Oceanography Exam #2
0.0(0)
Card Sorting
1/156
Earn XP
Last updated 2:47 PM on 3/6/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
157 Terms
1
New cards
deep-ocean currents
below the pycnocline, 90% of all ocean water, slow velocity
2
New cards
seawater density increases →?
dec temp, inc salinity
3
New cards
\
4
New cards
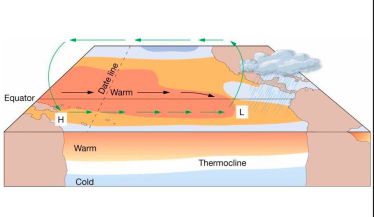
El Nino
* Walker Cell Circulation disrupted
* High pressure in eastern Pacific weakens
* Weaker trade winds
* Warm pool migrates eastward
* Thermocline deeper in eastern Pacific
* Downwelling
* Lower biological productivity → Peruvian fishing suffers
* High pressure in eastern Pacific weakens
* Weaker trade winds
* Warm pool migrates eastward
* Thermocline deeper in eastern Pacific
* Downwelling
* Lower biological productivity → Peruvian fishing suffers
5
New cards
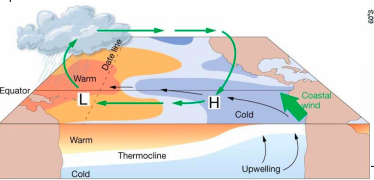
la nina
* Increased pressure difference across equatorial Pacific
* Stronger trade winds
* Stronger upwelling in eastern Pacific
* Shallower thermocline
* Cooler than normal seawater
* Higher biological productivity
* Stronger trade winds
* Stronger upwelling in eastern Pacific
* Shallower thermocline
* Cooler than normal seawater
* Higher biological productivity
6
New cards
el nino warm phase occurs every __ years
2-10
7
New cards
occurence of enso events
highly irregular
phases usually last 12-18 months
phases usually last 12-18 months
8
New cards
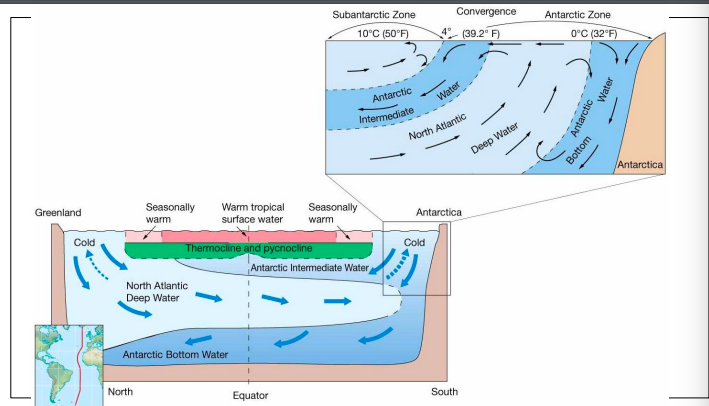
Thermohaline circulation
deep ocean circulation driven by temperature and salinity caused density differences in water
\
originates in high latitude surface ocean
cooled, now dense surface water sinks and changes little
\
originates in high latitude surface ocean
cooled, now dense surface water sinks and changes little
9
New cards
sources of deep water
antarctic bottom water, north atlantic deep water,
10
New cards
antarctic bottom water
* Densest water in the open ocean
* Rapid freezing produces cold, high density water
* Spreads into all ocean basins
* Rapid freezing produces cold, high density water
* Spreads into all ocean basins
11
New cards
north atlantic deep water
* Cools, sea ice forms, water sinks
* North Atlantic in the Norwegian Sea
* Labrador Sea
* Dense salty Mediterranean Sea
* Less Dense than Antarctic Bottom Water
* North Atlantic in the Norwegian Sea
* Labrador Sea
* Dense salty Mediterranean Sea
* Less Dense than Antarctic Bottom Water
12
New cards
subtropical convergence
downwelling
doesn’t produce deep water
doesn’t produce deep water
13
New cards
antarctic and arctic convergence
downwelling
antarctic intermediate water
antarctic intermediate water
14
New cards
oceanic common water
pacific and indian oceans
* don’t have a source of NHDW
* north pacific water = low salinity
* indian ocean = too warm
AABW and NADW mix to form OCW → lines bottom of Pacific and Indian
* don’t have a source of NHDW
* north pacific water = low salinity
* indian ocean = too warm
AABW and NADW mix to form OCW → lines bottom of Pacific and Indian
15
New cards
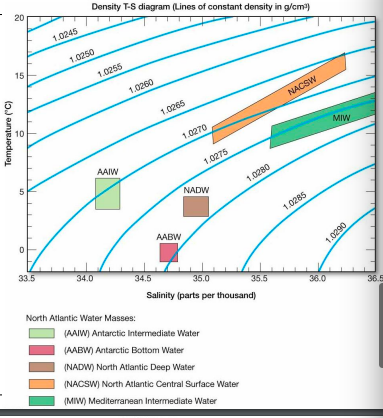
T-S Diagram
temp and salinity
lines of equal density
lines of equal density
16
New cards
study
17
New cards
converging surface water - downwelling
* Surface waters move toward each other
* Water piles up
* Low biological productivity
* Water piles up
* Low biological productivity
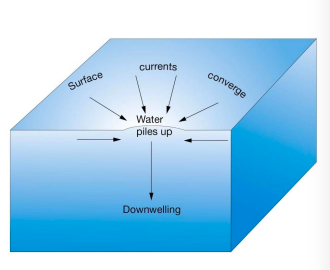
18
New cards
coastal upwelling
* Ekman transport moves surface seawater offshore.
* Cool, nutrient-rich deep water comes up to replace displaced surface waters.
* Western U.S. and cool San Francisco temperatures
* Cool, nutrient-rich deep water comes up to replace displaced surface waters.
* Western U.S. and cool San Francisco temperatures
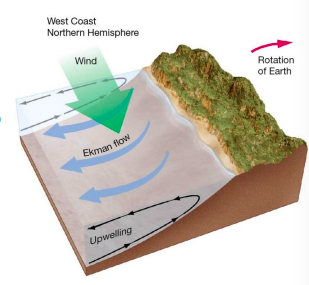
19
New cards
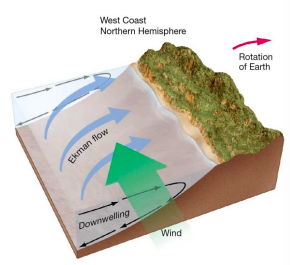
coastal downwelling
* Ekman transport moves surface seawater toward shore.
* Water piles up, moves downward in water column
* Lack of marine life
* Water piles up, moves downward in water column
* Lack of marine life
20
New cards
other coastal upwelling causes
* Offshore winds
* Seafloor obstruction
* Coastal geometry change
* Lack of pycnocline → High latitude oceans
* Seafloor obstruction
* Coastal geometry change
* Lack of pycnocline → High latitude oceans
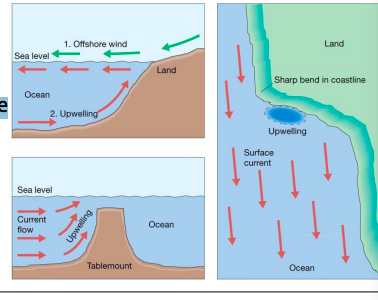
21
New cards
walker circulation cell
normal conditions
* Air pressure across equatorial Pacific is higher in eastern Pacific
* Strong southeast trade winds
* Pacific warm pool on western side of ocean
* Thermocline deeper on western side
* Upwelling off the coast of Peru
* Air pressure across equatorial Pacific is higher in eastern Pacific
* Strong southeast trade winds
* Pacific warm pool on western side of ocean
* Thermocline deeper on western side
* Upwelling off the coast of Peru
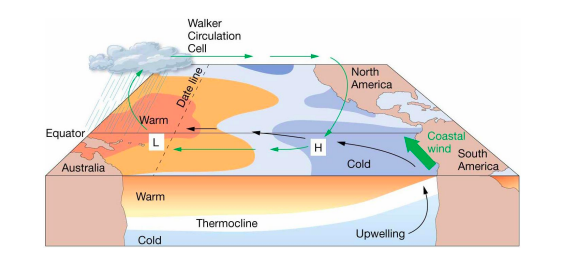
22
New cards
Discuss how the dipolar nature of a water molecule makes it such an effective solvent of ionic compounds.
The dipolar characteristic of water is really the key component to why water is such an effective solvent of ionic compounds. This is because the water molecules reduce the attraction between opposing ions, allowing the water molecules to stick to polar chemical compounds. This reduced attraction between the opposing ions allows for ionic compounds to separate easily.
23
New cards
Why are the freezing and boiling points of water higher than would be expected for a compound of its molecular makeup?
The freezing and boiling points of water are much higher than expected because the water molecule is made up of hydrogen bonds and van der Waals forces, which are really hard to break. These bonds require additional heat energy to overcome, and therefore leave the freezing and boiling points of water much higher than similar chemical compounds.
24
New cards
Describe how excess heat energy absorbed by Earth’s low latitude regions is transferred to heat deficient higher latitudes through a process that uses water’s latent heat of evaporation.
Water has a high specific heat, so when evaporation happens at the low latitude regions, a large amount of heat energy is absorbed. These vaporized molecules(from the evaporation) then travel to the heat deficient higher latitude regions through an atmospheric convection cell. Once the vaporized molecules reach the higher latitude region, they are condensed due to the decreased temperatures in these areas. The heat energy that was absorbed is released due to the condensation, which carries the excess heat energy from low latitude regions to the high latitude regions.
25
New cards
What physical conditions create brackish water in the Baltic Sea and hypersaline water in the Red Sea?
In the Baltic Sea, the physical conditions that create brackish water are freshwater and seawater. The freshwater from rivers or high rainfall mix with the seawater and create a ‘diluted’ salinity, or somewhat salty mix. In areas like the Red Sea, the physical conditions that create hypersaline water are high evaporation rates and limited open-ocean circulation. This causes excessive salt water, and occurs often in seas and inland bodies of water.
26
New cards
Describe three processes by which dissolved components are added to seawater and four processes by which dissolved components are removed from seawater.
Three processes where dissolved components are added to seawater are stream runoff or river discharge, volcanic eruptions, and hydrothermal activity at the mid-ocean ridge. Runoff transports dissolved components directly into the seawater and a large part of these components accumulate over time. Volcanic eruptions can add dissolved components into seawater by dispersing sulfur and other compounds through ashes and wind. Lastly, hydrothermal activity at the mid-ocean ridge can both add and remove dissolved components in seawater. Four processes where dissolved components are removed from seawater are absorption and precipitation, sea spray, biological processes and hydrothermal activity at the mid-ocean ridge. Precipitation and absorption remove dissolved components through the water cycle process. Sea spray releases small particles of dissolved compounds into the atmosphere, usually after a wave break. Lastly, biological processes like the production of hard parts by marine organisms also removes dissolved compounds from seawater.
27
New cards
Describe the halocline, including where it occurs in the ocean.
A halocline is a layer of rapidly changing salinity with depth. It goes from about 300 to 1,000 meters below the surface. In the low-latitude curve, the salinity decreases, while in the high-latitude curve, the salinity increases.
28
New cards
Using the processes that affect seawater salinity, explain why there is such a large range of salinity variation at the surface but such a narrow range of salinity at depth.
There is a large range of salinity variation at the surface compared to the depths because of all of the salinity-altering processes that occur at the surface rather than the depths. Processes like precipitation, formation of sea ice, evaporation, runoff, and sea ice melting have no effects on the deeper water, compared to the surface water.
29
New cards
Describe the pycnocline, including where it occurs in the ocean.
A pycnocline is a layer of rapidly changing density. It goes from about 300 meters to 1,000 meters below the surface, like how the halocline does.
30
New cards
If there is a net annual heat loss at high latitudes and a net annual heat gain at low latitudes, why does the temperature difference between these regions not increase?
The temperature difference doesn’t increase because atmospheric winds and ocean currents transfer heat from low latitudes to high latitudes. The heat gained and heat lost evens eachother out.
31
New cards
The Coriolis effect causes moving objects to curve to the right in the Northern Hemisphere and to the left in the Southern Hemisphere. Describe the underlying cause of the Coriolis Effect.
The underlying cause of the Coriolis Effect is the difference in speed of the Earth’s rotation at different latitudes. The object itself isn’t moving along a curve,but rather a straight path. Because of Earth’s rotation, however, the object seems to move along a curved path, i.e. the Coriolis Effect
32
New cards
Discuss the patterns and trends that you notice when looking at the global wind belts and boundaries shown in Figure 6.12 and Table 6.2
The most distinct pattern I see is that the northern hemisphere and the southern hemisphere are essentially mirrored images. The air masses, pressure, andwinds seem to be oscillating every 30 degrees or so.
33
New cards
Name the major wind belts in each hemisphere and identify the boundaries between these wind belts
The major wind belts in each hemisphere are the NE and SE trade winds, the prevailing westerlies, and the polar easterlies. Between the NE and SE trade winds is the Doldrums boundary. Around the 30 degree latitudes/ between the SE trade winds and the Prevailing Westerlies and between the NE trade winds and the Prevailing Westerlies are the Horse latitudes boundaries. The Polar Front boundaries are located between the Polar Easterlies and the Prevailing Westerlies of both hemispheres, so around 60 degrees. Lastly, the Polar high pressure boundaries are located at 90 degrees, between the Polar Easterlies.
34
New cards
Describe the difference between a sea breeze and a land breeze. What causes them to form? When do they occur?
a.) A sea breeze is when the rising air creates a low-pressure region over the land,pulling the cooler air over the ocean toward land during the afternoon
b.) A land breeze is when the land surface cools (at night) and creates a high-pressure region that causes the wind to blow from land
b.) A land breeze is when the land surface cools (at night) and creates a high-pressure region that causes the wind to blow from land
35
New cards
Describe the difference between sea ice, icebergs, and shelf ice, including how each is formed.
Sea ice is basically just frozen seawater. It starts out as small crystals that buildup creating a slush. This slush begins to form into a thin sheet, while wind stress and wave action breaks it down into what is called pancake ice, which eventually then forms into ice floes, or layers of ice. Icebergs are found at sea, but originate by breaking off from glaciers on land. They are formed by vast ice sheets onland. These sheets grow from snow pile-up and form outward towards the sea.Once the ice sheet reaches the sea, it either breaks and produces the iceberg
that way, or it floats on top of the water and breaks up from currents, wind, orwaves. Lastly, shelf ice is a thick floating sheet of ice that is formed at the edge of glaciers and breaks off to produce vast plate-like icebergs
that way, or it floats on top of the water and breaks up from currents, wind, orwaves. Lastly, shelf ice is a thick floating sheet of ice that is formed at the edge of glaciers and breaks off to produce vast plate-like icebergs
36
New cards
How many subtropical gyres exist worldwide? List each subtropical gyre and then list the main currents that exist within each subtropical gyre.
1. There are five subtropical gyres worldwide.
1. North Atlantic Subtropical Gyre
1. Currents: North Atlantic Current, Canary Current, North Equatorial Current, Gulf Stream
2. South Pacific Gyre
1. Currents: South Equatorial Current, East Australian Current, West Wind Drift, Peru Current
3. North Pacific Subtropical Gyre
1. Currents: North Pacific Current, California Current, North Equatorial Current, Kuroshio Current
4. South Atlantic Subtropical Gyre
1. Currents: South Equatorial Current, Brazil Current, West Wind Drift, Benguela Current
5. Indian Ocean Subtropical Gyre
1. Currents: South Equatorial Current, Agulhas Current, West Wind Drift, West Australian Current
37
New cards
Explain why the subtropical gyres in the Northern Hemisphere move in a clockwise fashion while the subpolar gyres rotate in a counterclockwise pattern
The subtropical gyres in the Northern Hemisphere move in a clockwise direction because of the anticyclonic flow of the high-pressure air cells. Same occurs for the subpolar gyres but instead the air cells are low-pressure and move in a cyclonic flow.
38
New cards
Explain why upwelling areas are associated with an abundance of marine life.
Upwelling areas are associated with an abundance of marine life because the upwellings bring high concentrations of nutrients. These nutrients allow for marine life to thrive, and an increase in productivity.
39
New cards
Describe the changes in atmospheric pressure, precipitation, winds, and ocean surface currents during the two monsoon seasons of the Indian Ocean.
1. During the Northeast Monsoon, there is rapidly cooling air which causes high atmospheric pressure. This high pressure causes dry air, which means little precipitation. The winds during this season flow from Southwest Asia off the land and over onto the ocean. These offshore winds cause the North Equatorial Current to flow from east to west, and the Somali Current flows south along Africa’s coast. The equatorial countercurrent is also established.
2. During the Southwest Monsoon, there is low atmospheric pressure which influences the heavy precipitation on land during this season. The winds reverse, blowing from the ocean onto the land. During this season, the North Equatorial Current gets replaced by the Southwest Monsoon Current, flowing in the opposite direction. The Somali Current also reverses and flows northward.
40
New cards
Describe the changes in atmospheric and oceanographic phenomena that occur during El Nino/La Nina events including changes in: atmospheric pressure, wind direction and intensity, walker circulation, weather, equatorial surface currents, coastal upwelling/downwelling, abundance of marine life, sea surface temperature, the pacific warm pool, sea surface elevation, and the position of the thermocline
1. El Nino
1. Atmospheric pressure
1. Reduced pressure along the coast of South America
2. Wind direction and intensity
1. Southeast trade winds diminish
2. When El Nino is really strong, the trade winds will blow in the opposite direction
3. Walker circulation
1. Reduced difference between the high and low pressure regions
1. Between the southeastern Pacific high-pressure cell and the Indonesian low-pressure cell is weakened
4. Weather
1. Difficult to predict
2. More storms
5. Equatorial surface currents
1. Current strengthens
6. Coastal upwelling/downwelling
1. No more upwelling
2. Downwelling can occur when the warm water is being pushed against the western coast of South America
7. Abundance of marine life
1. Decrease in productivity and marine life abundance
8. Sea surface temperature
1. Increase in sea surface temperature in Eastern Pacific waters
9. The Pacific Warm Pool
1. Equatorial water isn’t being pushed into the warm water near Indonesia, so instead it gets pushed eastward along the equator
1. Can reach the coasts of North and South america
10. Sea surface elevation
1. Usually rises
11. The position of the thermocline
1. Thermocline boundary between warm surface water and cool depth water flattens
2. La Nina
1. Atmospheric pressure
1. Larger pressure difference across the Pacific ocean
2. Wind direction and intensity
1. Strengthened trade winds
3. Walker circulation
1. Strengthens
4. Weather
1. Difficult to predict
5. Equatorial surface currents
1. Weakens?
6. Coastal upwelling/downwelling
1. Increased upwelling
7. Abundance of marine life
1. Increased nutrients → increased productivity → increased marine life
8. Sea surface temperature
1. Cooler than normal across the equatorial South Pacific
9. The Pacific Warm Pool
1. Shrinks to the west
10. Sea surface elevation
1. Sea levels drop
11. The position of the thermocline
1. Shallows out in the Eastern pacific
41
New cards
Discuss the origin of thermohaline vertical circulation. Why do deep currents form only at high latitudes?
These thermohaline vertical circulations start up in high latitude surface oceans and they happen when the cooler, denser surface water sinks. The salinity of this water increases with the formation of sea ice. The reason deep currents form only at high latitudes is because they are driven by the differences in density and temperatures, and the low-latitude water is too warm and never dense enough to sink I think.
42
New cards
Antarctic Intermediate Water can be identified throughout much of the South Atlantic based on its temperature, salinity, and dissolved oxygen content. Why is it colder and less salty - and why does it contain more oxygen - than the surface water mass above it and the North Atlantic Deep Water below it?
This water is colder because of the formation at the Antarctic Convergence. It is less salty because the water that is being sunk is at a higher latitude. There is more oxygen in this water because it’s colder water than that of the North Atlantic Deep Water and the surface water mass.
43
New cards
Geometry of Water
hydrogen atoms separated by 105°
covalent bonds
covalent bonds
44
New cards
Water polarity and what gives it this polarity?
dipole
the geometry
the geometry
45
New cards
Hydrogen bonds contribute to..
Cohesion
High surface tension
Water’s thermal properties
Water’s density
High surface tension
Water’s thermal properties
Water’s density
46
New cards
How is water the universal solvent?
Water molecules stick to other polar chemical compounds, which reduces attraction between ions of opposite charges
\
Electrostatic attraction between oppositely charged ions
* Reduced by water
* Causes Na+ and Cl to separate
* hydration
\
Electrostatic attraction between oppositely charged ions
* Reduced by water
* Causes Na+ and Cl to separate
* hydration
47
New cards
water has ___ melting and boiling points because why?
high
additional heat energy needed to overcome hydrogen bonds and van der Waals forces
additional heat energy needed to overcome hydrogen bonds and van der Waals forces
48
New cards
Heat Capacity
amount of heat required to raise the temperature of 1 gram of a substance 1°C
49
New cards
Water has a ___ heat capacity because why
high
can take in or lose heat without changing temperature
can take in or lose heat without changing temperature
50
New cards
specific heat
– heat capacity per unit mass
51
New cards
latent heat
hidden heat
52
New cards
water has ___ latent heat
high
53
New cards
when water undergoes a change of state, what happens to the heat?
large amounts of heat absorbed or released
54
New cards
latent heat of melting
E needed to break intermolecular bonds that hold water molecules in place in ice crystals
55
New cards
latent heat of vaporization
* E added at boiling point to break intermolecular bonds
* Change state from liquid to vapor
* Break all hydrogen bonds
* Change state from liquid to vapor
* Break all hydrogen bonds
56
New cards
Latent heat of evaporation
Evaporation: conversion of liquid to gas below the boiling point
57
New cards
Latent Heat of Condensation
Water vapor cools, condenses, releases heat to surrounding air
58
New cards
Latent Heat of Freezing
Heat released when water freezes
59
New cards
precipitation and evaporation latitudes
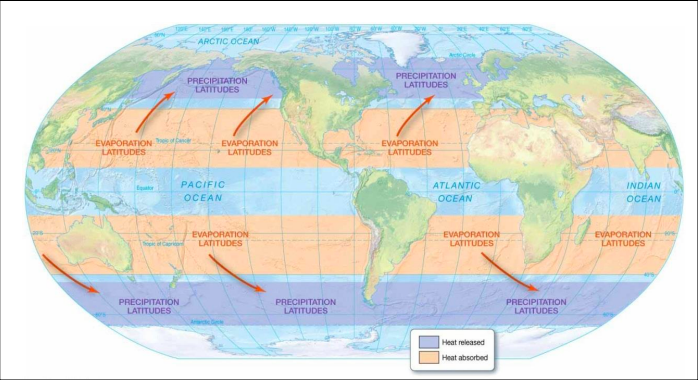
60
New cards
Global thermostatic marine effect
Oceans moderate temperature changes from day to night and during different seasons
61
New cards
Global thermostatic continental effect
Land areas have greater range of temperatures from day to night and during different seasons
62
New cards
Global thermostatic effects
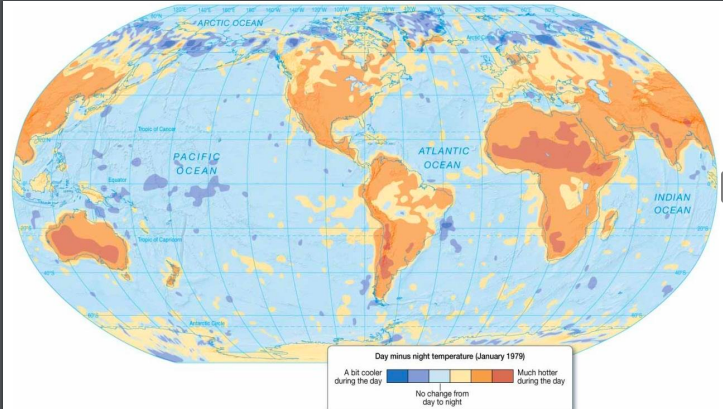
63
New cards
Water density
* Generally the density of water increases as temperature decreases
* From 4°C to 0°C the density of water increases.
* Unique property of water
* Ice is less dense than liquid water.
* Changes in molecular packing
* Water expands \~9% in volume as it freezes
* From 4°C to 0°C the density of water increases.
* Unique property of water
* Ice is less dense than liquid water.
* Changes in molecular packing
* Water expands \~9% in volume as it freezes
64
New cards
Density
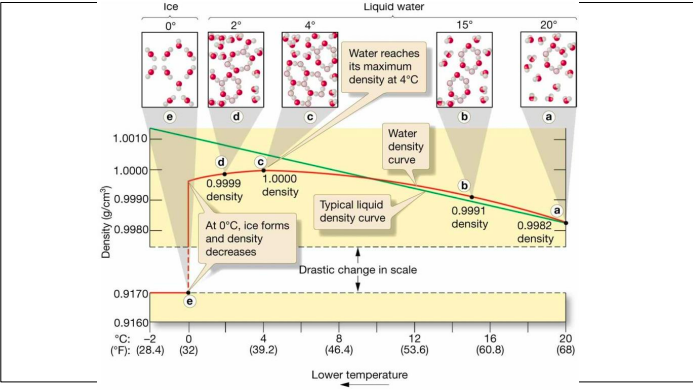
65
New cards
Increased pressure or adding dissolves substances ____ the max density temp
decreases
66
New cards
Dissolved solids also reduce the ____-
freezing point of water
\
most seawater never freezes
\
most seawater never freezes
67
New cards
Salinity
Total amount of solid material dissolved in water
Includes dissolved gases, Excludes organic substances
Includes dissolved gases, Excludes organic substances
68
New cards
Typical Ocean water salinity
35 parts per thousand
69
New cards
How is salinity found and how is it expressed
Ratio of mass of dissolved substances to mass of water sample
Expressed in ppt (parts per thousand) ‰
Expressed in ppt (parts per thousand) ‰
70
New cards
Components on salinity in water
Chloride, sodium, sulfate, magnesium, calcium, potassium, other
71
New cards
Is salinity constant?
no
72
New cards
Hypersaline
evaporation dominated
73
New cards
Brackish
Influx of fresh water from runoff, precipitation
74
New cards
What causes differences in salinity?
Seasonal variations → flux of freshwater, dry/humid
75
New cards
What processes decrease salinity?
runoff, melting sea ice, melting icebergs and precipitation
76
New cards
What processes increase salinity?
sea ice formation, evaporation
77
New cards
Comparison of major dissolved components in streams with those in seawater
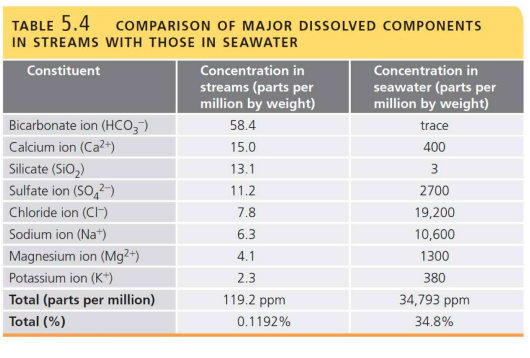
78
New cards
Residence time
Average length of time a substance remains dissolved in seawater
79
New cards
Ions with long residence times are ____
in high concentrations in seawater
• (Na+ 260 million year)
• (Na+ 260 million year)
80
New cards
Ions with short residence times are ___
in low concentration in seawater
81
New cards
Steady-state
Average amount of element of interest remains constant
\
Oceans haven’t increased in salinity over time (rate at which an element is added = rate at which it is removed)
\
Oceans haven’t increased in salinity over time (rate at which an element is added = rate at which it is removed)
82
New cards
Why has open ocean maintained a constant composition?
rapid mixing relative to long residence times
83
New cards
Ions with long residence times __ and those with short __
accumulate; removed
84
New cards
Conservative constituents of seawater occur in __ proportions because they have a __
constant; long RT
85
New cards
Conservative constituent examples
Na, K, Mg, Ca, Cl, sulfate, borate
86
New cards
nonconservative constituents have a __ residence time and are associated with _ cycles
short; seasonal, biological or short geological
87
New cards
nonconservative constituents examples
dissolved nitrate and phosphate
88
New cards
RT of seawater constituents
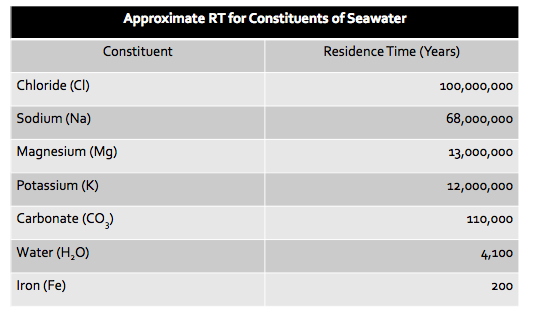
89
New cards
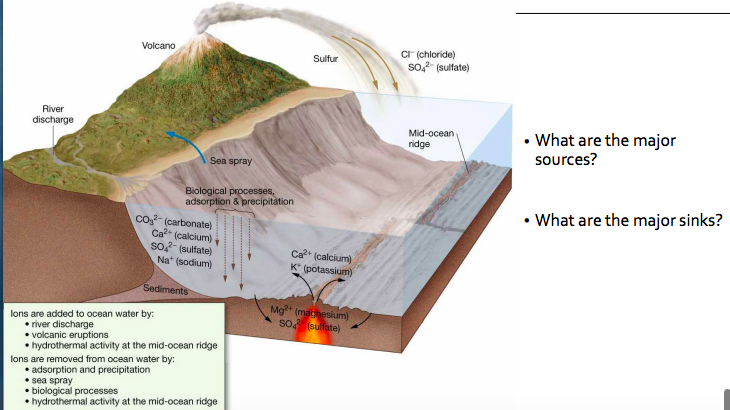
90
New cards
High latitudes are associated with __ salinity
low
91
New cards
equatorial region is associated with __ salinity
low
92
New cards
mid latitudes are associated with __ salinity
high
93
New cards
salinity and temp vs latitudes
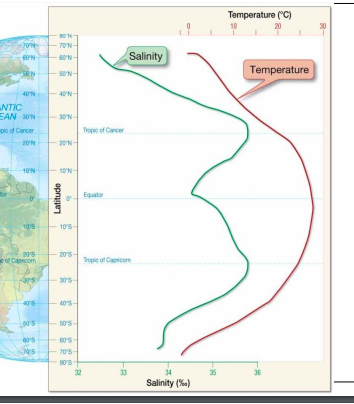
94
New cards
halocline
layer of rapidly changing salinity with depth
\~300-1000 meters below surface
\
* the deep ocean is relatively constant
\~300-1000 meters below surface
\
* the deep ocean is relatively constant
95
New cards
pH scale
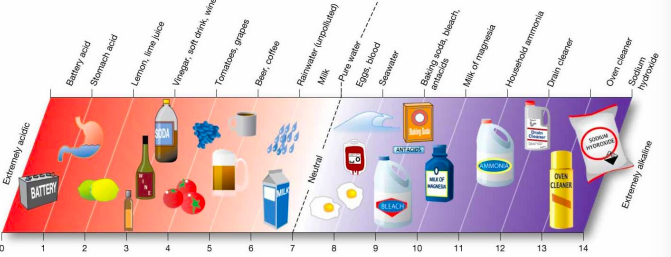
96
New cards
Ocean pH
slightly alkaline - surface water avg. is 8.1
ocean water pH dec with depth
ocean water pH dec with depth
97
New cards
pH, depth, and CCD
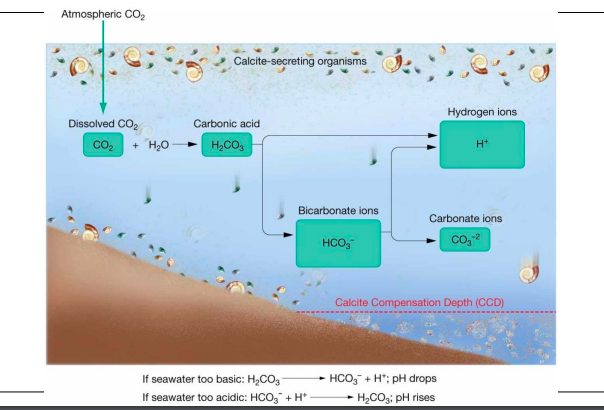
98
New cards
Freshwater density
1\.000 g/cm3
99
New cards
ocean surface water density
1\.022 to 1.030 g/cm3
100
New cards
density differences impacts
creates deep-ocean currents, ocean mixing