C.Bio - Cell Signalling
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
What causes neutrophils to be able to track and 'chase' moving bacteria?
The bacteria release chemoattractant signals, which attract the neutrophil to chase them. To achieve this neutrophil needs to have receptors for this chemoattractant (ligand)
( A similar phenomenon occurs when amoeba are attracted to the chemoattractant, cAMP)
( Another example: yeast forming shmoos in response to alpha mating factor)
How are cells instructed?
Through a combination of extracellular signals.
The pure survival of the cells requires signaling (growth factors need to be present, and the cell requires multiple growth factor receptors to keep it alive)
To grow and divide, the cell needs signals (Exogenous signals that trigger growth and division)
Differentiation requires signaling
Absent of all these factors, the cell will die.
Even cell death can require signals; in some cases, though, the cell death is programmed (apoptosis) (ex: T cells releasing signals that trigger cancer cells to die)
metabolic, changes, secretion, cell movement, etc. also require extracellular signals
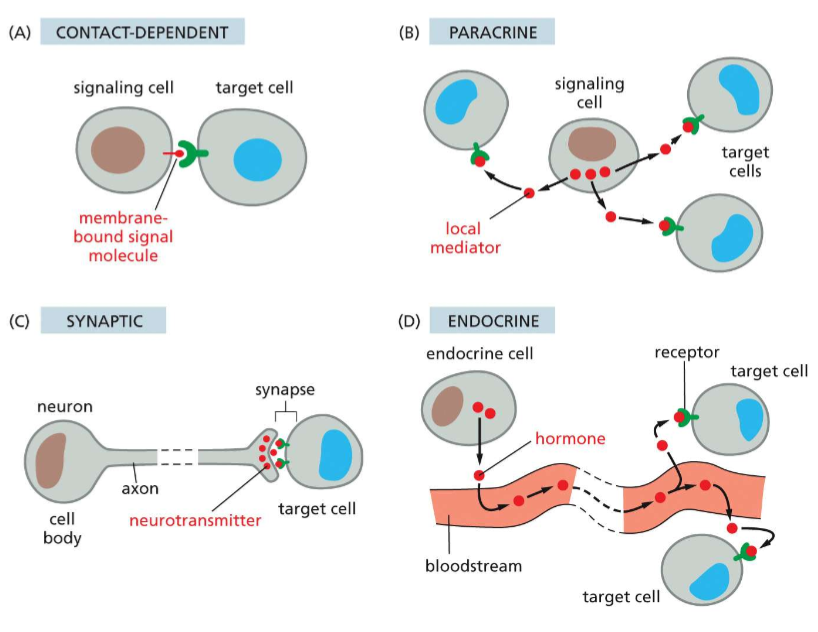
What are the four types of signaling/ signal transfer?
A) Contact-dependent signaling
ver short distances to the target cells. The signaling molecules survive only in the microenvironment in which they are released
C) Synaptic signaling--signals are transmitted across a synapse. (ex: a neuron sends an action potential through the axon. This releases neurotransmitters that bind to receptors on target cells, like muscles or another neuron)
D) Endocrine signaling-- signaling molecules secreted over long distances (like hormones)cell-cell contact is the initiator cell signaling process ( some ligands can even be transmembrane proteins) (used in epithelial cells)
B) Paracrine signaling-- signaling molecules/ligands are released by the signaling cell and travel locally o
In the statement, "when things change, cells respond," what can things refer to? What can respond refer to?
Things (ligands): nutrients, growth factors, force, light (photons), smell, other cells (cell surface ligands), extracellular matrix (when there is plenty of ECM, the cell grows and reproduces; if there is a lack of ECM, the cell goes into apoptosis)
Respond (effectors carry out response): growth, division, proliferation, differentiation, mating, metabolism change, movement, death
At the most simplistic basis, what are the 4 main molecules carrying out the work of cell signaling?
1) Ligand
2) Receptors
2) Signaling through receptors and intracellular signaling molecules (second messengers, kinases, GTPases, etc)
3) Effectors (transcription factors, cytoskeletons, metabolic enzymes, trafficking machinery, etc)
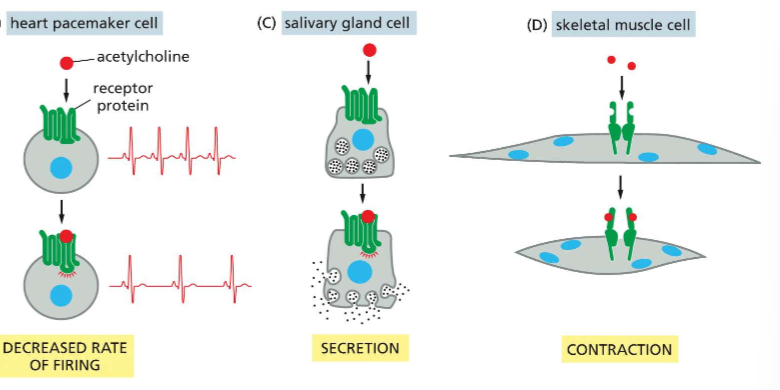
What occurs when different cell types encounter the same extracellular signal?
The extracellular signaling molecule elicits different responses in different cell types
Ex: Ligand = acetycholine
In heart pacemaker cells = decreased firing rate
In salivary gland cells = secretion
In skeletal muscle cells = contraction
Types of extracellular signaling molecules
proteins, peptides, amino acid, nucleotides, hormones, smell, taste, force, light
Types of receptors
transmembrane or cytoplasmic
(Most receptors are transmembrane; however cytoplasmic receptors can exist IF the ligand can pass through the plasma membrane; aka is nonpolar like steroids)
Describe the stages/ key molecules in cell signaling
1) Extracellular signaling molecule
2) Receptor Protein
3) Intracellular signaling molecules
4) Effector proteins
5) Cell physiological response
Types of intracellular signaling molecules
Large: Enzymes ( kinases, phosphatases, GTPases, etc.)
Small: Second messengers ( cAMP, cGMP, Ca2+, diacylglycerol, etc.)
Types of effector proteins
Transcription factors, cytoskeletons, metabolic enzymes, vesicular trafficking machinery (ex: insulin, which is also a growth factor, stimulates exocytosis of glucose transporters onto the plasma membrane)
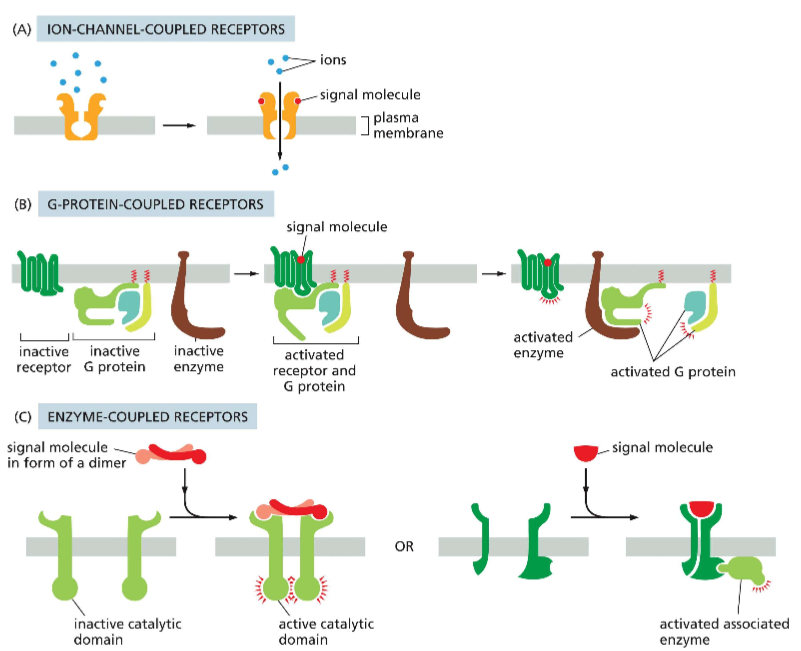
What are the 3 major classes of cell-surface receptor proteins
A) Ion-channel coupled receptors: an ion channel has regulatory domains that act as a receptor for an ion. This controls the opening or closing channels (ex: the role glutamate in sending action potentials)
B) G-protein coupled receptors (GPCR): have 7 transmembrane domains. When they are bound to a ligand, they form an active tetrameric protein that causes a series of downstream events
C) enzyme-coupled receptors: the receptors are typically also kinases. When the ligand is bound, the receptors form a dimer and have active catalytic properties, which cause them to phosphorylate each other, causing downstream events
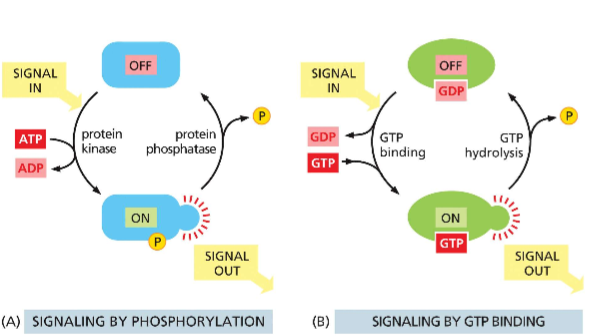
How do phosphorylation and GTP binding mediate cell signaling
for small GTPases
transition from GTP-bound (active) to GDP-bound (inactive) is done by hydrolysis (done by GAP)
transition from GDP-bound (inactive) to GTP-bound (active) is done by GDP (exchange via GEF)These different states have conformational differences which define function
Which 3 amino acids can act to phosphorylate within kinases?
Serine, Threonine and Tyrosine
they all contain a hyrdroxyl group-receptor for the GAMMA phosphate group
Results in conformational changes
What are small GTPases?
Enzymes that bind to and hydrolyze GTP to GDP ( have an intrinsic way of turning themself off)
90% of pancreatic cancers have ___
An active RAS mutation that causes RAS GTPase to constitutively active/on
What is the difference between the confirmation of RAS GTPases in the GTP-bound vs GDP-bound form?
In the GTP-bound (active) form, the Switch I ( Thr-35 containing) and Switch II (Gly-60) regions of the protein interact with the gamma phosphate group, causing the protein to close. In the GDP-bound state, there is no gamma phosphate interaction, and the protein is open (inactive).
THESE STRUCTURAL CHANGES ALTER THE PROTEIN'S AFFINITY FOR BINDING PARTNERS
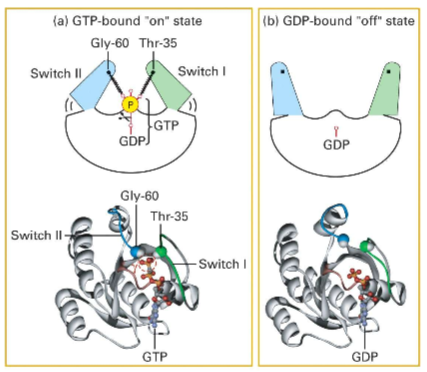
What is the of the P-loop?
The P-loop is located between the first beta strand and the first alpha helix. It interacts with the phosphates (phosphoryl groups) on GTP, especially the beta and gamma phosphates. The phosphates essentially hold the loop
What are the functions/ general roles of GTPases?
They serve as a molecular "switch" for the activity of their downstream effectors and a molecular "clock" (timing when activation occurs, duration, and resetting )
ALL OF THESE ROLES HAVE PHYSIOLOGICAL CONSEQUENCES
GEF (Guanine Exchange Factor) is an ____ for an inactive GTPase
effector
The proteins that active monomeric GTPases recruit with when active are called their ________
downstream effectors
(GAP is also another binding partner)
What is the role of GAP?
To promote GTP hydrolysis ability of the GTPase/ catalyzes the reaction. This allows the protein to be converted from active to inactive
How can ATP binding serve as a molecular switch in muscle cells?
Muscle contraction is mediated by ATP binding, changing to conformation of myosin and actin in muscle cells
Protein____ and _____ binding can serve as molecular "switches" in cells
phosphorylation
nucleotide
Both events cause CONFORMATIONAL CHANGES
The __ terminus of the G-protein coupled receptor exists in the extracellular space. The ___ terminus is in the cytoplasm.
N ; C
Describe the process of GPCR signaling
1) When no ligand is present the GPCR receptor is inactive and exists separately from the membrane-associated trimeric G protein complex (Alpha, beta, gamma)
2) Upon signal molecule binding there is a conformational change in GCPR in the cytoplasmic domain. GPCR begins to interact/bind with the alpha domain of the trimeric G-complex
3) G- alpha and G-gamma+ G-beta begin to separate, stimulating responses in different downstream effectors (ex: enzymes)
What is the difference in trimeric G-protein confirmation when GPCR is ligand bound vs. not?
When not ligand, the alpha helix domain of the trimeric G protein ( which lies in the RAS domain (G-alpha) is GDP-bound. This makes the trimeric G-protein inactive.
On GPCR and G-alpha interaction, GDP is unloaded and exchanged for GTP, causing the dissociation of the G-alpha-activated subunit from the G-beta-gamma-activated subunit. This allows both subunits to activate downstream effectors!
GPCR serves as a ___ for G proteins
GEF
By binding to G-alpha, GPCR activates the release of GDP and diffusion of GTP into the alpha helix (GTP loading) binding pocket of G-alpha
Why is GCPR able to control so many biological processes?
There are many different G proteins, each with different downstream effectors. This allows a variety of GPCRs to control a variety of biological processes
Adenylyl Cyclase is activated by ____
binding to an activated G-alpha subunit ( adenylyl cyclase is one of the G protein's downstream effectors). It experiences a CONFORMATIONAL CHANGE instructed by G-alpha = activates
What is the function of adenylyl cyclase?
To hydrolyzes ATP into cAMP. cAMP acts as a second messenger. Playing an important regulatory role in different types of cells
How is cAMP deactivated?
Using cyclic AMP phosphodiesterase
How does cAMP concentration change in response to signals?
It rapidly increases!
What molecule mediates the downstream effects of cAMP?
PKA (cAMP-Dependent Protein Kinase). By binding to and activating PKA, cAMP controls kinase activation, regulating downstream events. Activation of PKA results in the phosphorylation of downstream effectors.
Describe the process of PKA activation
1) In it's inactive state, PKA has two regulatory subunits and two inactive catalytic subunits. Regulatory subunits inhibit the catalytic activity of PKA
2) When 4 cAMP binds to the regulatory subunit of cAMP, a conformation change occurs in the regulatory subunit.
3) The affinity of the regulatory subunits for the catalytic subunits decreases, resulting in a binding partner switch. The catalytic subunits are released and active!
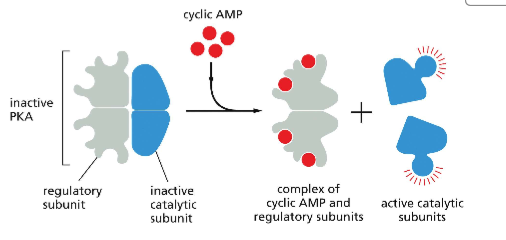
Why is it advantageous to the cell to need for cAMP for activation of 1 PKA?
It allows for greater regulation of cell signaling. This stipulation results in the cell needing a high enough concentration of cAMP to activate PKA. This prevents incidental activation of signaling pathways due to arbitrary biological 'noise'
sometimes adenylyl cyclase activation can happen without GPCR and can 'leak' through
Describe PKA interaction with CREB (one of the many PKA substrates)
CREB ( cAMP-response element binding protein) is a transcriptional regulator. When it is phosphorylated by PKA, it will be activated. Activated CREB binds to the cyclic AMP response element (CRE), a transcriptional regulatory element, and CREB-binding protein (CBP) is recruited. This results in gene transcription.
Describe the structure of phosphatidylinositols
They are signaling lipids that exist on the inner leaflet of the plasma membrane. It has two lipids chains which are inserted directly into the plasma membrane, a carbon backbone, phosphate and a ring structure with an -OH on every position.
The first position is linked to a phosphate, which links the ring to the lipid tails; the other positions can have a phosphate or a combination of phosphates, allowing for a multitude of molecules to be derived from the same backbone.
This allows the PIP to serve as landmarks, recuiting different molecules based on phosphorylation pattern
Which PIP is most enriched in the plasma membrane?
PI(4,5)P2
Which PIP is most enriched in the Golgi?
PI(4)P
Which PIP are enriched in the endomsome membrane?
PI(3)P, PI(5)P and PI(3,5)P2
What are the roles of PI and PIP kinases? What specificity is integrated into this process?
PI kinases phosphorylate PI (phosphatidylinositol). There are different PI kinases for the phosphorylation of different positions on the ring. Ex: to phosphorylate the 4 position, the kinase necessary is PI4 kinase.
PIP kinase phosphorylation PI with a P already at one of the ring positions
What enzyme facilitates the reversal of phosphorylation?
Phosphatases
What is the role of phospholipase?
It cuts the inositol group from the lipid. This results in a diacylglycerol ( lipid backbone + OH group) and modified inositol (ex IP3)
How are phospholipases activated?
Phospholipases can be activated as a result of G-protein activation. They are downstream effectors of some G-proteins.
Describe the G-protein signal transduction pathway involving PI(4,5)P2 and and phospholipase
1) A signaling molecule activates GPCR
2) The activated G protein activates phospholipase C-beta
3) Phospholipase cleaves the lipid-phosphate bond on PI(4,5)P2 , separating it into diacylglycerol and inositol 1,4,5-triphosphate ( IP3)
4) IP3 opens the IP3 receptor on IP3-gated Ca2+ release channel, allowing Ca2+ to leave from where it is stored in the lumen of the ER; diffuses from high to low concentration
5) Diacylglycerol recruits protein kinase C; active PKC complex is formed when Ca2+ binds to diacylglycerol + PKC active PKC complex is formed when Ca2+ binds to diacylglycerol + PKC
Why does Ca2+ have to be tight controlled in the cell?
Ca2+ serves as a cofactor for alot of proteins ( ex: proteases). This tight regulation of cell calcium results in Ca2+ concentration being low within the cytoplasm and higher outside the cell. Activation of a Ca2+ pump (ATPase that pumps Ca2+ against its gradient) is used to turn off Ca2+-activated protein activity
Describe the signal transduction pathway that leads to smell
Odor--> Activated Olfactory GPCR ( concentrated into modified cilia) --> activated G-proteins (G olf) --> activates adenylyl cyclase --> produces cAMP--> opens cAMP-gated Ca2+ channels--> neuron depolarization--> action potential to the brain
Describe the signal transduction pathway for sight
On the plasma membrane of the retina, there is a GPCR called rhodopsin. When light (ligand = photon) hits this GPCR, it causes a conformational change in the GPCR. This stimulates the activity of transducin (G-protein), followed by activation of phosphodiesterase and cGMP hydrolysis, which closes cGMP-gated Na2+/Ca2+ channels
TRANSDUCTION FROM PHOTO ENERGY, TO CHEMICAL, TO ELECTRICAL
What does it mean to desensitize a signal transduction pathway, and is it achieved?
Desensitization is the turning off signal transduction, even when the ligand is still present. One way this is done is by getting rid of the cell surface GPCR via endocytosis: following this, the receptor can either be recycled or degraded in the lysosome
Describe the role of GRK in GPCR Desensitization
When GPCR is activated, it stimulates the GRK (GPCR Kinase) to phosphorylate the cytoplasmic tail/ intracellular loop of the GPCR. This recruits arrestin to the phosphorylated GPCR, activating GPCR endocytosis and degradation
The GAP for G-proteins is called ____
RGS (Regulator of G-protein signaling). This phosphorylates the GTP held in the alpha subunit to GDP, resulting in reformation of the trimeric G protein (where alpha, beta and gamma are together)
In cancer cells it is often found that ___ receptors are overactive
EGF; Epidermal growth factor stimulates cell survival, growth, proliferation, or differentiation of various cell types. When overactive it results in excessive cell growth.
Any stage in this pathway being constitutively active =
cancer cell ( ex: a point mutation that inhibits GTPase activity would lead to Ras being constitutively active)
What are the receptors for enzyme-coupled signal transduction called? What do their intracellular and extracellular domains look like?
RTKs. Intracellular domains are long with plenty of space for phosphorylation. Extra cellular domains ready for dimerizing ligands