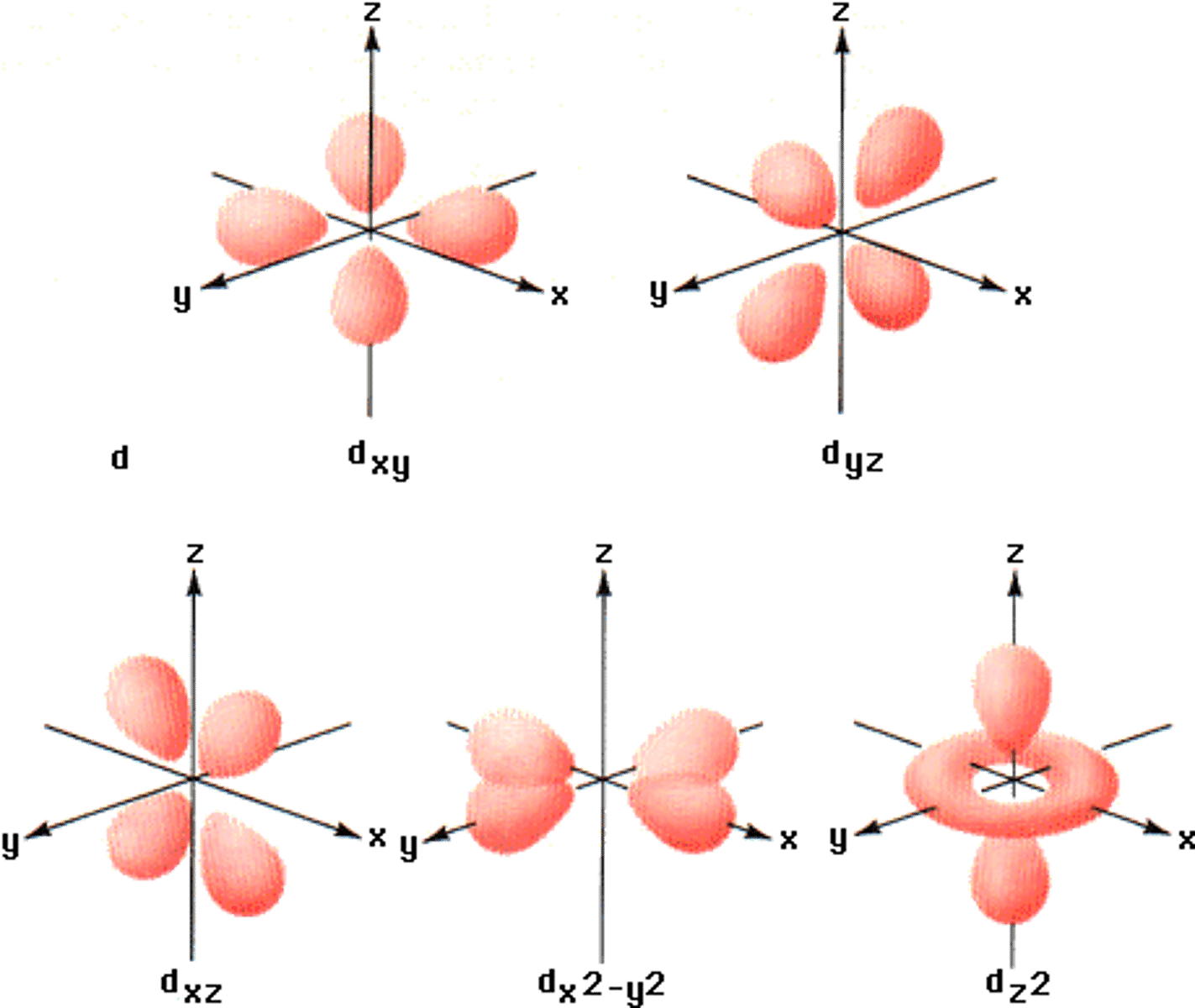
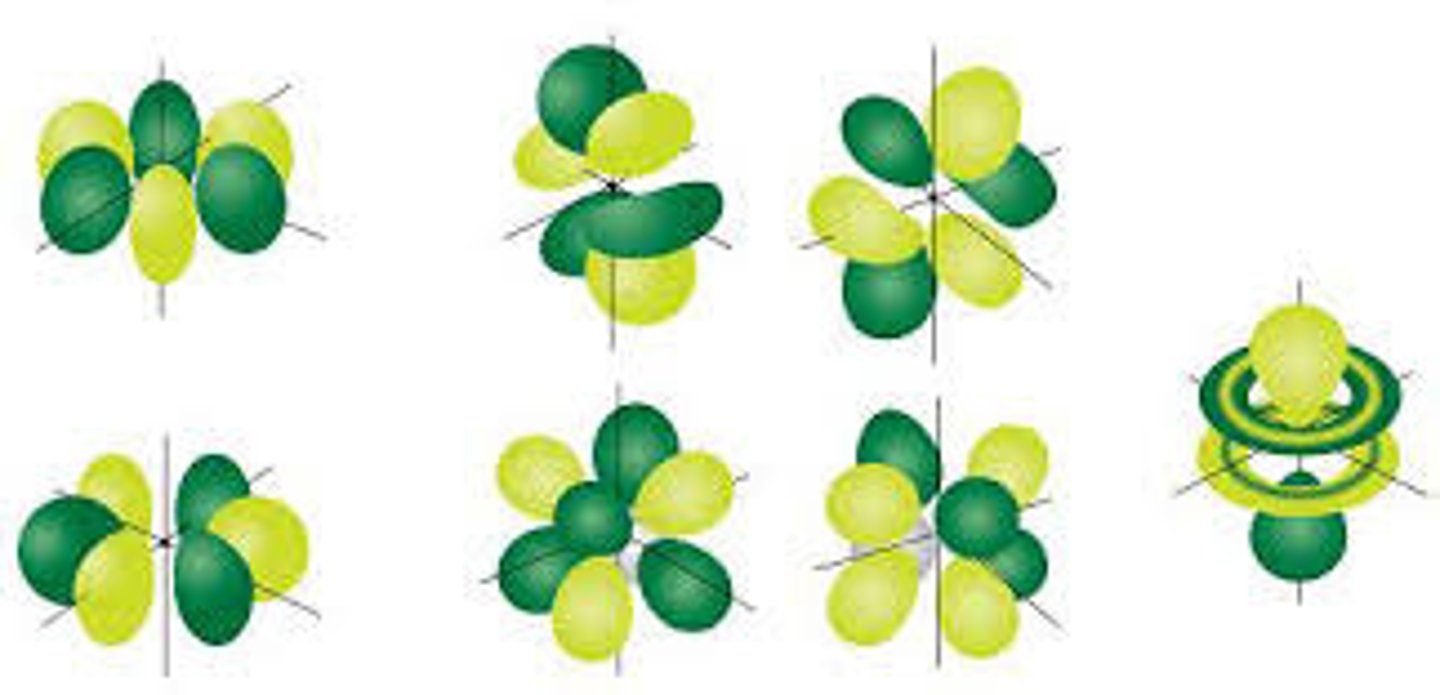
shape of f orbital
flower (8 lobes)
diamagnetic
all electrons are paired
-repelled by external magnetic field
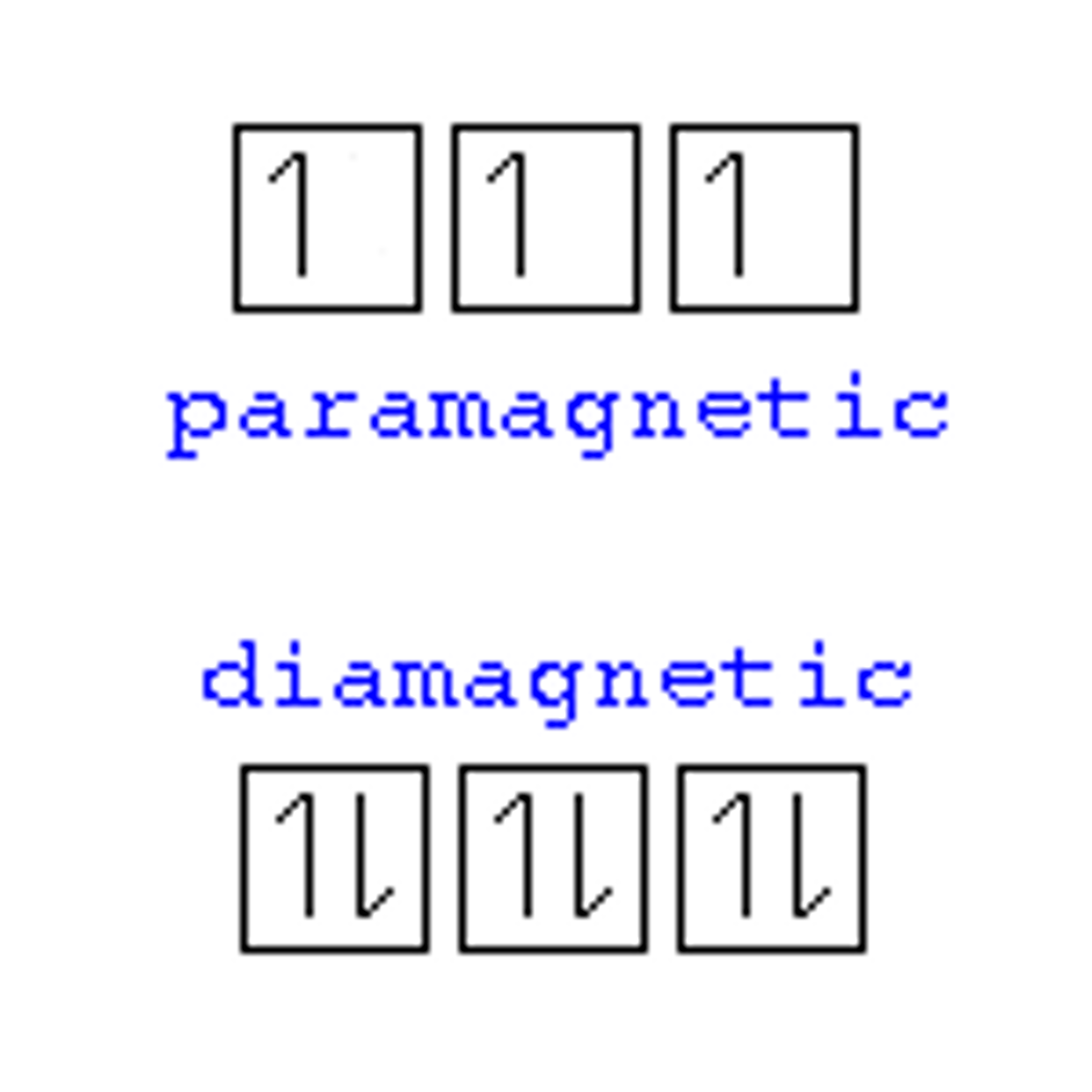
paramagnetic
1 or more unpaired electrons
-pulled into an external magnetic field
Aufbau Principle
An electron occupies the lowest-energy orbital that can receive it

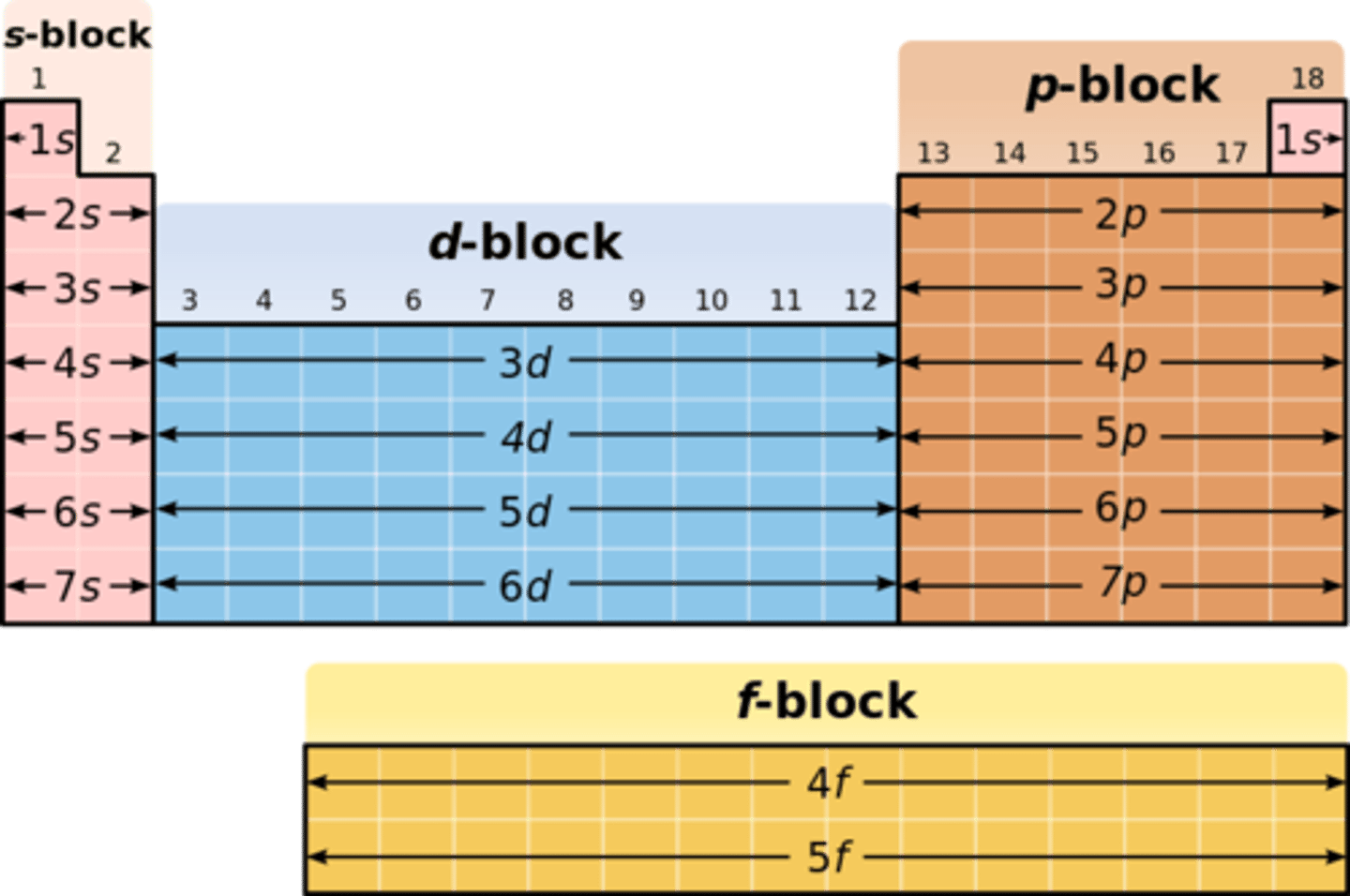
atomic orbitals on the periodic table
s- alkali and alkaline earth
p- non-metals
d- metals
f- metals
alkali metals
Group 1, 1 electron in outer level, very reactive, soft, silver, shiny, low density
-Lithium, Sodium, Potassium, Rubidium, Cesium, Francium
alkaline earth metals
metallic elements in group 2 of the periodic table which are harder than the alkali metals and are also less reactive
Berylium, magnesium, calcium, strontium, barium, radium
transition metals
Groups 3-12, 1-2 electrons in the outer energy level, less reactive than alsali-earth metals, shiny, good conductor of thermal energy and electrical current, high density
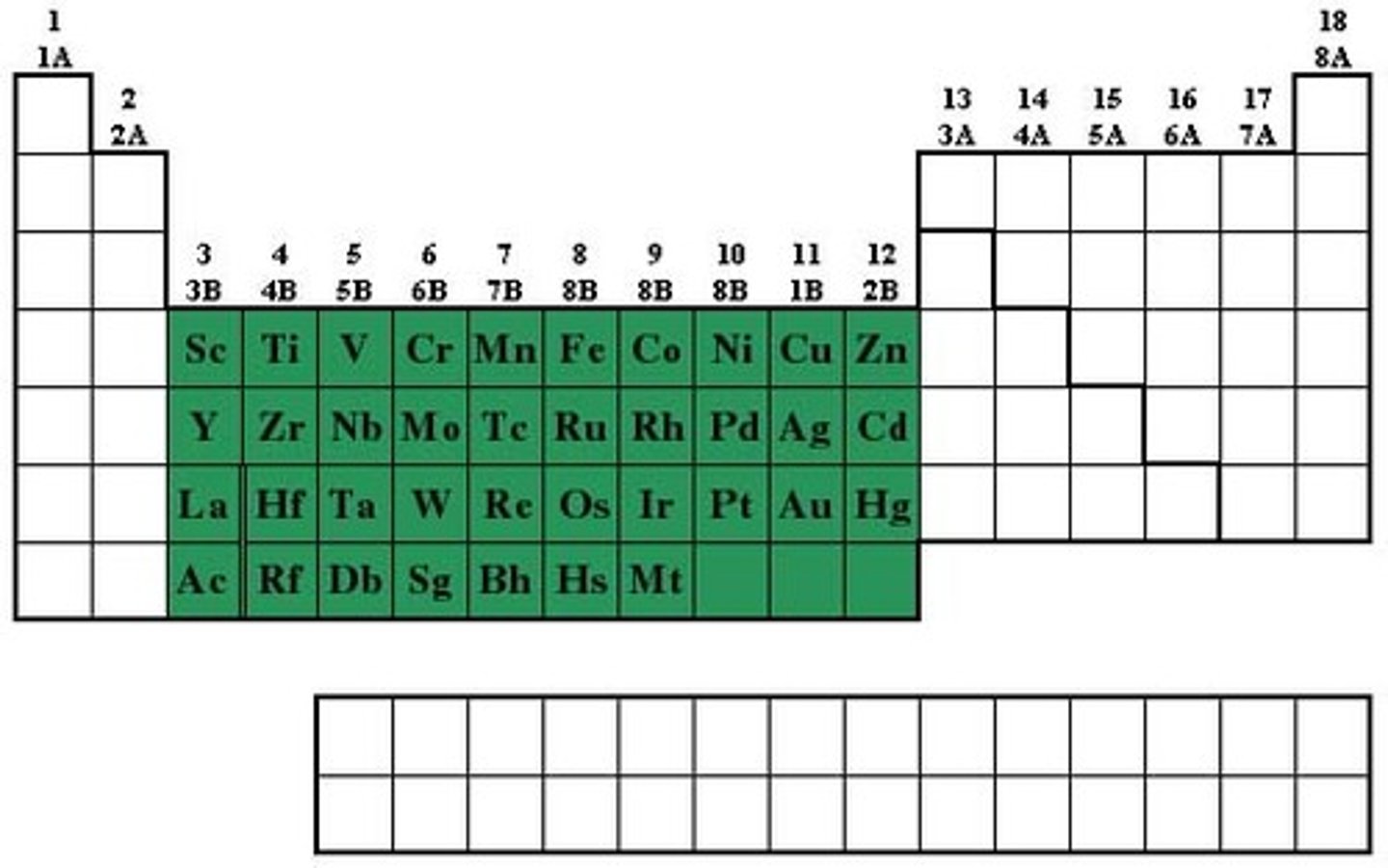
post transition metals
The are soft (or brittle), have poor mechanical strength, and have melting points lower than those of the transition metals. Being close to the metal-nonmetal border, their crystalline structures tend to show covalent or directional bonding effects, having generally greater complexity or fewer nearest neighbors than other metallic elements.
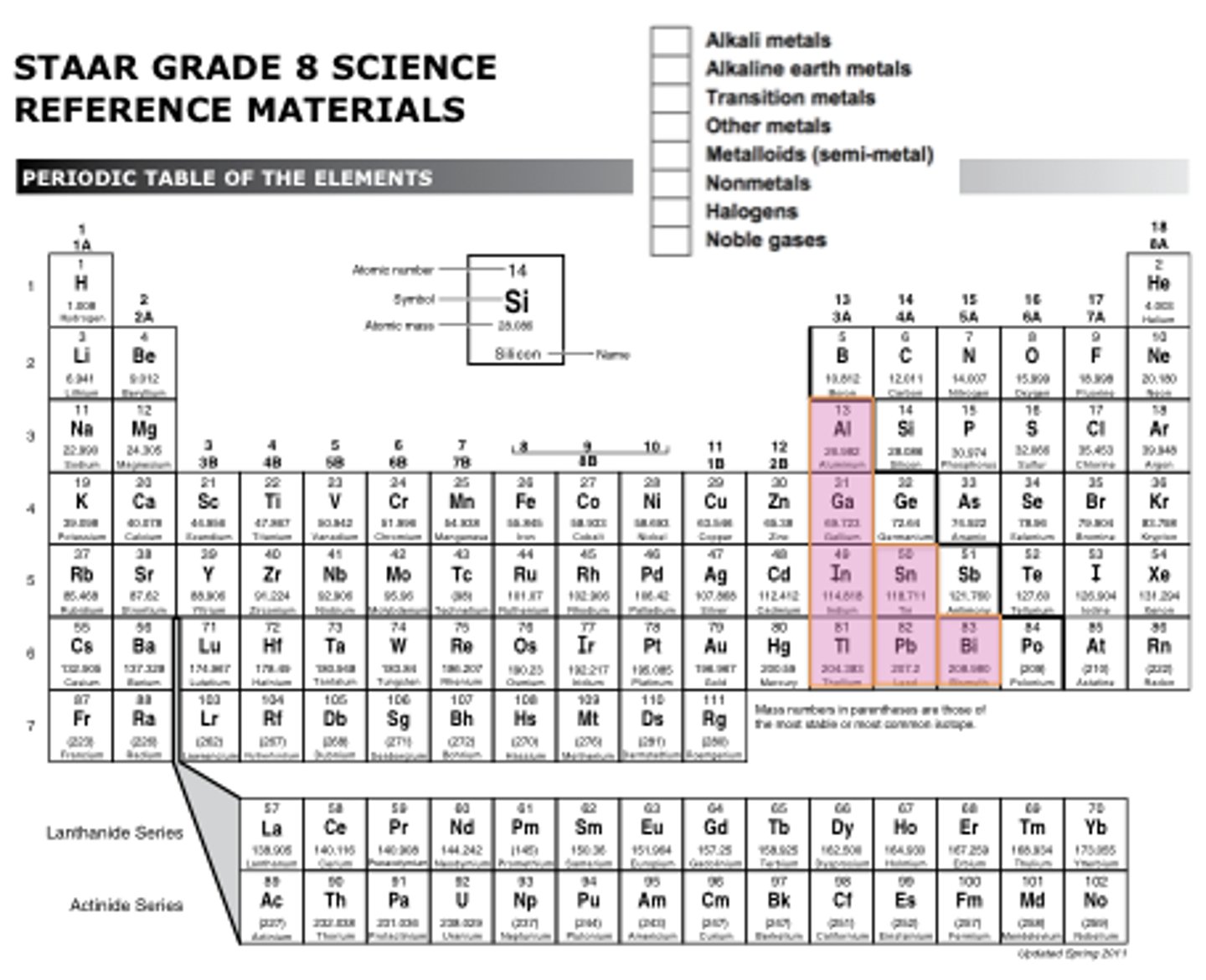
metalloids
Elements that have properties of both metals and nonmetals.
-boron, silicon, germanium, arsenic, antimony, tellurium, polonium
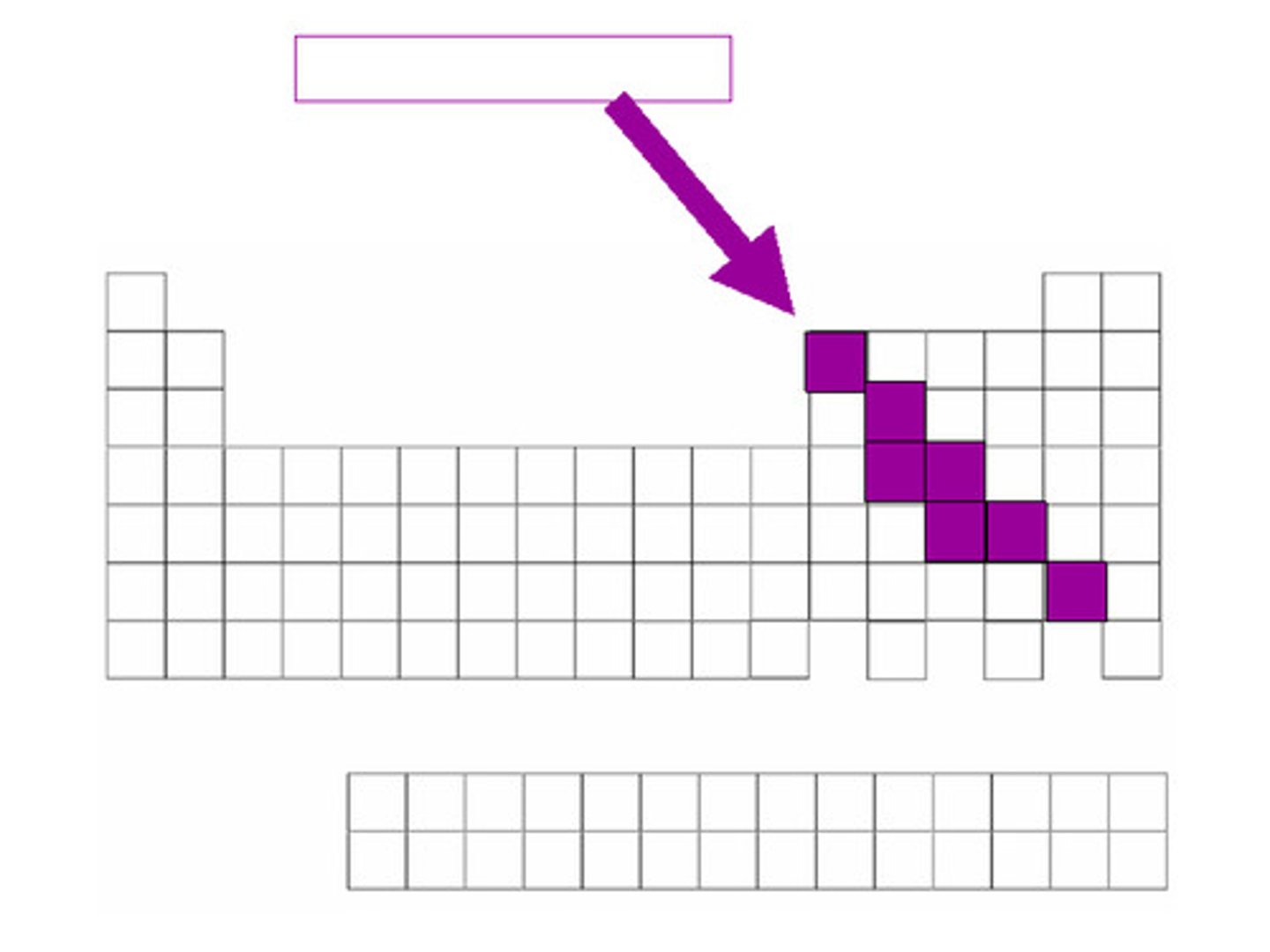
non-metals
Low conductivity, not ductile, not malleable, brittle, dull, gas at room temp
-carbon, nitrogen, oxygen, phosphorus, sulfur, selenium
halogens
group 17/group 7A
-flourine, chlorine, bromine, iodine, astatine, tennesine
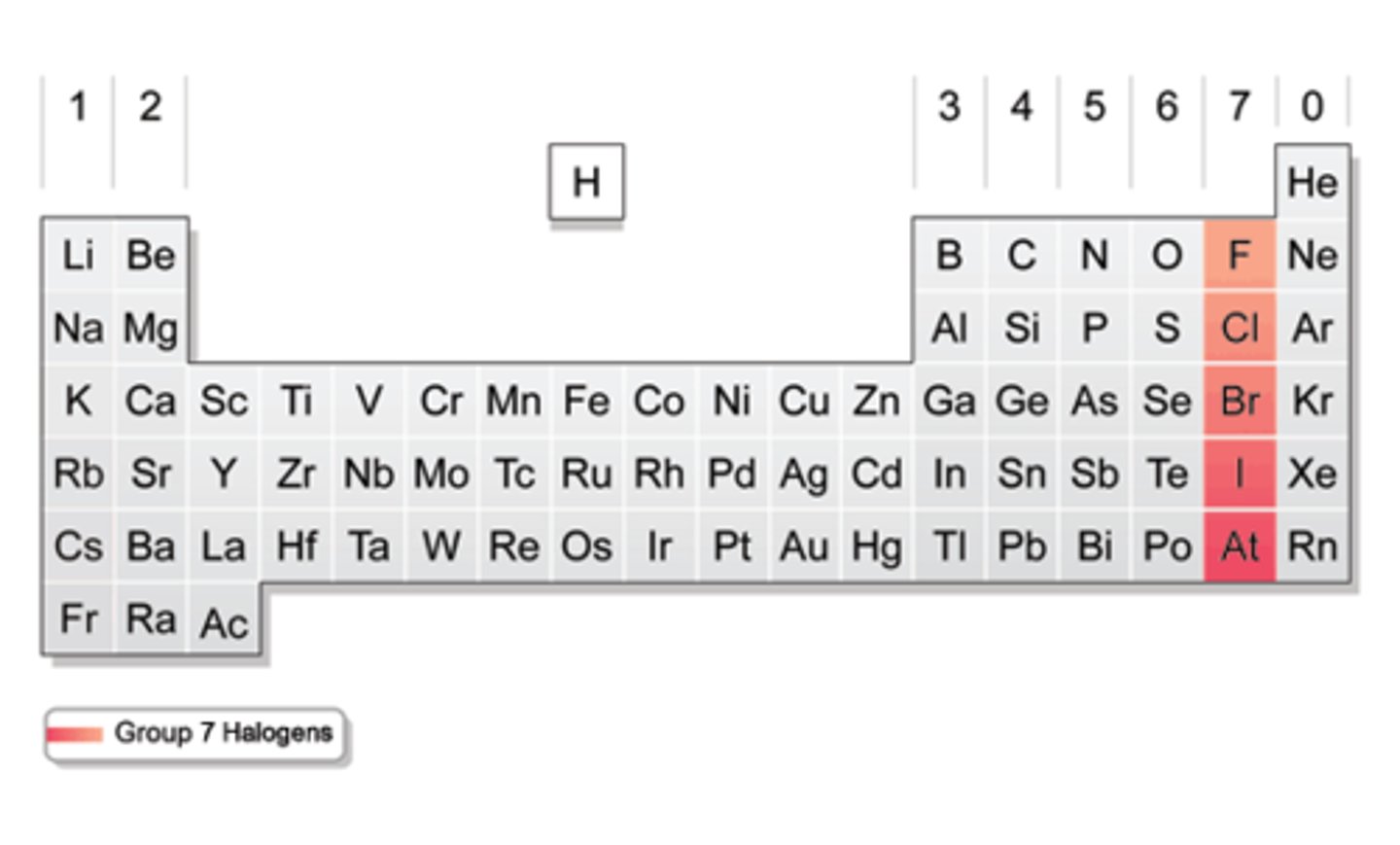
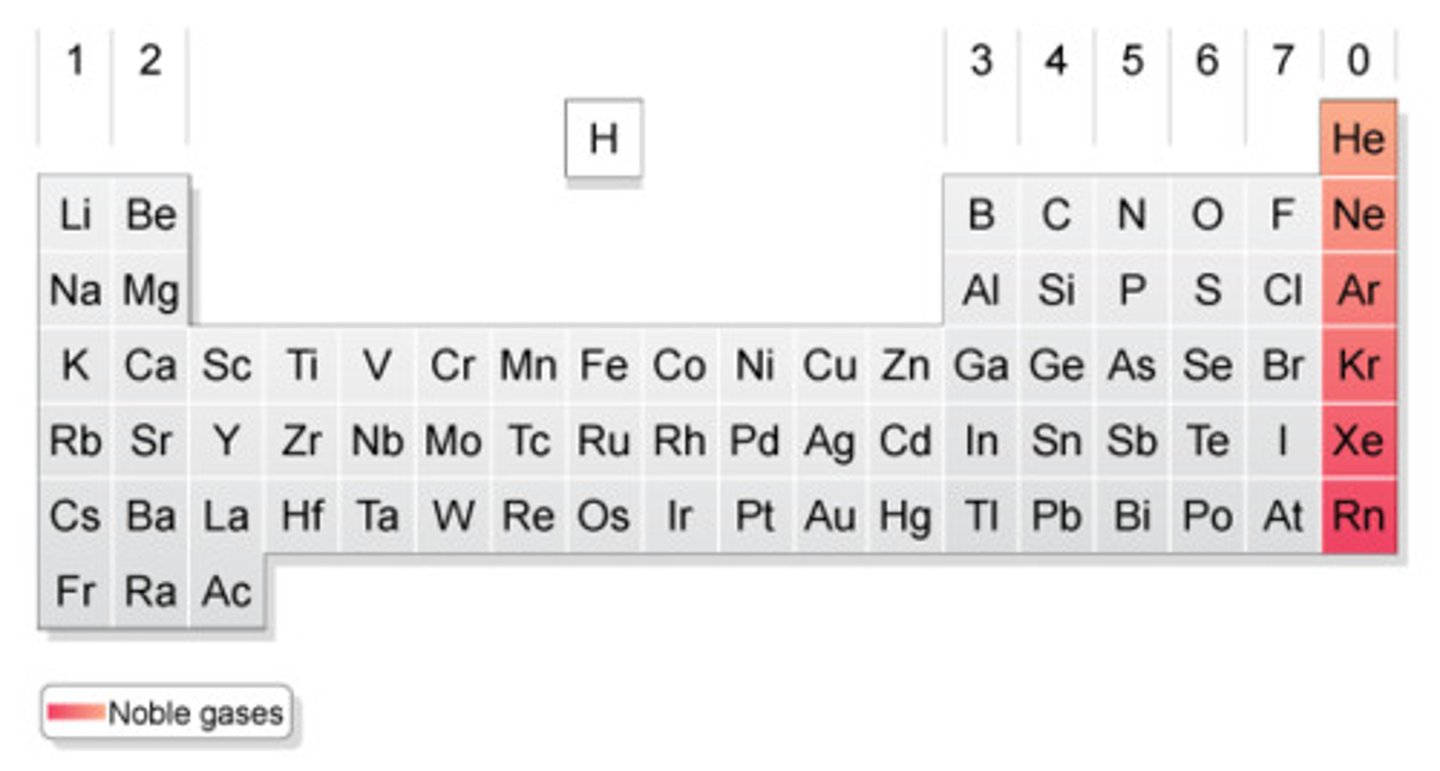
noble gases
Group 18/group 8A
helium, neon, argon, krypton, xenon, radon, oganesson
periodic table trend: Zeff (effective nuclear charge)
-pull between the nucleus and valence electrons
-unchanged going up or down the groups
-from left to right increases across periods
-means: higher effective nuclear charge causes greater attractions to the electrons, pulling the electron cloud closer to the nucleus which results in a smaller atomic radius.
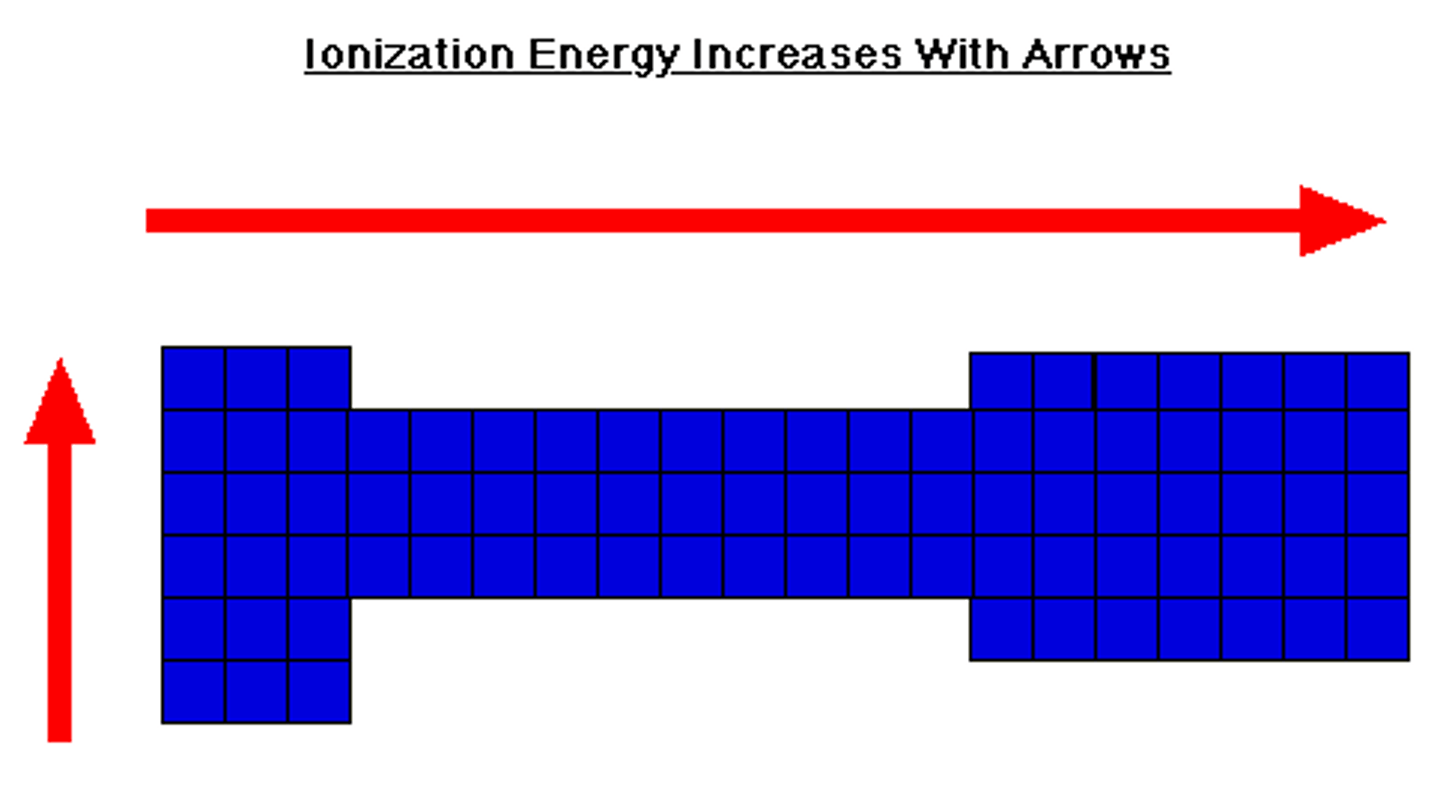
periodic table trend: ionization energy
-increases going up a group
-increases from left to right across periods
-meaning: the amount of energy required to remove an electron from an isolated atom or molecule increases following the above trends
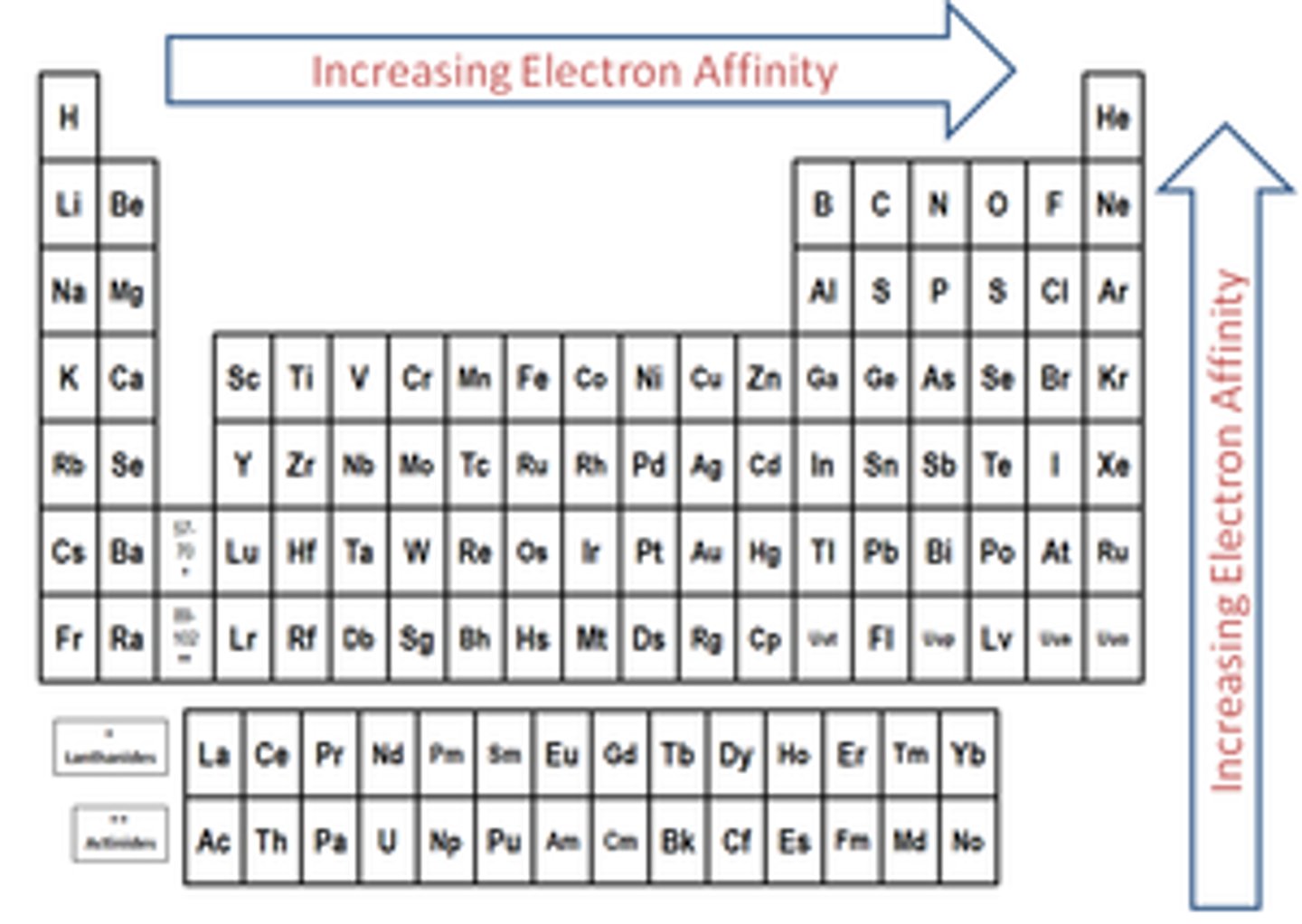
periodic table trend: electron affinity
-gain electrons
-deltaH of the reaction < 0 when gaining electrons but EA is reported as positive values
-noble gases have no affinity for electrons (it would take energy to force an electron on them)
-increases going up a group
-increases from left to right across periods
periodic table trend: electronegativity
-force the atom exerts on an electron in a bond
-of the noble gases only Kr and Xe have an EN
-increases going up a group
-increases from left to right across periods
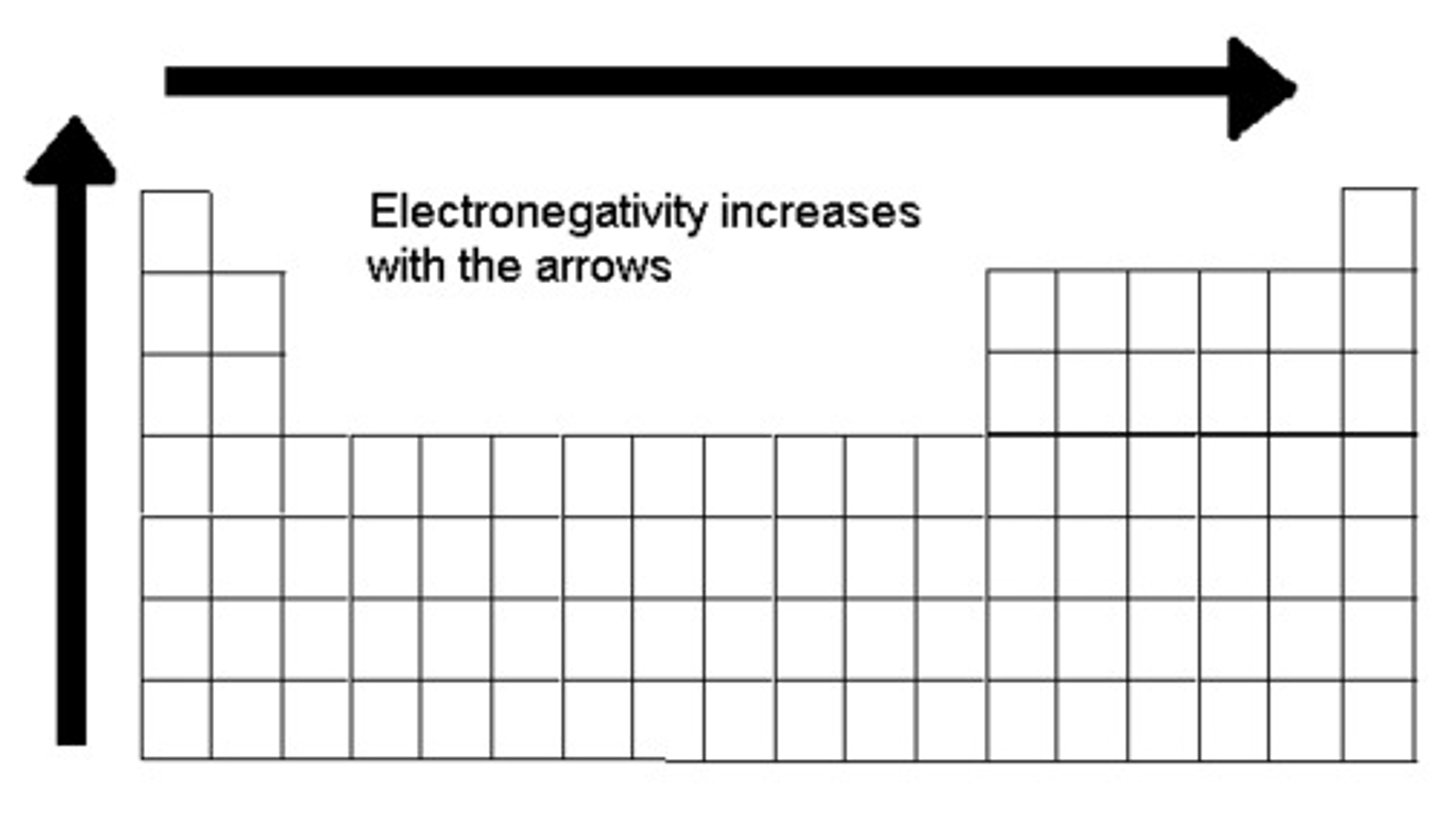
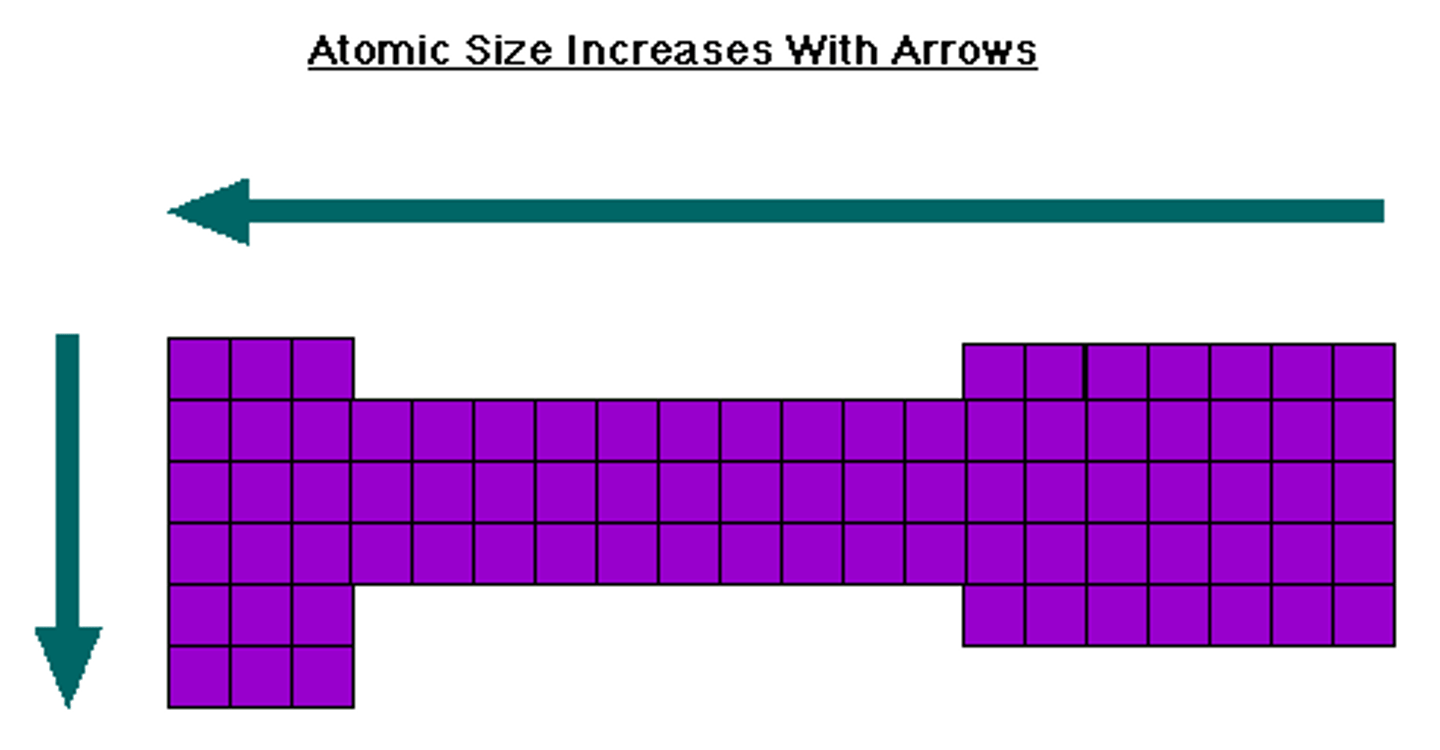
periodic table trend: atomic size
-increases going down a group
-increases going from right to left across periods
-this trend is basically the opposite of all others
-only this direction cations < neutral < anions
electronegativity of H
exact: 2.2
about: 2.0
electronegativity of C
exact: 2.55
about: 2.5
electronegativity of N
exact: 3.04
about: 3.0
electronegativity of O
exact: 3.44
about: 3.5
electronegativity of F
exact: 3.98
about: 4.0
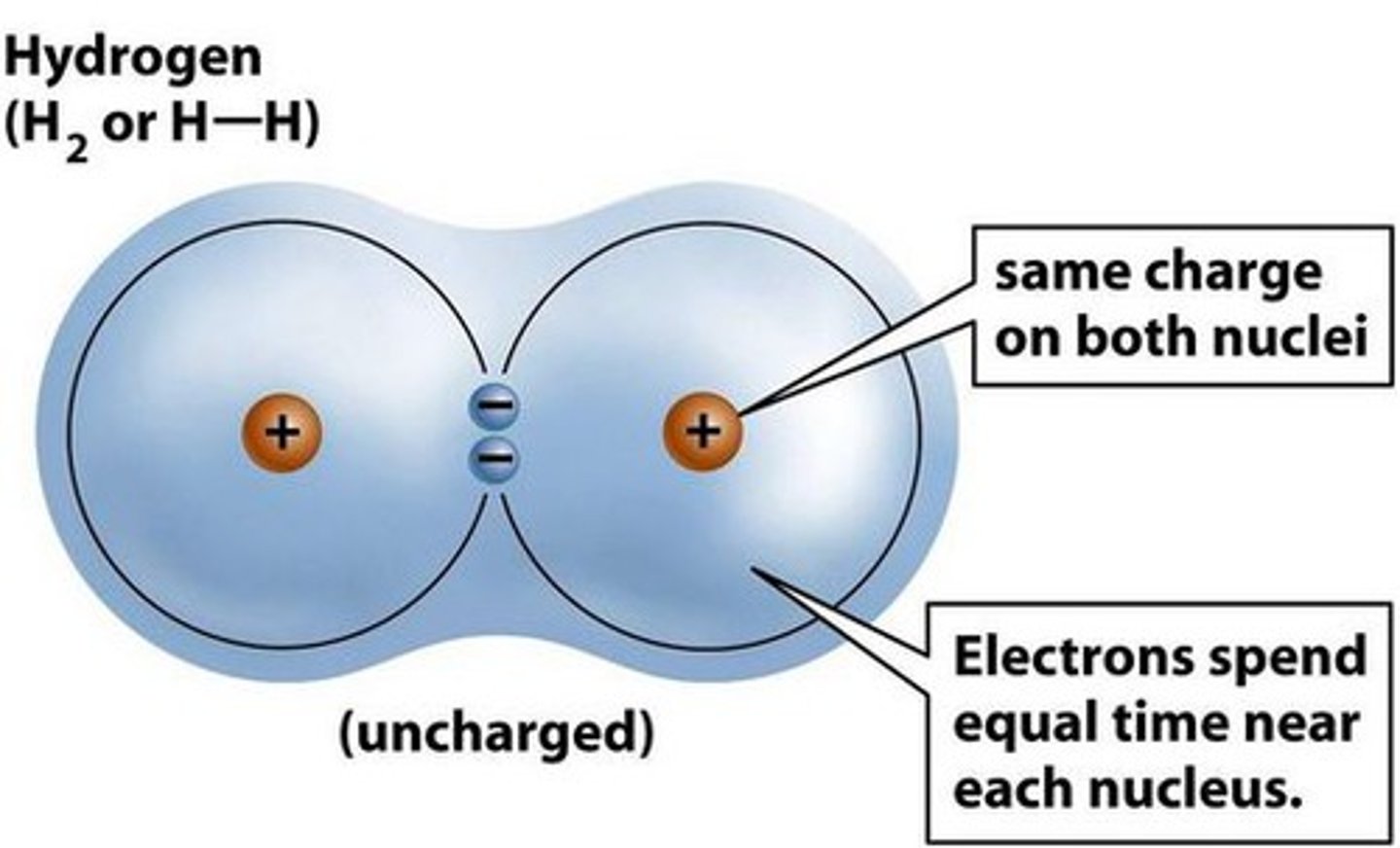
covalent bond
formed via the sharing of electrons between two elements of similar EN
bond order
refers to whether the covalent bond is a single, double, or triple bond
-as bond order increases bond strength and bond energy increase but bond length decreases

nonpolar bond
delta EN < 0.5
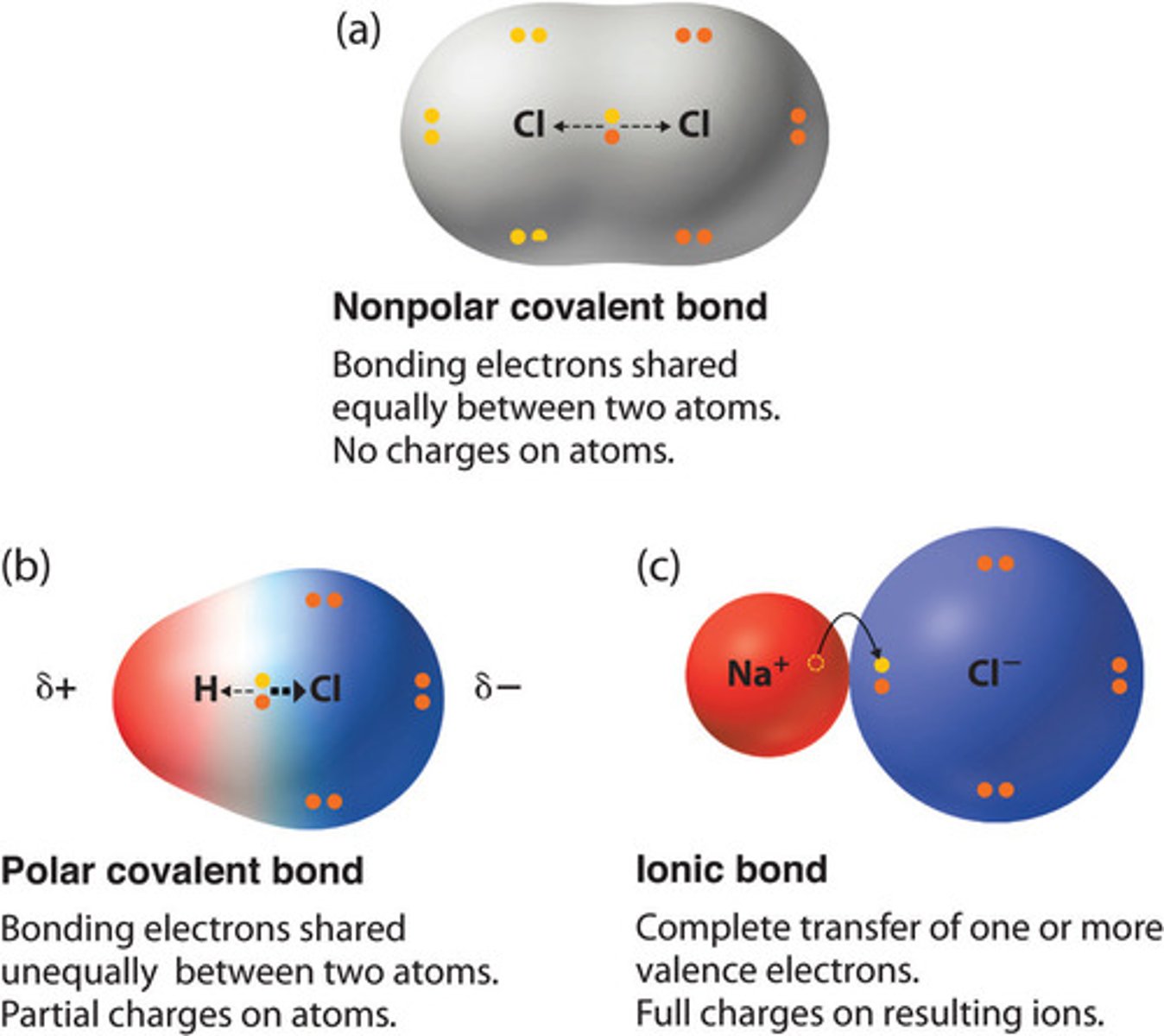
polar bonds
delta EN is between 0.5 and 1.7
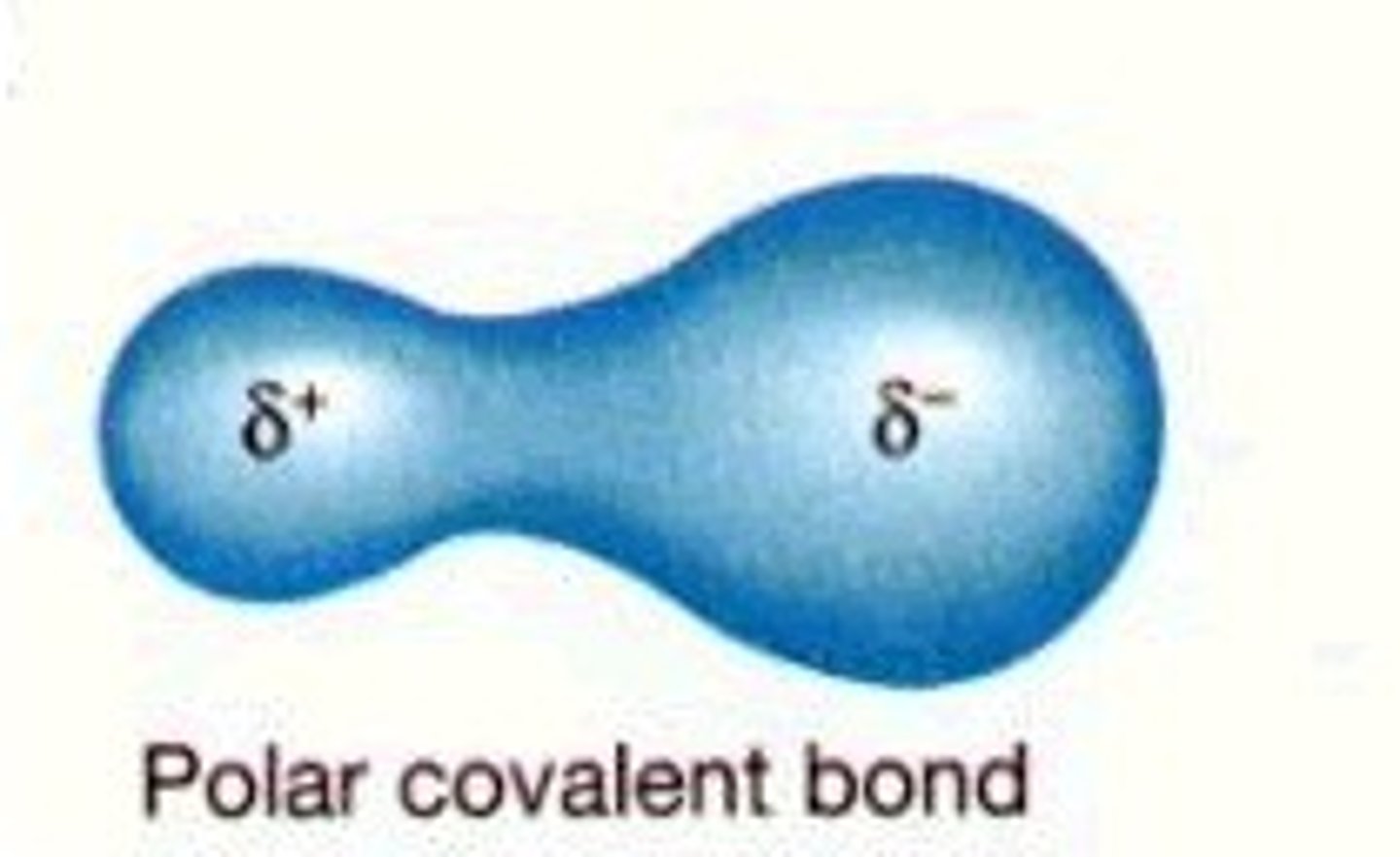
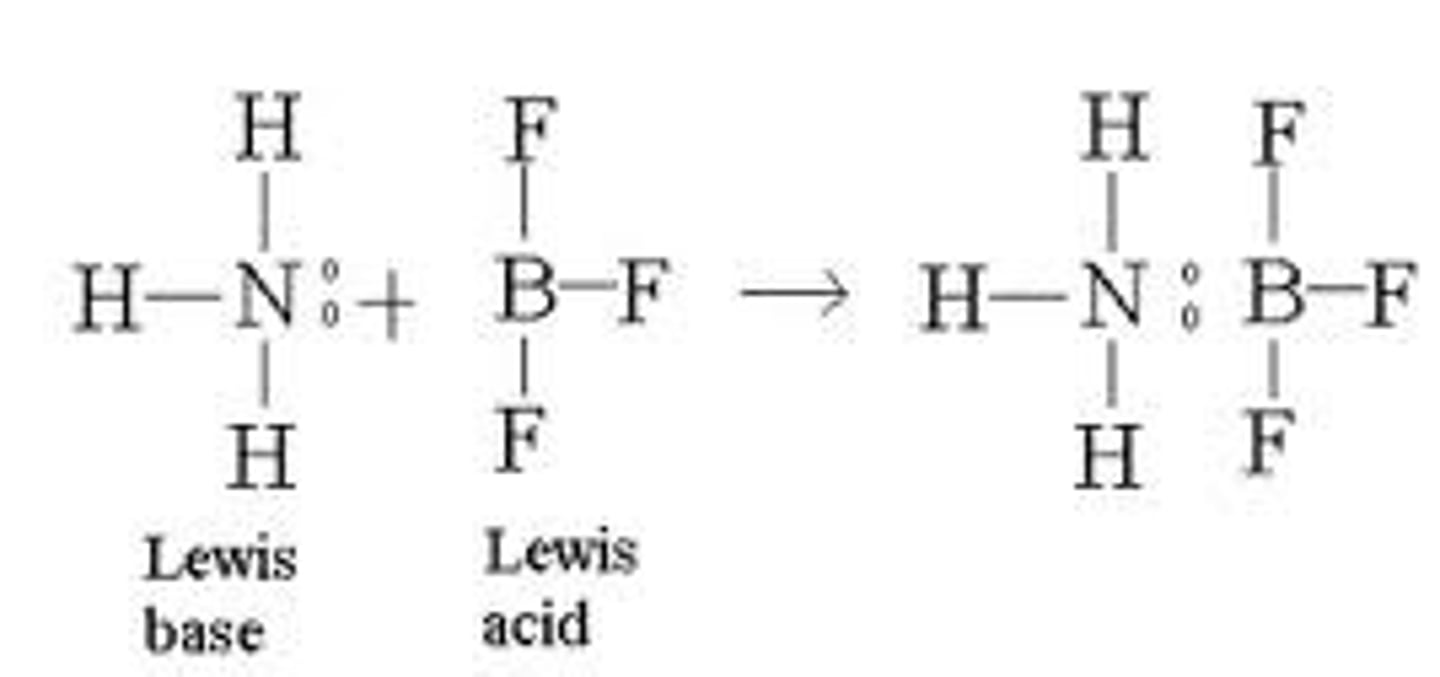
coordinate covalent bonds
a single atom provides both bonding electrons
-most often found in lewis acid-base chemistry
-lewis base: donates electrons
-lewis acid: accepts electrons
intramolecular forces
from strongest to weakest:
-hydrogen bonds
-dipole dipole
-london dispersion
note: van de walls forces are a general term that includes dipole dipole and london dispersion forces
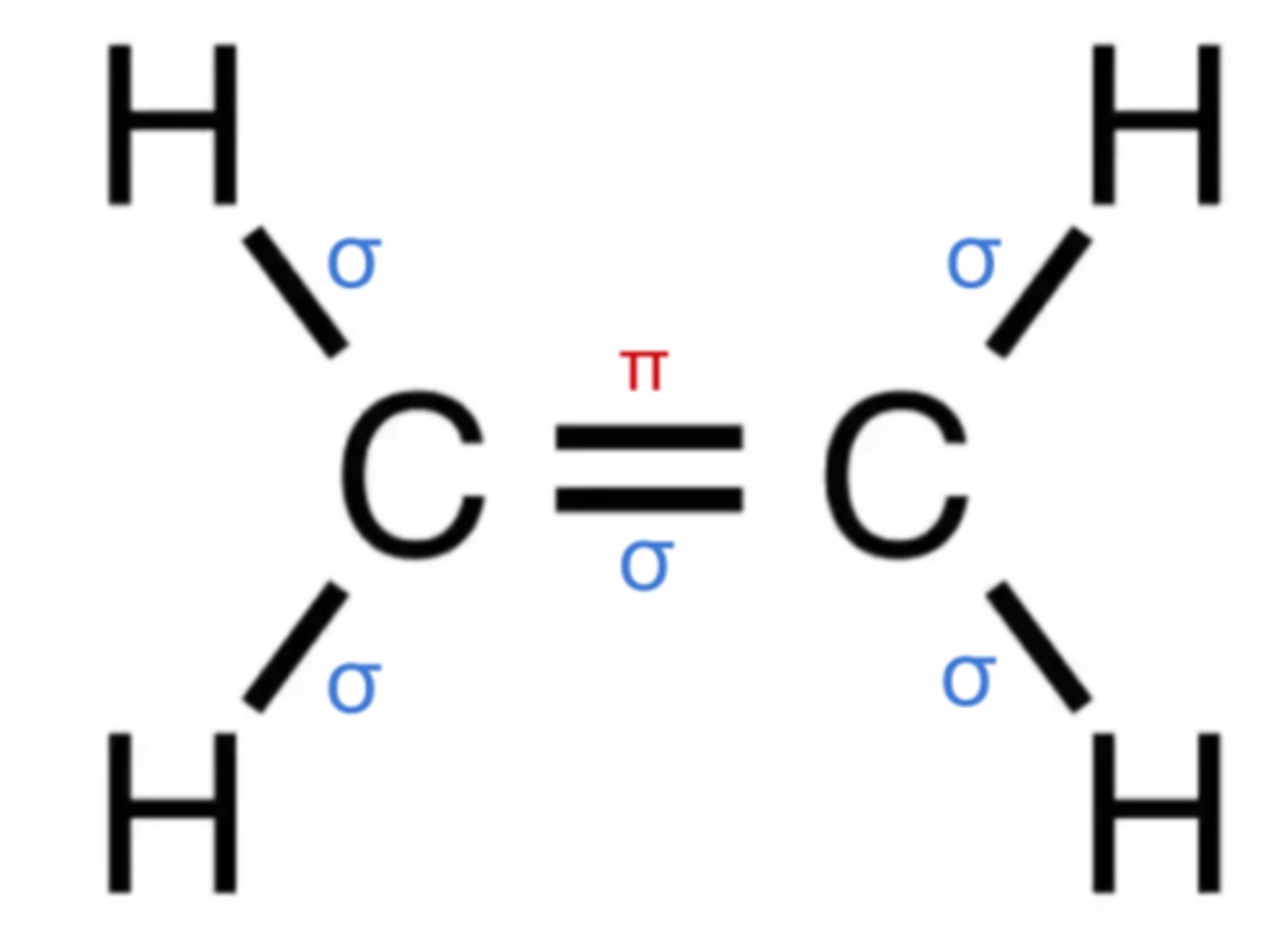
sigma bonds and pi bonds
single bond= sigma bond
double= 1 sigma bond, 1 pi bond
triple = 1 sigma bond, 2 pi bond
bond type of according to delta EN
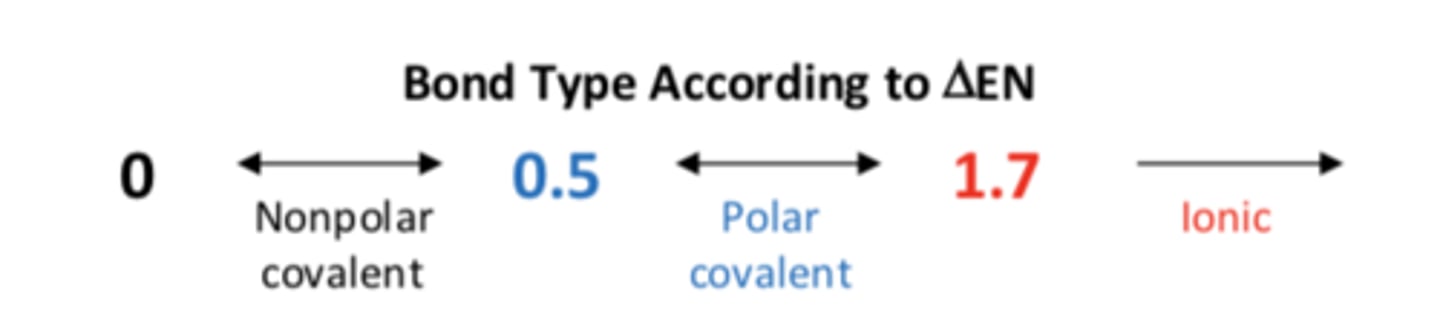
ionic bond
formed via the transfer of one or more electrons from an element with a relatively low IE to an element with a relatively high electron affinity
delta EN > 1.7
cation
positive +
anion
negative -
crystalline lattices
large, organized arrays of ions
formal charge
# of valence electrons - ( #nonbonding electrons + #pair of bonding electrons)
sp
e- groups around central atom: 2
bonded pairs: 2, 1
lone pairs: 0, 1
electron geometry: linear
molecule shape: linear, linear
bond angle: 180 degree
sp2
e- groups around central atom: 3
bonded pairs: 3, 2, 1
lone pairs: 0, 1, 2
electron geometry: trigonal planar
molecule shape: trig planar, bent, linear
bond angle: 120 degree
sp3
e- groups around central atom: 4
bonded pairs: 4, 3, 2, 1
lone pairs: 0, 1, 2, 3
electron geometry: tetrahedral
molecule shape: trig planar, trigonal pyramidal, bent, linear
bond angle: 109.5 degree
sp3d
e- groups around central atom: 5
bonded pairs: 5, 4, 3, 2
lone pairs: 0, 1, 2, 3
electron geometry: trigonal bipyramidal
molecule shape: trigonal bipyramdial, seesaw, T-shaped, linear
bond angle: 90 & 120 degree
sp3d2
e- groups around central atom: 6
bonded pairs: 6, 5, 4
lone pairs: 0, 1, 2, 3
electron geometry: octahedral
molecule shape: octahedral, square pyramidal, square planar
bond angle: 90 degree
electron geometry
bonded and lone pairs treated the same
molecular shape
lone pairs take up less space than a bond to another atom
equivalent mass
mass of an acid that yields 1 mole of H+ or mass of a base that reacts with 1 mole of H+
GEW (gram equivalent weight)
(molar mass)/(mol H+ or e-)
-unit is grams
equivalents =
(mass of compound)/(GEW)
-unit is grams
normality
(equivalents of solute)/(liters of solution)
or M x n (number of equivalents)
-concentration of equivalents in solution
for acids
the number of equivalents (n) is the number of H+ available from a formula unit
molarity
(normality)/(mol H+ or e-)
unit is mol/L
empirical
simplest whole-number ratio of atoms
molecular
multiple of empirical formula to show exact number of atoms of each element
combination reaction
-two or more reactants forming one product
ex: 2H2 + O2 -> 2H2O
decomposition
-single reactant breaks down
-2HgO (s) -> 2Hg + O2
combustion
-involves a fuel, usually a hydrocarbon, and O2
-commonly forms CO2 and H2O
-ex: CH4 + 2O2 -> CO2 + H2O
single displacement
-an atom/ion in a compound is replaced by another atom/ion
-Cu + AgNO3 -> Ag + CuNO3
double-displacement (metathesis)
-elements from two compounds swap places
-ex: CaCl2 + 2AgNO3 -> Ca(NO3)2 + 2AgCl
neutralization
a type of double replacement reaction
-acid + base -> salt + H2O
-HCl + NaOH -> NaCl + H2O
for elements (usually metals) that can form more than one positive ion, the charge is indicated by a Roman numeral in parentheses following the name of the element
Fe2+ -> iron(II)
Fe3+ -> iron(III)
Cu+ -> copper (I)
Cu2+ -> copper (II)
older method of naming them is: -ous and -ic to the atoms with lesser and greater charge, respectively
Fe2+ -> ferrous
Fe3+ -> ferric
Cu+ -> cuprous
Cu2+ -> cupric
monoatomic anions drop the ending of the name and add -ide
H- -> hydride
F- -> flouride
O2- -> oxide
S2- -> sulfide
N3- -> nitride
P3- -> phosphide
oxyanions= polyatomic anions that contain oxygen
more oxygen = -ate
less oxygen = -ite
NO3- -> nitrate
NO2- -> nitrite
SO4 2- -> sulfate
SO3 2- -> sulfite
in extended series of oxyanions, prefixes are also used
more oxygen= hyper (per-)
less oxygen= hypo-
ClO- -> hypochlorite
ClO2- -> chlorite
ClO3- -> chlorate
ClO4- -> perchlorate
polyatomic anions that gain H+ to for anions of lower charge add the word hydrogen or dihydrogen to the front
HCO3- -> hydrogen carbonate or bicarbonate
HSO4- -> hydrogen sulfate or bisulfate
H2PO2- -> dihydrogen phosphate
-ic
-acid name
-have more oxygen than -ous
-ous
-acid name
-have fewer oxygen than -ic
chemical kinetics
m = 0
order: zeroth order
rate law: R= k
integrated rate law: [A]= [A]0-kt
half life: t1/2 = ([A]0/2k)
units of rate constant: M/s
![<p>order: zeroth order</p><p>rate law: R= k</p><p>integrated rate law: [A]= [A]0-kt</p><p>half life: t1/2 = ([A]0/2k)</p><p>units of rate constant: M/s</p>](https://knowt-user-attachments.s3.amazonaws.com/880d2eec-d7a9-42e2-bdea-8caaa248d93a.jpg)
m = 1
order: first order
rate law: R= k[A]
integrated rate law: [A]= [A]0 x e^-kt
half life: t1/2 = (ln(2)/k)
units of rate constant: 1/s
![<p>order: first order</p><p>rate law: R= k[A]</p><p>integrated rate law: [A]= [A]0 x e^-kt</p><p>half life: t1/2 = (ln(2)/k)</p><p>units of rate constant: 1/s</p>](https://knowt-user-attachments.s3.amazonaws.com/ead36125-8bef-4c2b-81ae-13e1809bf924.png)
m = 2
order: second order
rate law: R= k [A]^2
integrated rate law: 1/[A] = 1/[A]0 + kt
half life: t1/2 = (1/k[A]0)
units of rate constant: 1/M*s
![<p>order: second order</p><p>rate law: R= k [A]^2</p><p>integrated rate law: 1/[A] = 1/[A]0 + kt</p><p>half life: t1/2 = (1/k[A]0)</p><p>units of rate constant: 1/M*s</p>](https://knowt-user-attachments.s3.amazonaws.com/b49cdf44-101f-486f-a11f-86a48dce82c2.png)
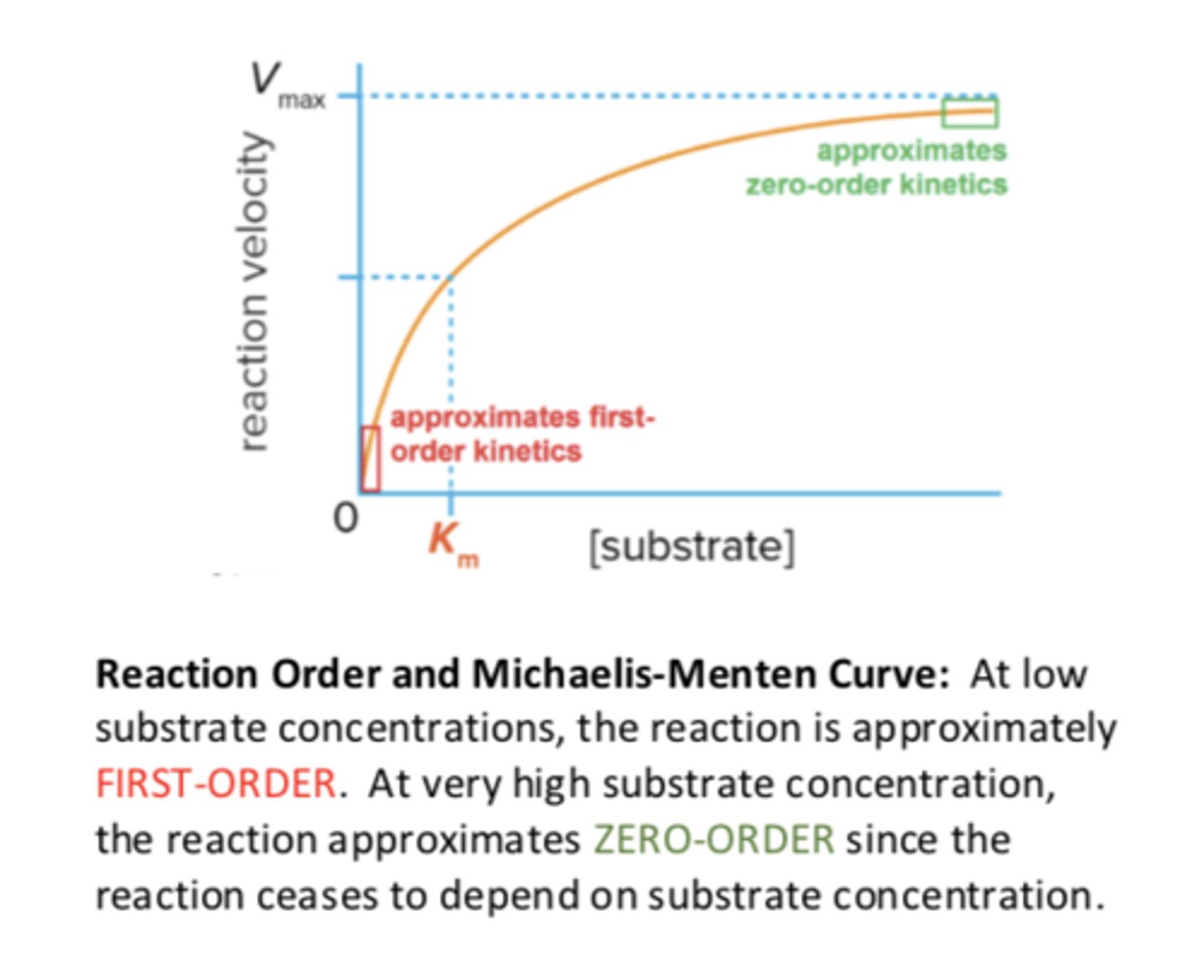
Reaction Order and Michaelis-Menten Curve
-at low substrate concentrations, the reaction is approximately FIRST-ORDER
-at very high substrate concentration, the reaction approximates ZERO-order since the reaction ceases to depend on substrate concentration
hydrolysis
using water to break the bonds in a molecule
gibbs free energy (delta G)
-delta G = Ea - Ea rev
-delta G
exergonic
+delta G
endergonic
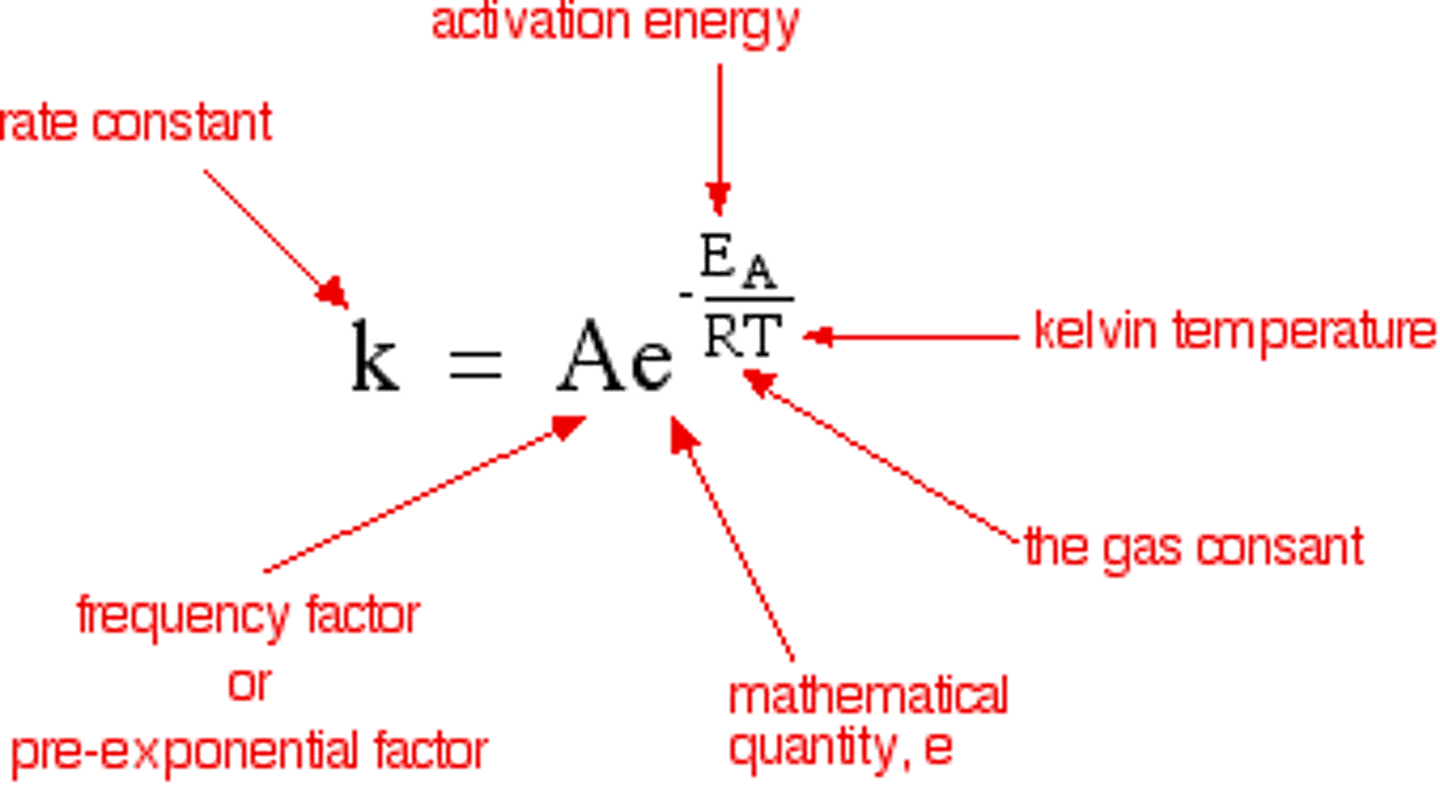
Arrhenius equation
k=Ae^(-Ea/RT)
k= rate constant
A= frequency factor
Ea = activation energy
R= gas constant = 8.314 J/mol*K
T= temp in K
Trends in Arrhenius equation
higher A = higher k
higher T = higher k
(exponent gets closer to 0 and exponent becomes less negative)
definition of rate
for aA + bB -> cC + dD
rate = (- delta [A])/ (adelta(t)) = (- delta [B])/ (bdelta(t))= (- delta [C])/ (cdelta(t)) = (- delta [D])/ (ddelta(t))
rate law
rate = k[A]^x[B]^y
radioactive decay
[A]t = [A]0 x e^kt