GEN CHEM 1 | VSEPR Theory
1/35
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Valence Shell Electron Pair Repulsion (VSEPR) Theory
electrons surrounding atoms exert repulsive forces against each other, which means a molecule will take a shape where this will be minimized
Central Atom
Represented by A, it is where all the bonds are attached to.
Electron Groups
consists of bonding groups and lone pairs
Bonding Groups
Represented by X, these are the bonds formed between the central atom and the other atoms
Lone Pairs
Represented by E, these are pairs of electrons that did not form a bond
Linear Electron Geometry
2 electron groups; sp hybridization; 180º
Trigonal Planar Electron Geometry
3 electron groups; sp² hybridization; 120º
Tetrahedral Electron Geometry
4 electron groups; sp³ hybridization; 109.5º
Trigonal Bipyramidal Electron Geometry
5 electron groups; sp³d hybridization and 120º (in plane) and 90º (above and below plane) for all VSPER classes except linear which has 180º
Octahedral Electron Geometry
6 electron groups; sp³d² hybridization and 90º
Linear Molecular Geometry
AX2 (2 bonds, 0 lone pairs)
AX2E3 (2 bonds, 3 lone pairs)
AX2E4 (2 bonds, 4 lone pairs)
Trigonal Planar Molecular Geometry
AX3 (3 bonds, 0 lone pairs)
Bent Molecular Geometry
AX2E (2 bonds, 1 lone pair)
AX2E2 (2 bonds, 2 lone pairs)
Tetrahedral Molecular Geometry
AX4 (4 bonds, 0 lone pairs)
Trigonal Pyramidal Molecular Geometry
AX3E (3 bonds, 1 lone pair)
Trigonal Bipyramidal Molecular Geometry | Composition
AX5 (5 bonds, 0 lone pair)
Seesaw Molecular Geometry | Composition
AX4E (4 bonds, 1 lone pair)
T-Shaped Molecular Geometry | Composition
AX3E2 (3 bonds, 2 lone pairs)
AX3E3 (3 bonds, 3 lone pairs)
Octohedral Molecular Geometry | Composition
AX6 (6 bonds, 0 lone pairs)
Square Pyramidal Molecular Geometry | Composition
AX5E (5 bonds, 1 lone pair)
Square Planar Molecular Geometry | Composition
AX4E2 (4 bonds, 2 lone pairs)
Predicting Molecular Geometry
Draw the Lewis Structure of the molecule
Count the number of bonds and lone pairs based on the central atom. Write these as the AXE notation.
Determine the shape based on the given notation
Draw the molecule based on the shape.
What is the line if the bond is on the plane of the paper?
Straight line
What is the line if the bond is coming out of the page towards us?
Triangle
What is the line if the bond is going into the page away from us?
Broken line
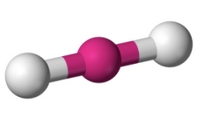
What molecular geometry is shown here?
Linear
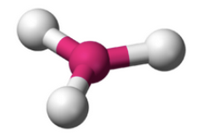
What molecular geometry is shown here?
Trigonal Planar
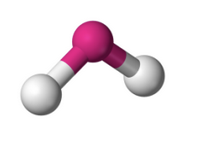
What molecular geometry is shown here?
Bent/Angular
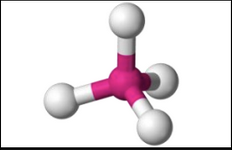
What molecular geometry is shown here?
Tetrahedral
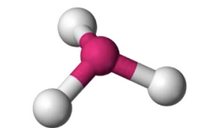
What molecular geometry is shown here?
Trigonal Pyramidal
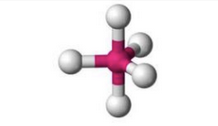
What molecular geometry is shown here?
Trigonal Bipyramidal
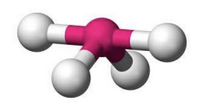
What molecular geometry is shown here?
Seesaw
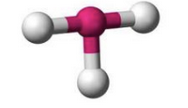
What molecular geometry is shown here?
T-Shape
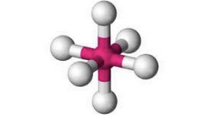
What molecular geometry is shown here?
Octahedral
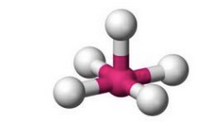
What molecular geometry is shown here?
Square Pyramidal
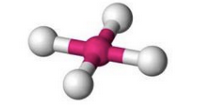
What molecular geometry is shown here?
Square Planar