Organic Chemistry AS Level chemistry
1/211
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
212 Terms
water impact
global warming
Molecular formula
: The formula which shows the actual number of each type of atom
empirical formula
shows the simplest whole number ratio of atoms of each element in the compound
algebraic formula for a homologous series e.g. CnH2n
General formula
Displayed formula
show all the covalent bonds and atoms present in a molecule
Skeletal formula
A simplified organic formula, with hydrogen atoms removed from alkyl chains, leaving just a carbon skeleton and associated functional groups.
formula that shows the arrangement of atoms in the molecule of a compound.
structural formula
Homologous series
Homologous series are families of organic compounds with the same functional
group and same general formula.
•They show a gradual change in physical properties (e.g. boiling point).
• Each member differs by CH2 from the last.
• same chemical properties.
alkane
CH3CH2CH2CH3
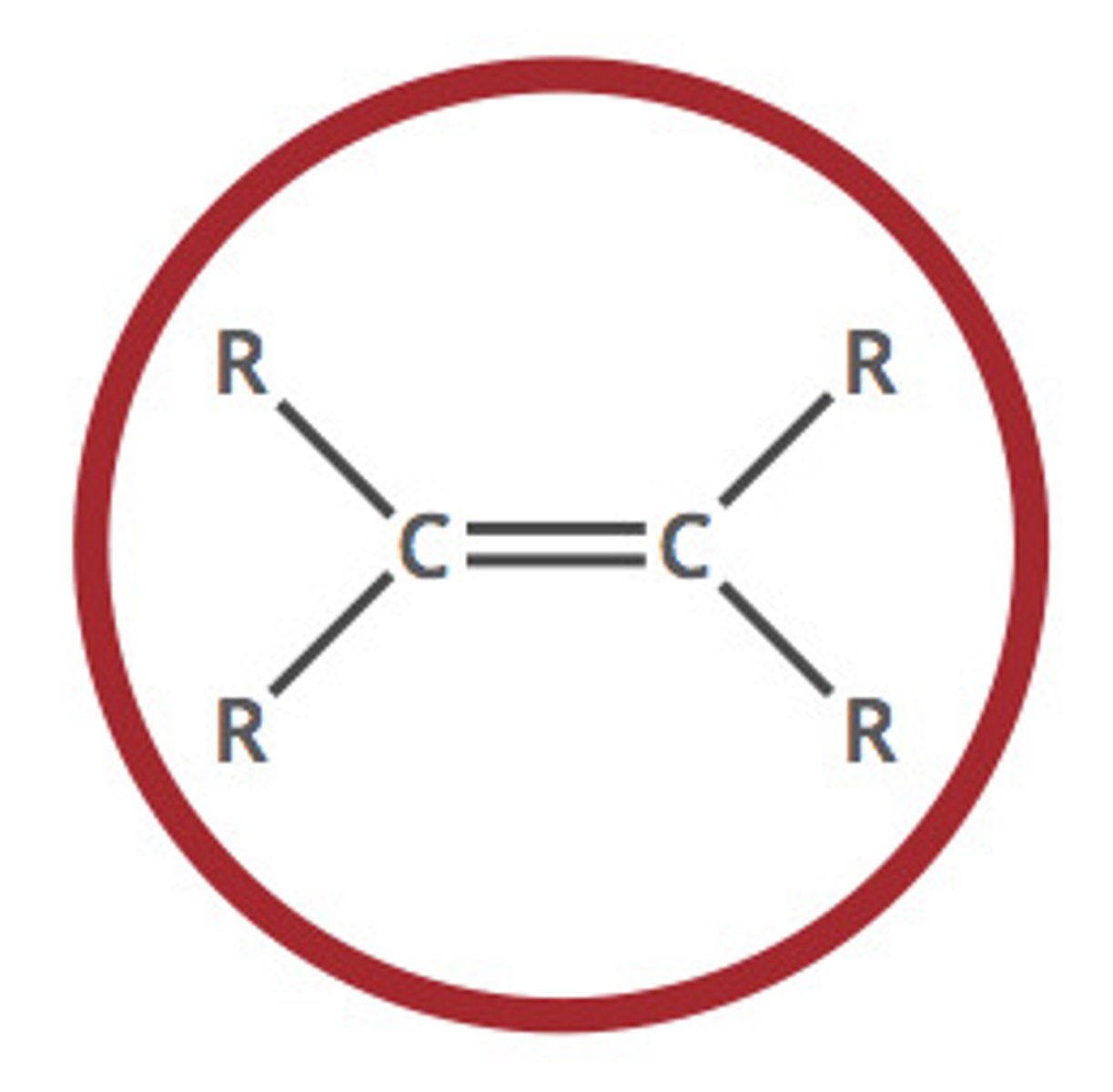
Alkenes
Hydrocarbons with one or more carbon-carbon double bonds
branched alkanes
Alkyl- (-yl)
Halogenoalkane
chloro-
bromo-
iodo-
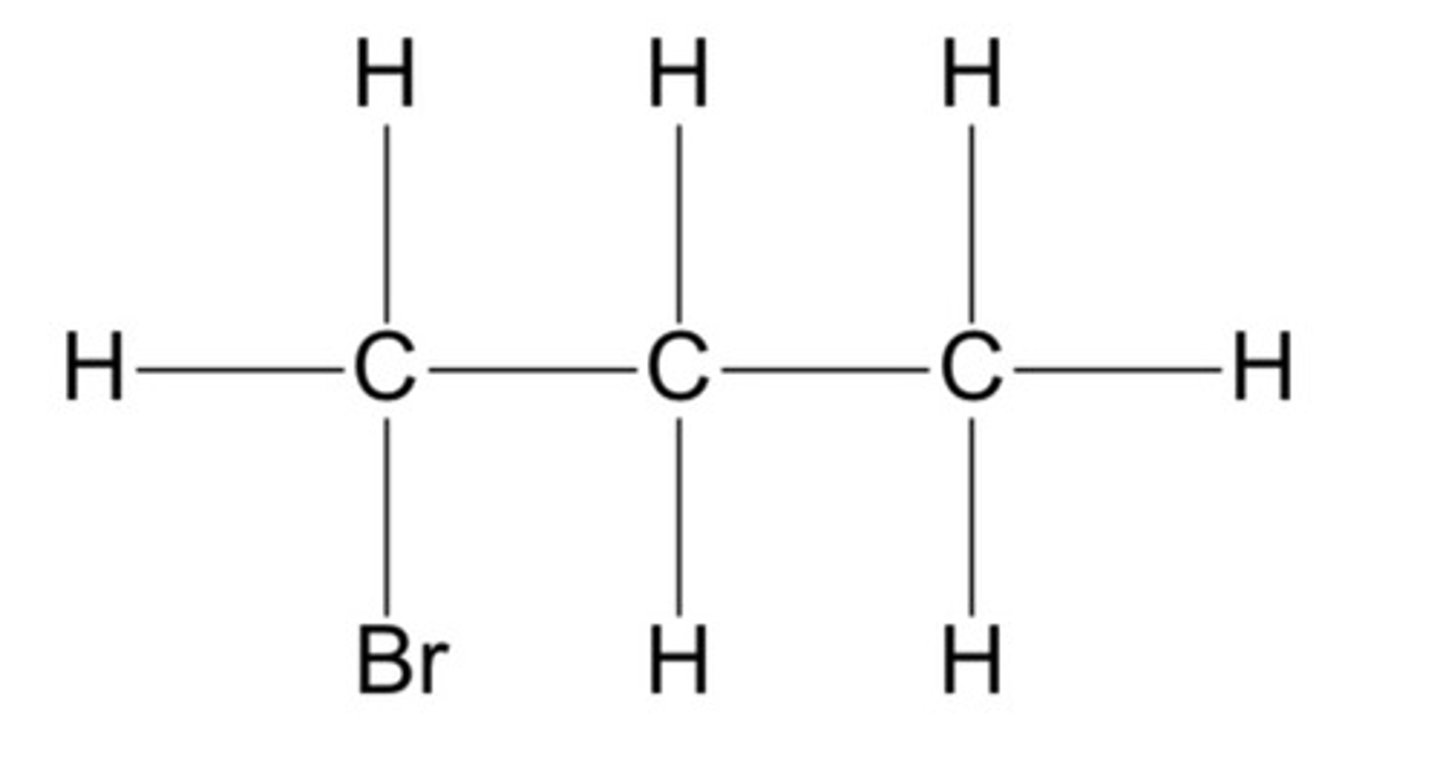
Aldehydes
suffix -al
prefix formyl-
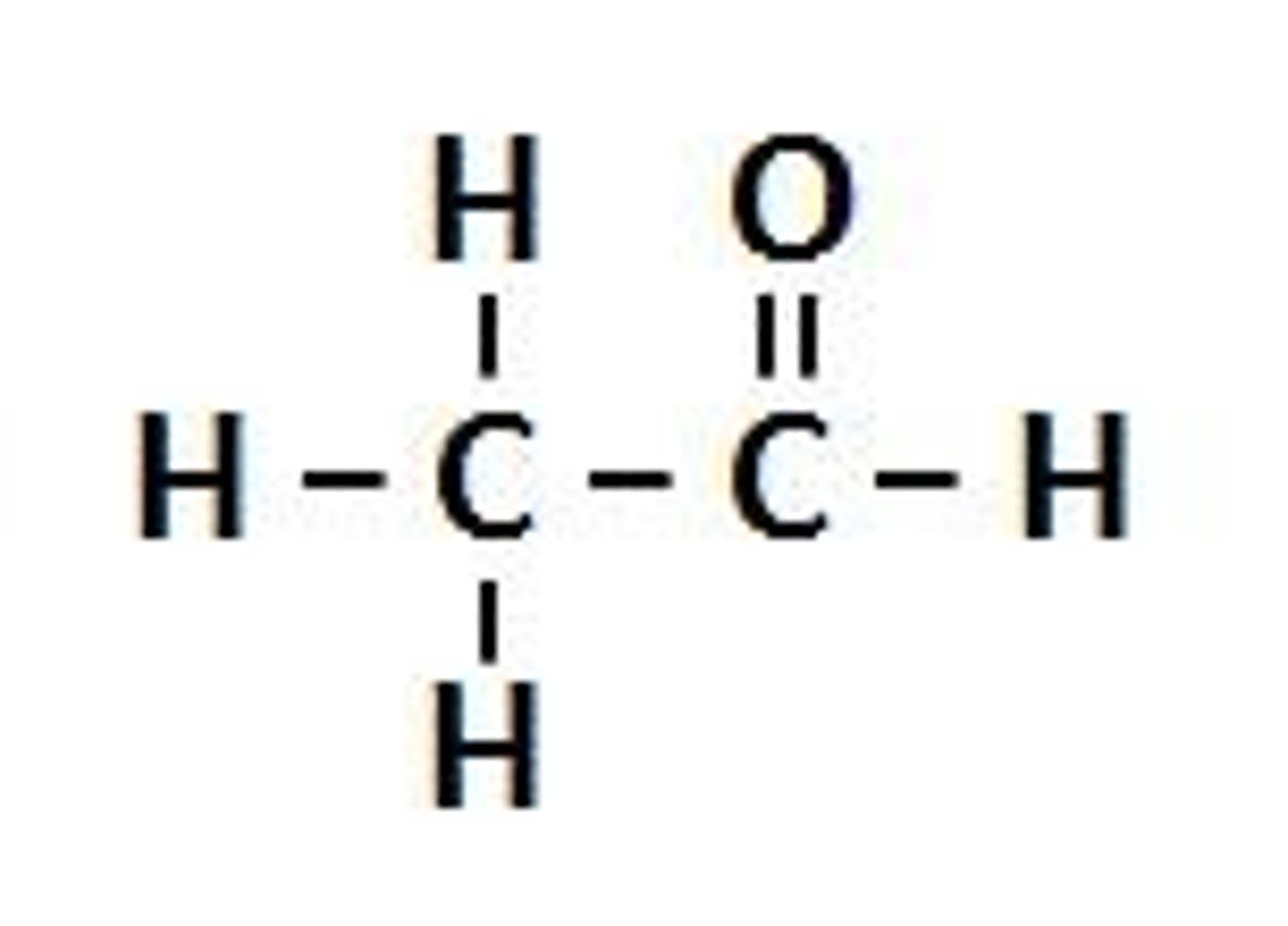
Ketones
suffix* -one
prefix oxo-
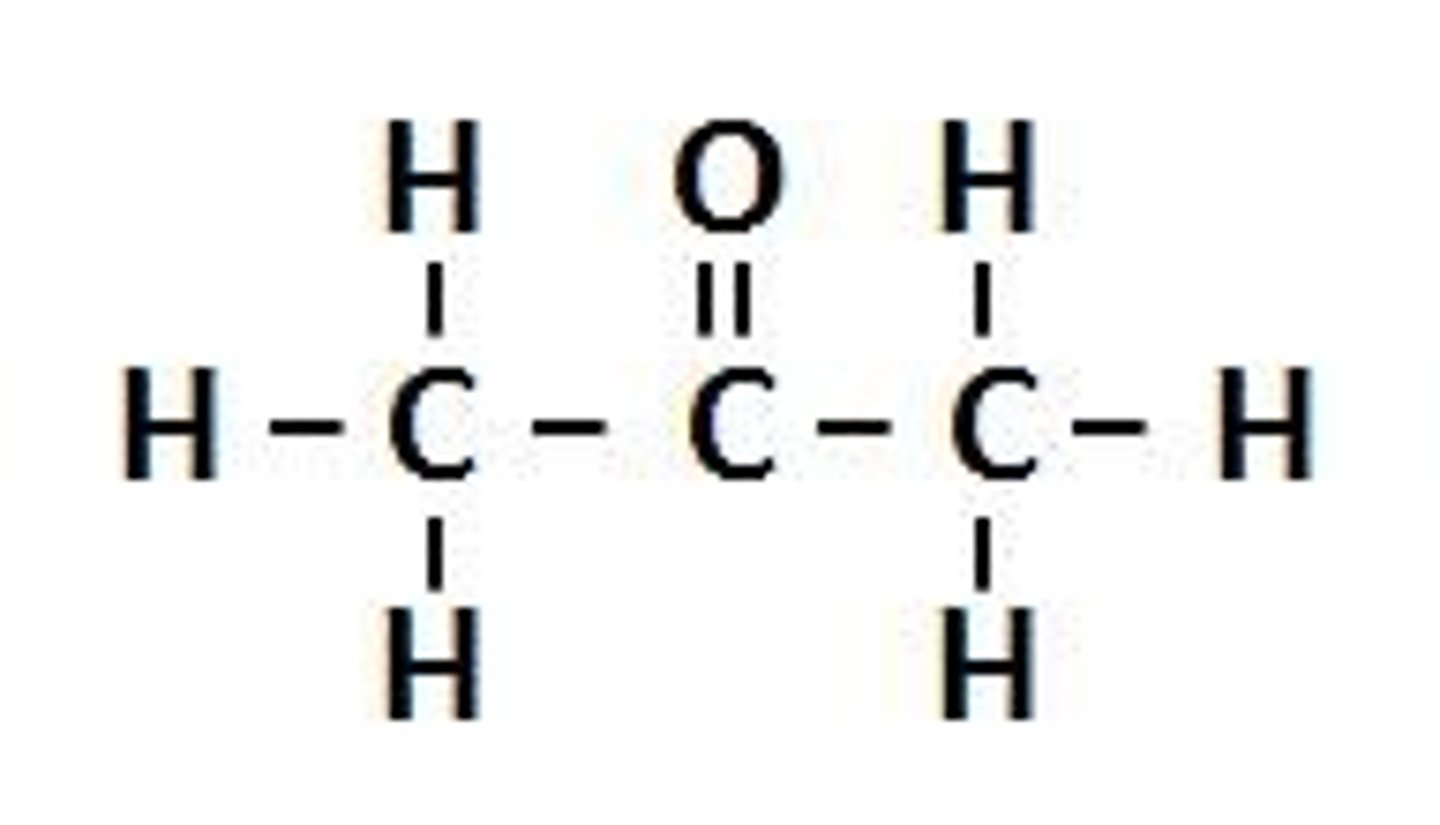
Carboxylic acids
suffix* -oic acid
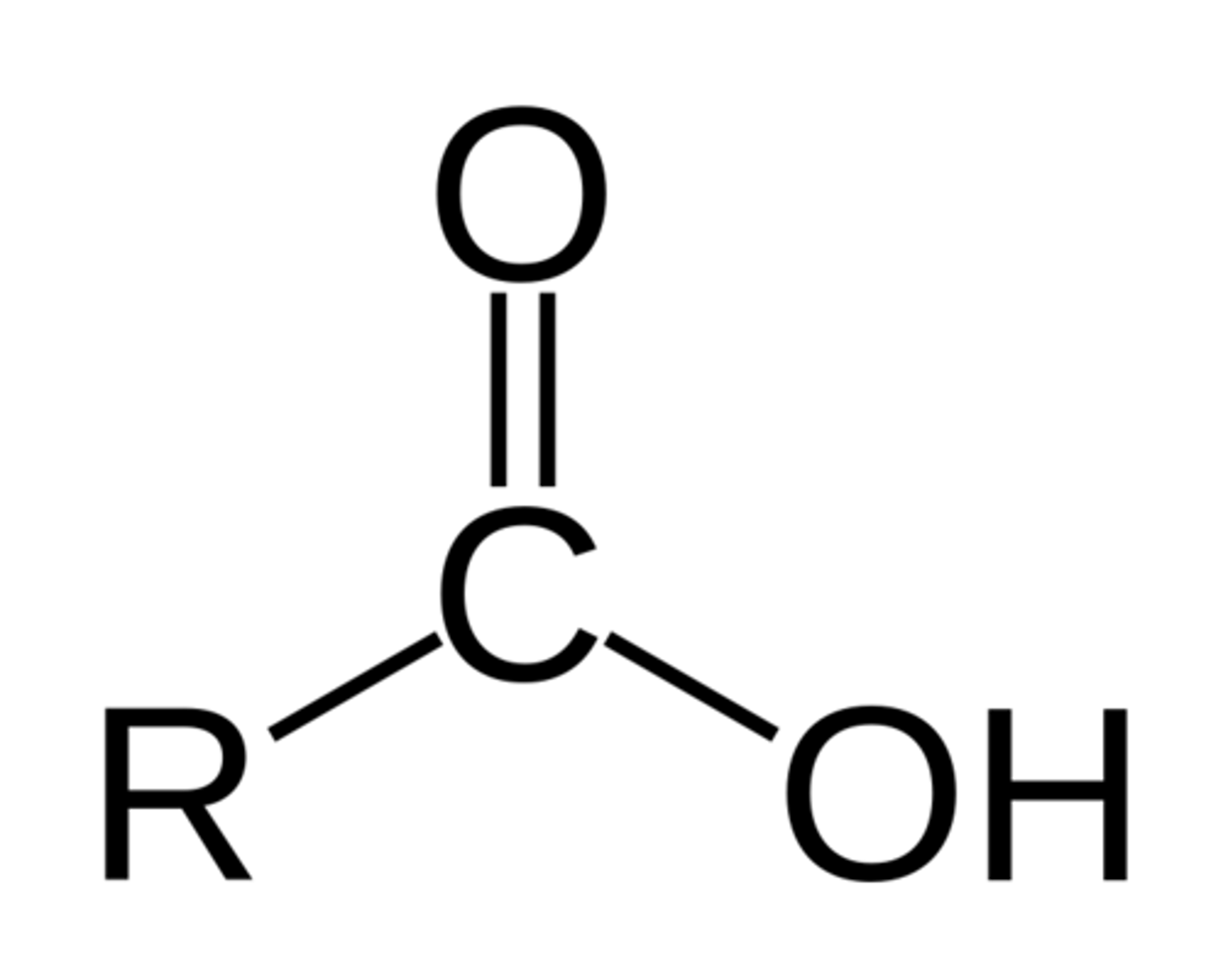
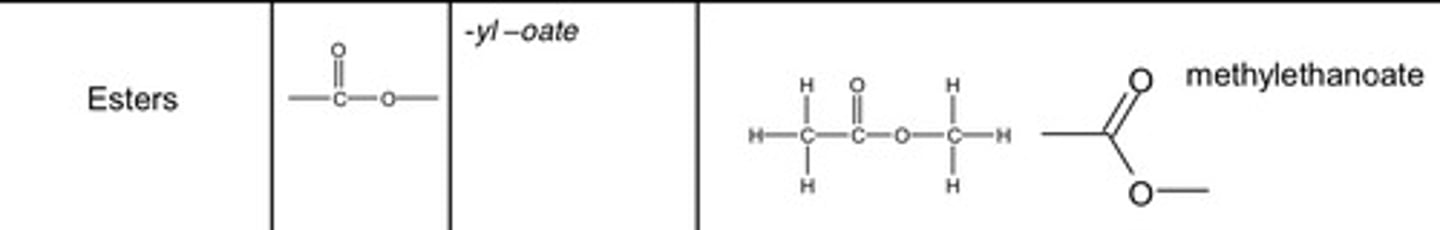
Esters
-yl-oate
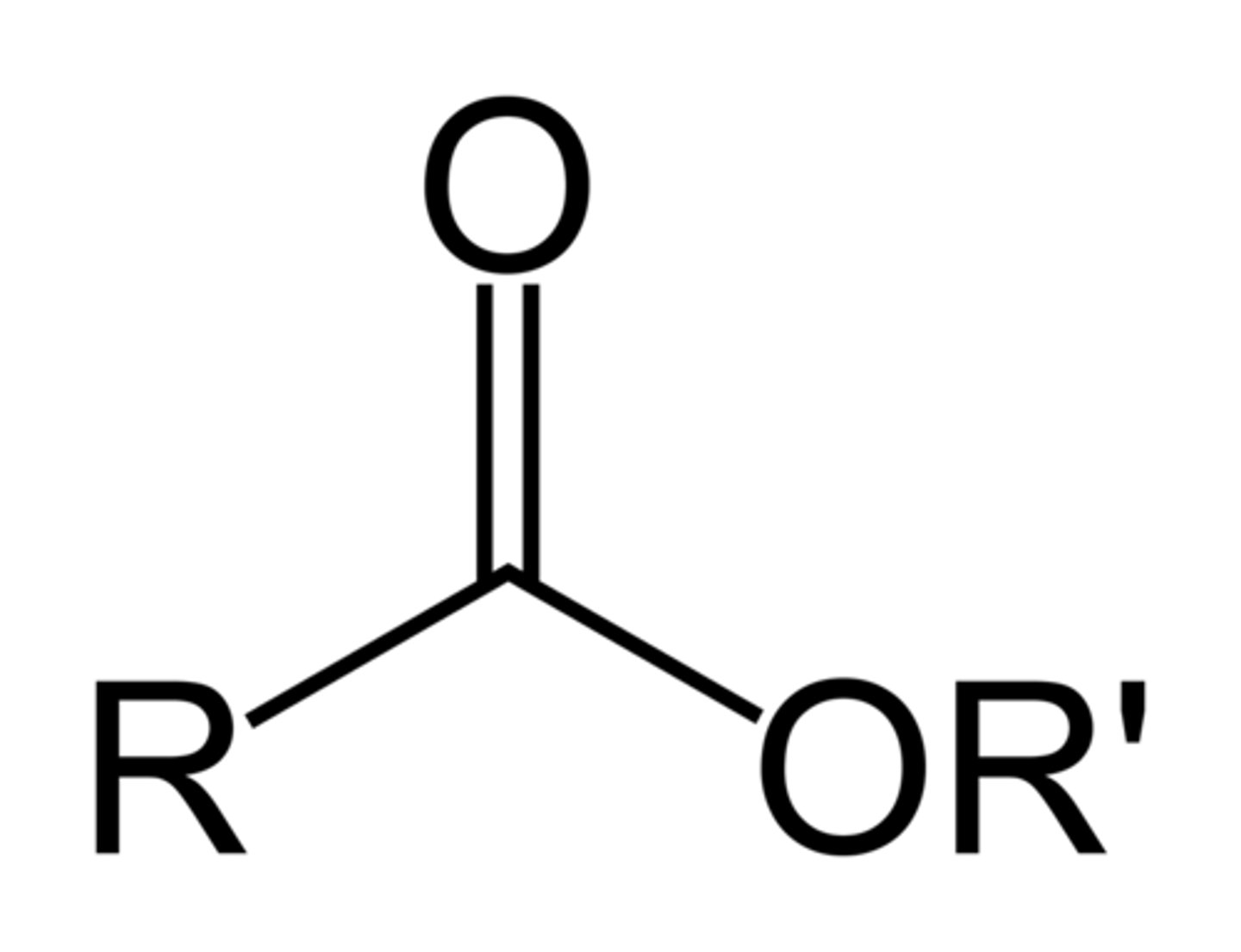
Alcohols
OH-
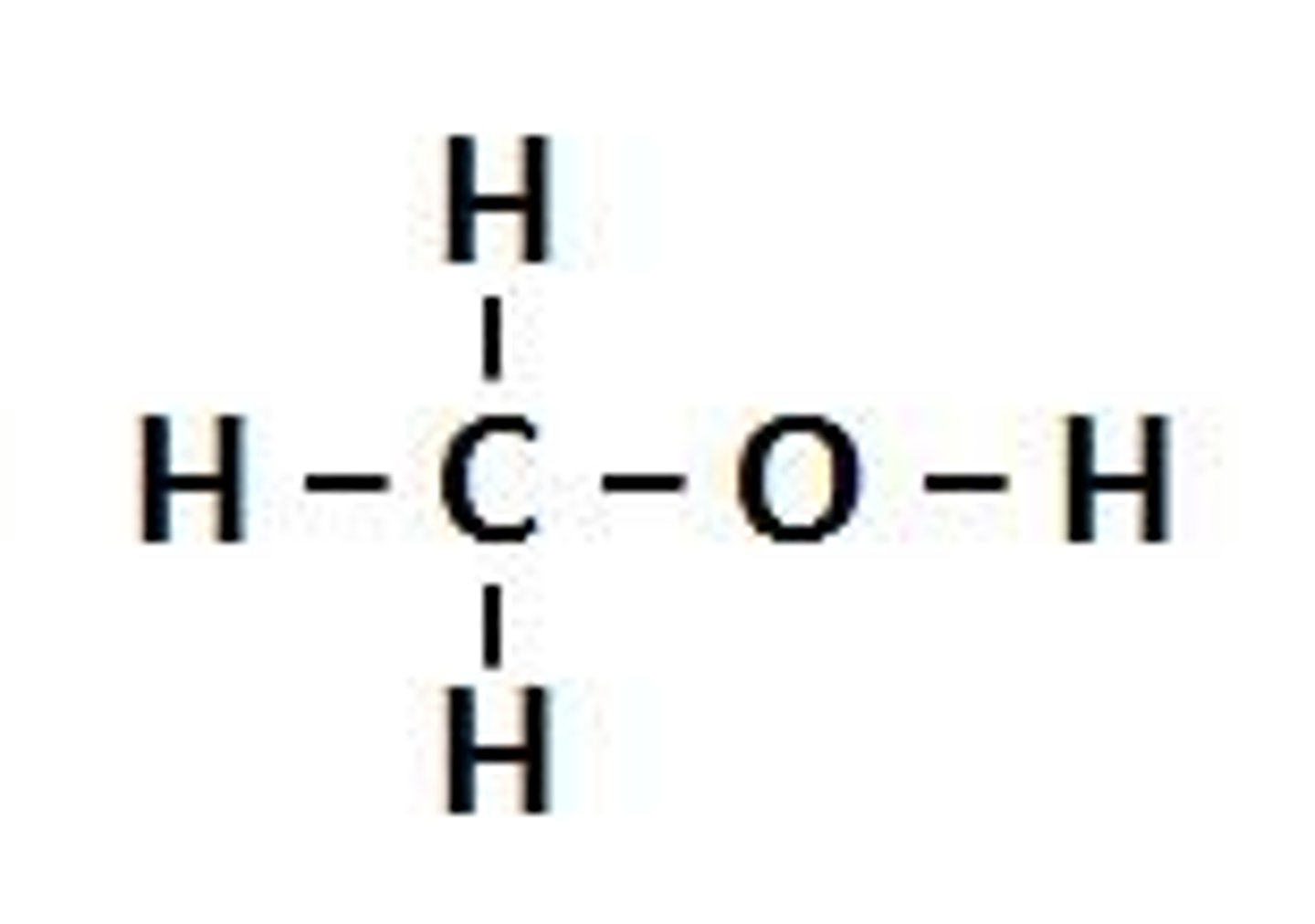
General rules for naming carbon chains
Count the longest carbon chain and name appropriately
Find any branched chains and count how many carbons they contain
Add the appropriate prefix for each branch chain
CH3
|
CH2
|
H3C - CH -- CH2 -- CH -- CH3
|
CH2
|
CH3
3,5-dimethylheptane
functional group rules
•When using a suffix, add in the following way :
If the suffix starts with a vowel- remove the -e from the stem alkane name
e.g. Propan-1-ol, butan-1-amine, ethanoic acid, ethanoylchloride, butanamide
If the suffix starts with a consonant or there are two or more of a functional group meaning di, or tri needs to be
used then do not remove the the -e from the stem alkane name
e.g. Propanenitrile, ethane-1,2-diol, propanedioic acid, propane-1,2,3-triol, pentane-2,4-dione.
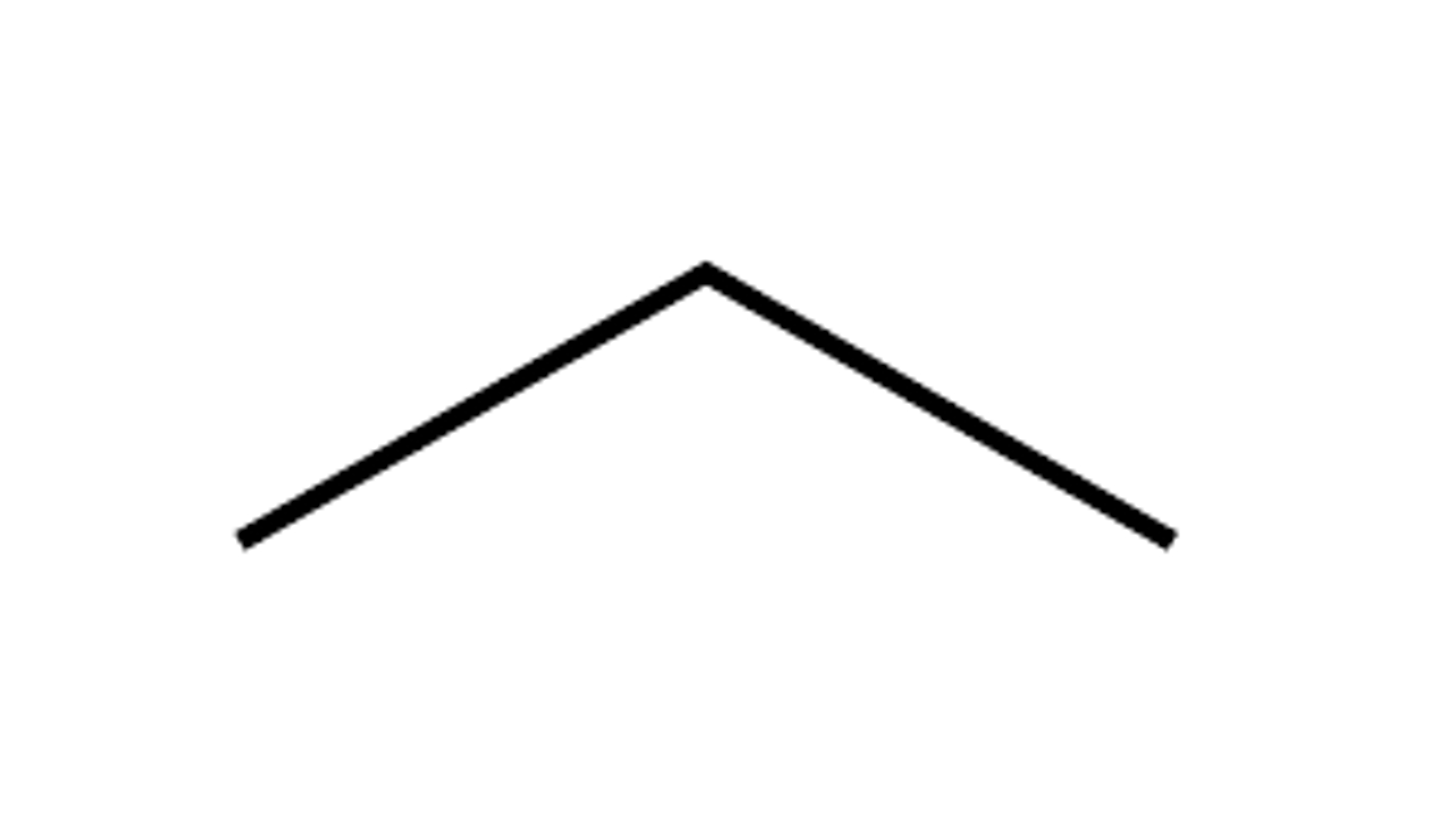
skeletal formula for propane
skeletal formula for propene
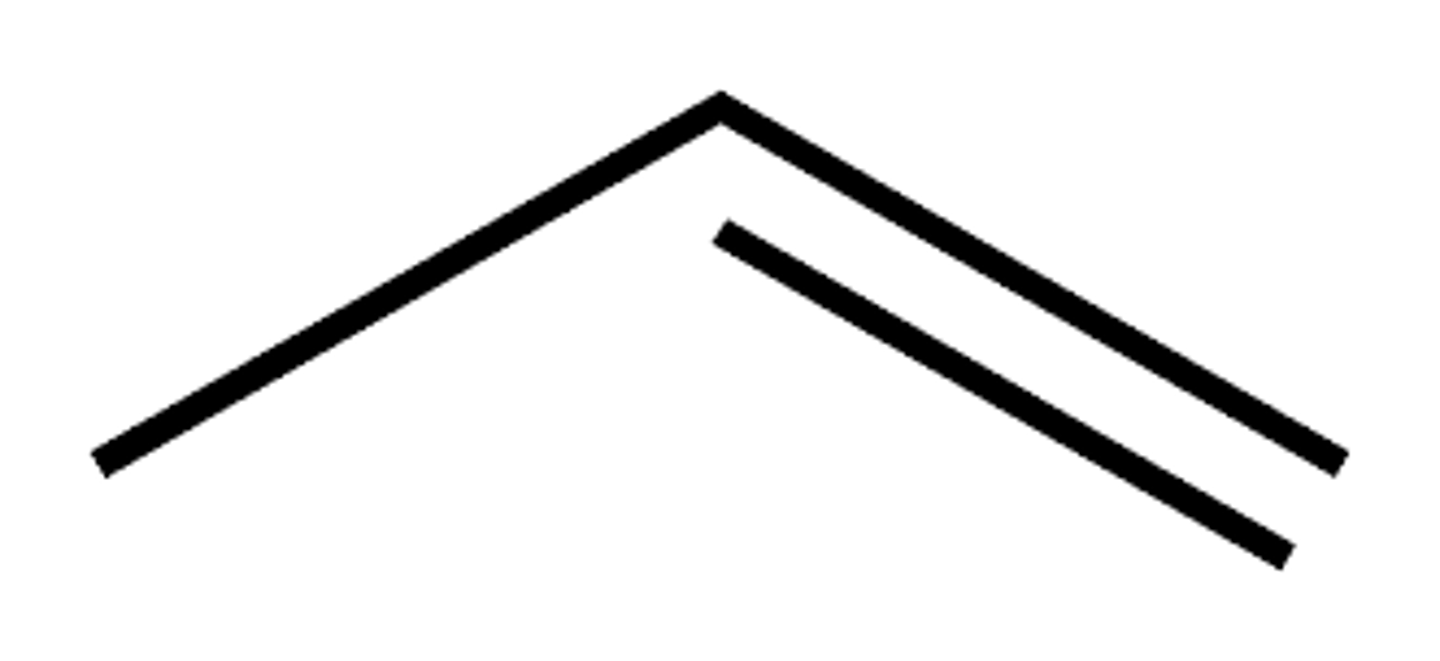
methanoic acid skeletal formula
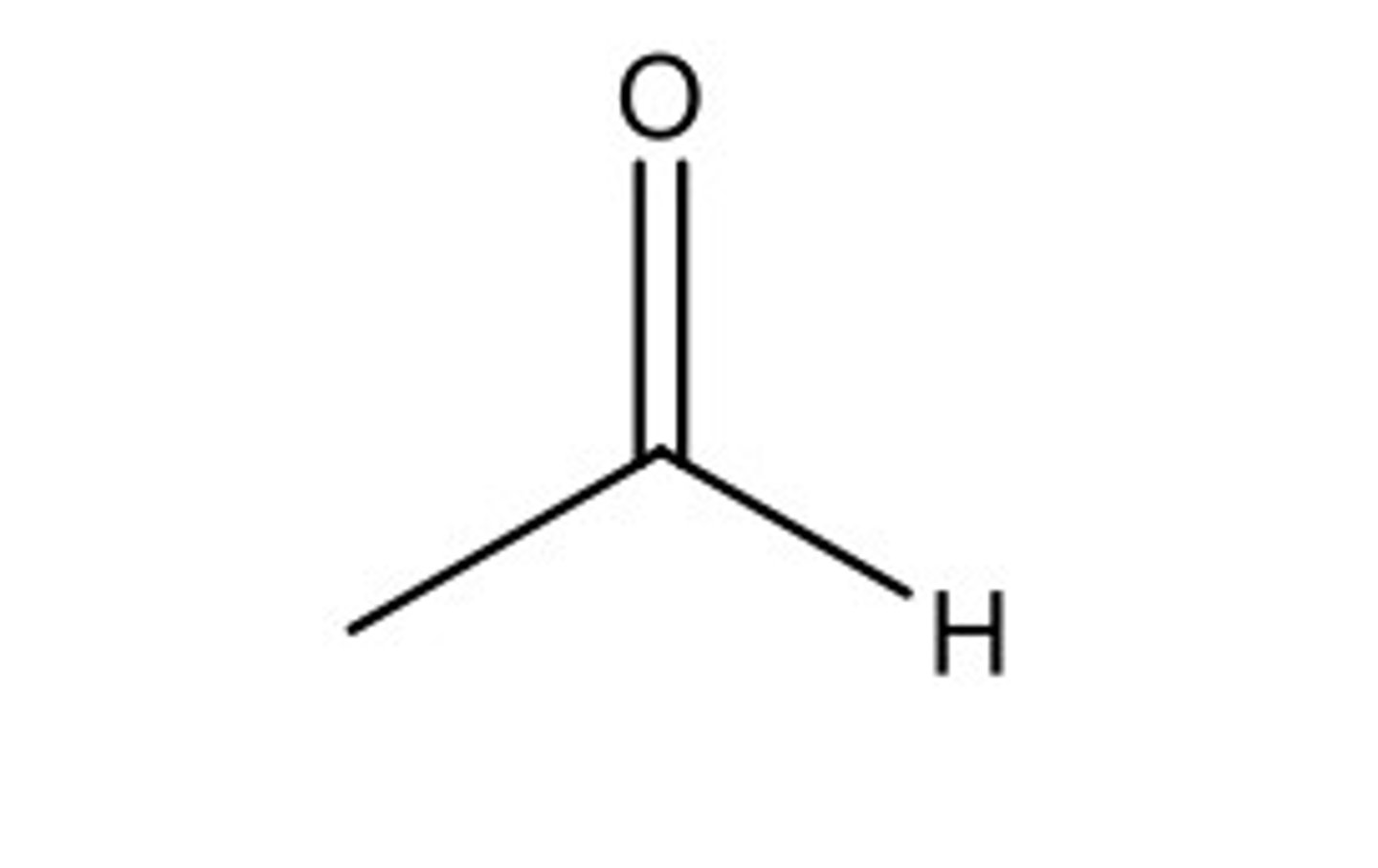
ethanal skeletal formula
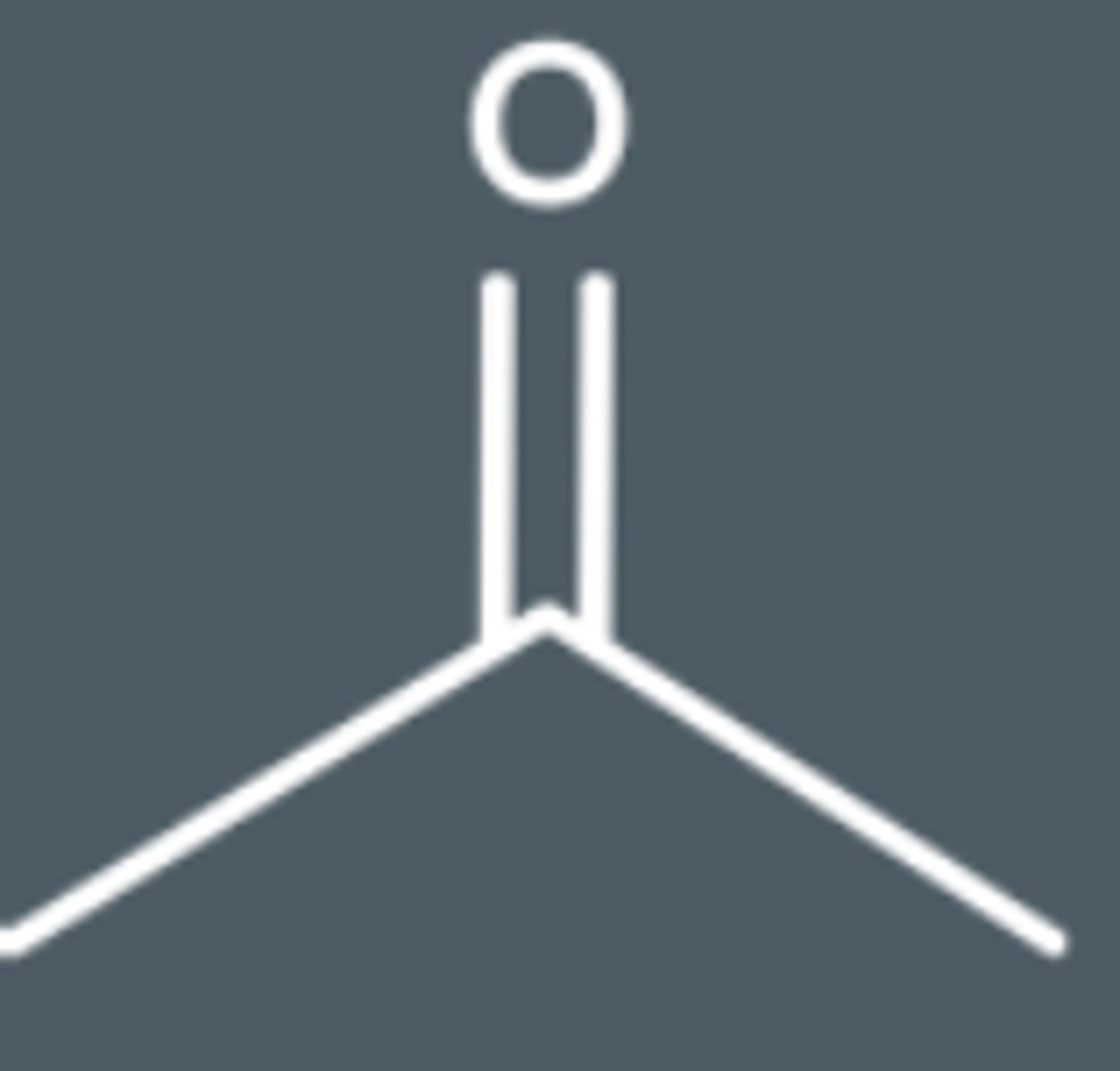
propanone skeletal formula
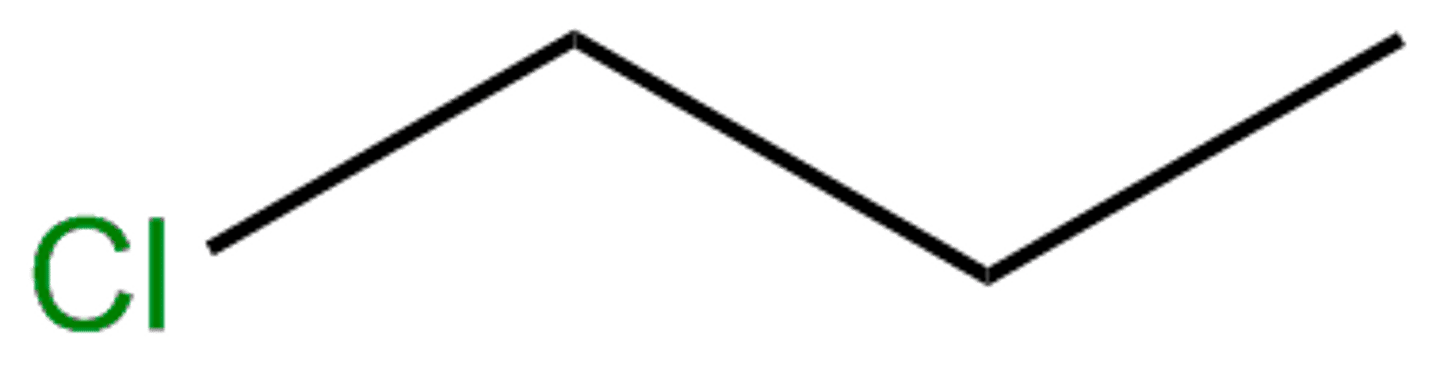
1-chloropropane
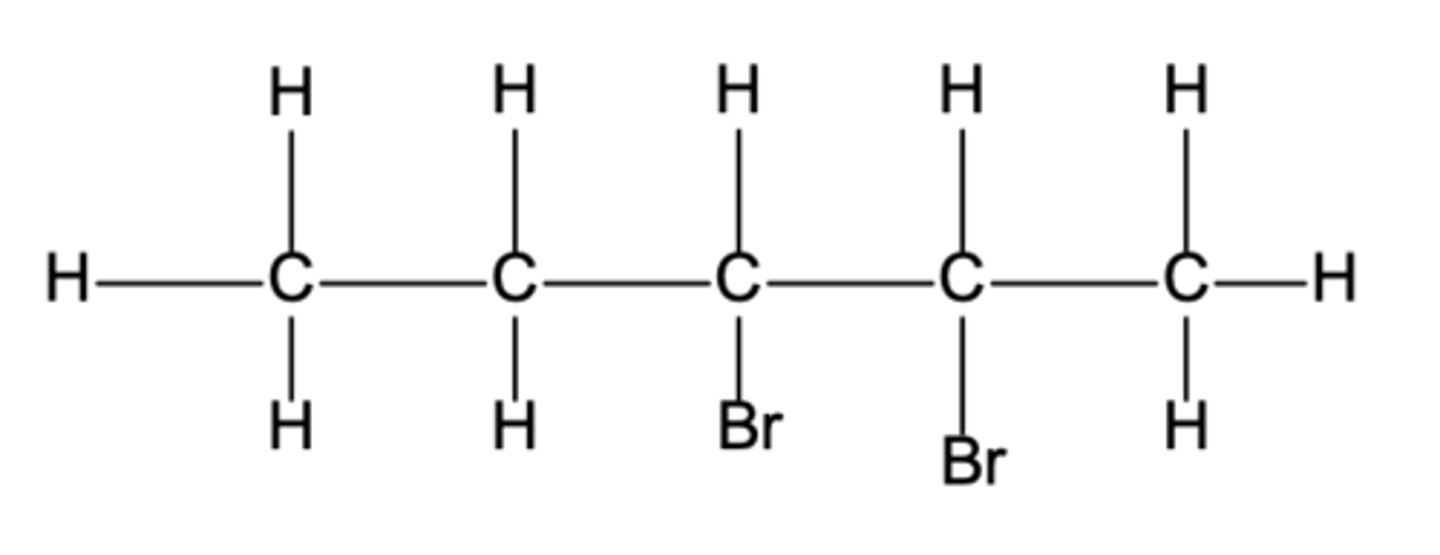
displayed formula for 2,3-dibromopentane.
displayed formula for 2,3-dibromopentan
methylethanoate skeletal formula
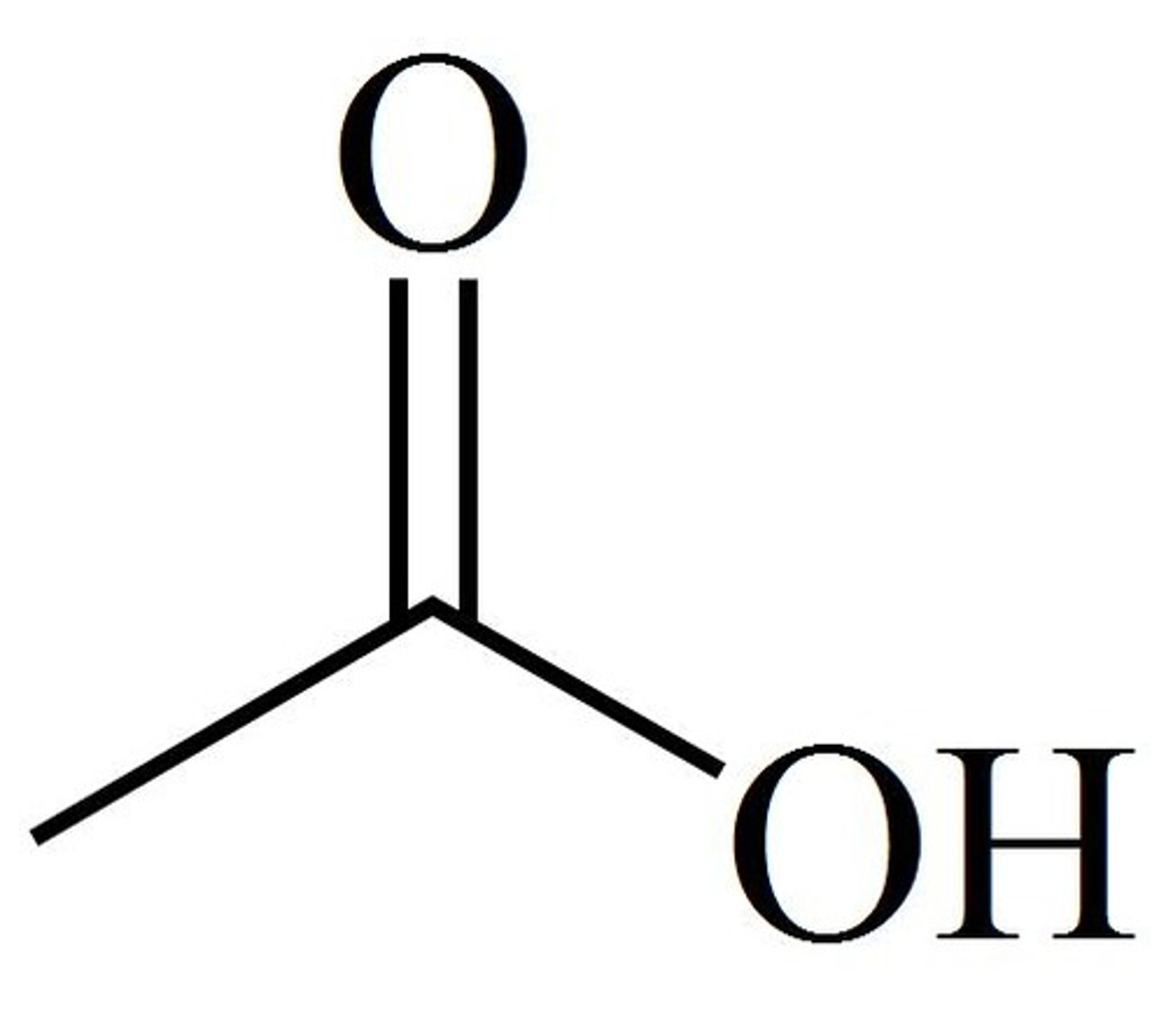
ethanoic acid
Where can structural isomers arise from
Structural isomerism can arise from •Chain isomerism •Position isomerism •Functional group isomerism Same molecular formula different structures (or structural formulae)
chain isomer
Compounds with the same molecular formula but different structures of the carbon skeleton
position isomers
Compounds with the same molecular formula but different structures due to different positions of the same functional group on the same carbon skeleton
position isomer examples
propan-1-ol and propan-2-ol
functional group isomers
Compounds with the same molecular formula but with atoms arranged to give different functional groups
Homologous series
A series of organic compounds with the same functional group but with each successive member differing by CH2
alkenes rules
The double bond will be between two carbons. Use the lower
number of the two to show the position of the double bond
The name for alkenes may include E or Z at start to show
the type of stereoisomer
If more than one double bond is present then suffix
ends diene or triene. The stem ends in a
The suffix-en for alkenes can go in front of other suffixes.
The alcohol and carboxylic acid groups have higher priority
than the alkene group so take precedence with numbering
alkanes rules
Class the halogen as a substituent on the C chain and use
the prefixes-fluoro,
-chloro,
-bromo, or-iodo. (Give the
position number if necessary)
2-bromobutane
alcohol
These have the ending-ol and if necessary the position
number for the OH group is added between the name
stem and the -ol
Ethane-1,2-diol
aldehydes
An aldehyde's name ends in-al
It always has the C=O bond on the first carbon of
the chain so it does not need an extra number. It is
by default number one carbon on the chain.
ketones
Ketones end in-one
When ketones have 5C's or more in a chain then
it needs a number to show the position of the
double bond. E.g. pentan-2-one
If two ketone groups then di is put before-
one and an e is added to the stem.
pentane-2,4-dione
What are stereoisomers?
Compounds with the same structural formula but with a different arrangement of the atoms in space. Stereoisomers have the same structural formulae but
have a different spatial arrangement of atoms.
Who can exhibit E-Z isomers and why
Alkenes can exhibit a type of isomerism
called E-Z stereoisomerism
E-Z isomers exist due to restricted
rotation about the C=C bond
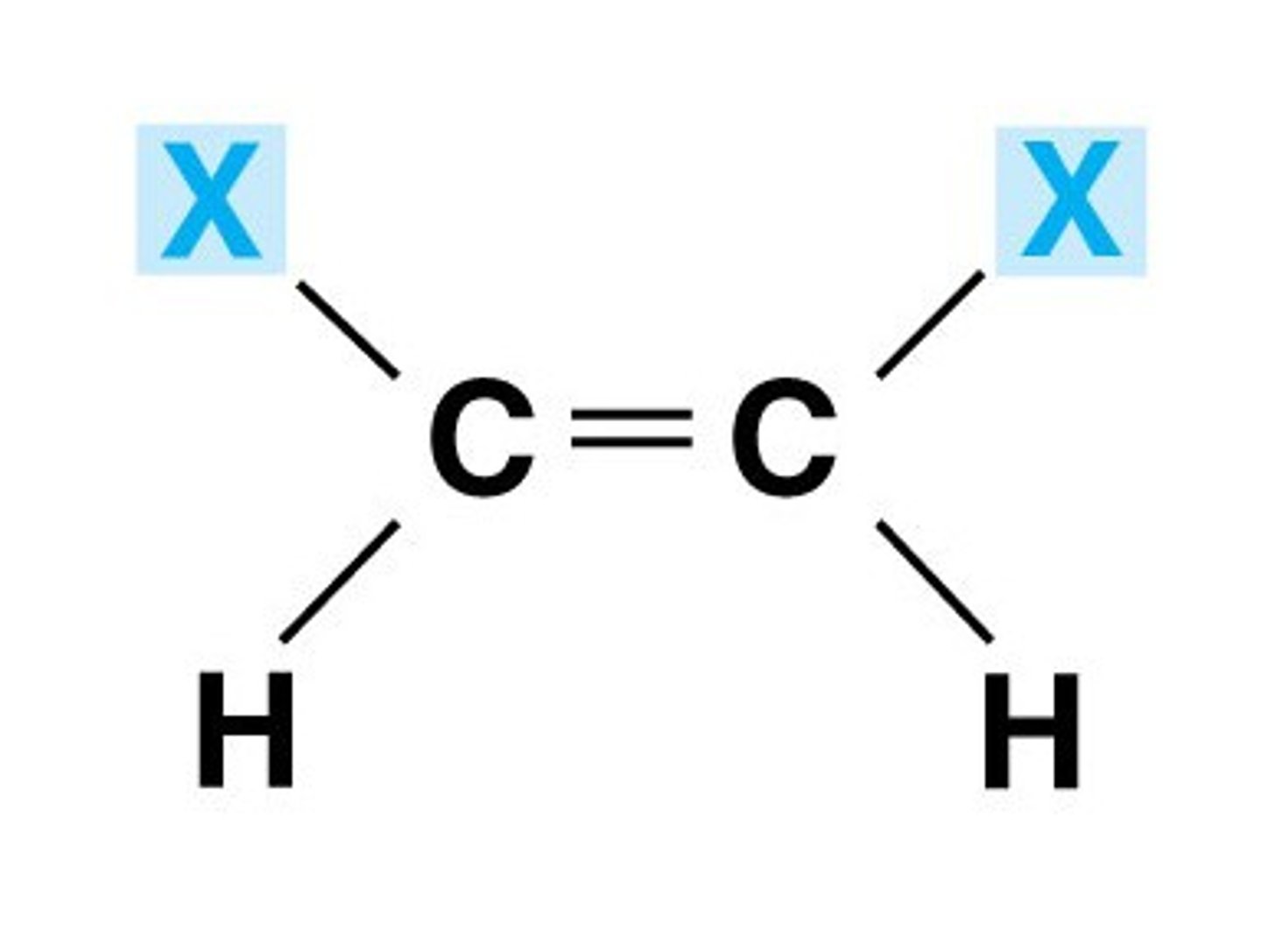
When do E-Z isomers arise?
E-Z stereoisomers arise when:
(a) There is restricted rotation around the C=C double bond.
(b) There are two different groups/atoms attached both ends of the
E isomer
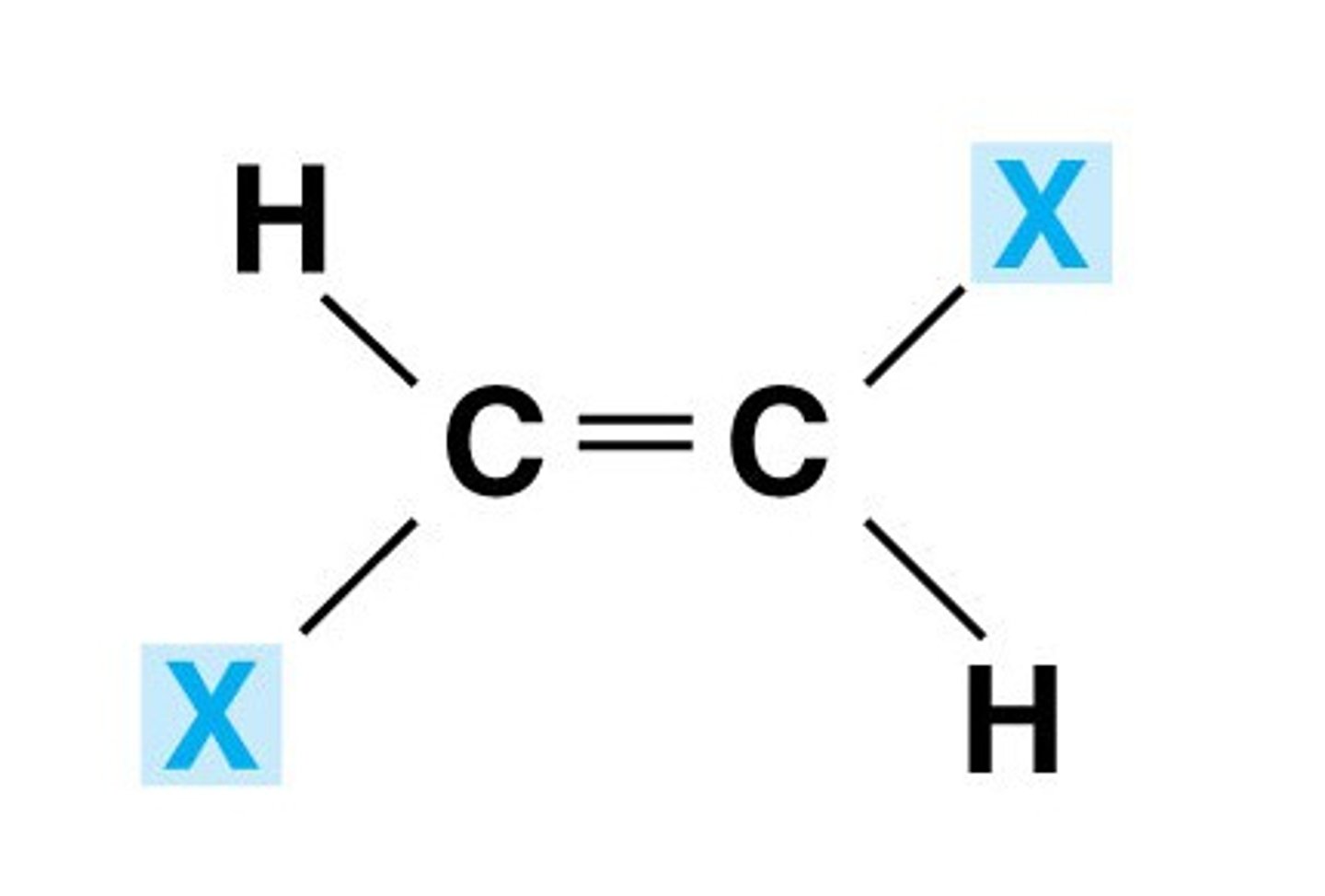
z isomer
Priority atom
The atom with the bigger
atomic number is classed as the priority atom
CIP Priority rules
1. Compare the atomic number of the
atoms directly attached to each side of the
double bond; the atom of higher atomic
number is given priority.
2. 2. If the atoms are the same, consider the
atoms at distance 2 from the double bond.
Make a list of each atom bonded to the one
directly attached to the double bond.
Arrange list in order of decreasing atomic
number. Compare the lists atom by atom; at
the earliest difference, the group containing
priority
the atom of higher atomic number is given
priority
chain isomer examples
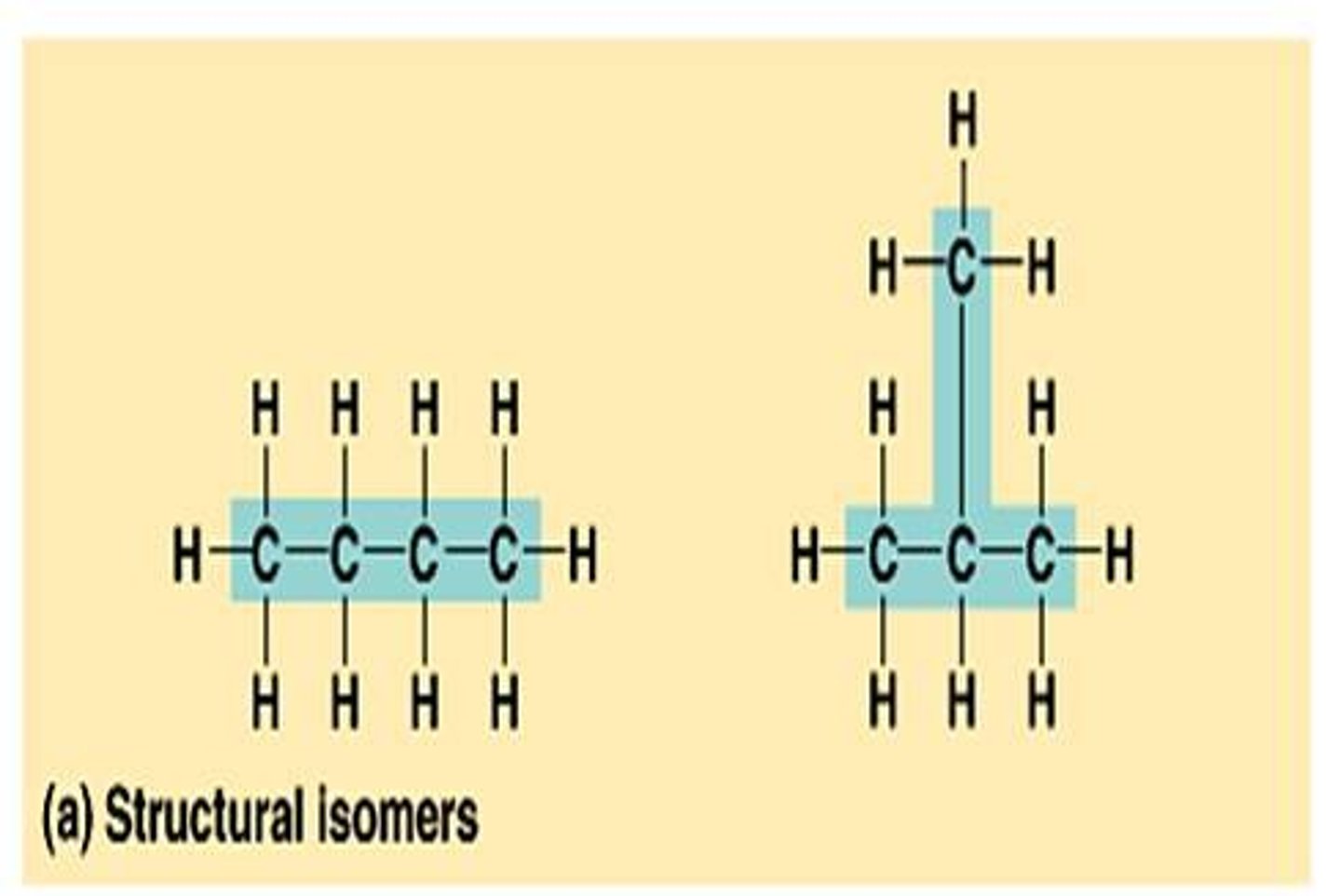
butane and 2-methyl propane
Explain the differences between structural isomerism and stereoisomerism. Use
examples to show how compounds with the molecular formula C4H8 exhibit
stereoisomerism and the three types of structural isomerism.
Stage 1
Difference between structural & stereoisomers
1a structural isomers = molecules with same molecular formula but different
structure
1b stereoisomers = molecules with same structural formula but different
arrangement of atoms in space
Stage 2
Stereoisomers
2a lack of rotation around C=C
2b structures of E- and Z-but-2-ene
2c correct identity of E and Z isomers
Stage 3
Structural isomers
3a different C chain, e.g. methylpropene & but-1-ene / but-2-ene
3b different position of functional group e.g. but-1-ene & but-2-ene
3c different functional group, e.g. cyclobutane & but-1-ene / but-2-ene /
methylpropene
general formula for alkene
CnH2n
unsaturated
double bonds, so they tend to undergo addition reactions
what does a carbon double bond consist of
a sigma (2 s-orbitals) bond and
a pi bond (2 p-orbitals)
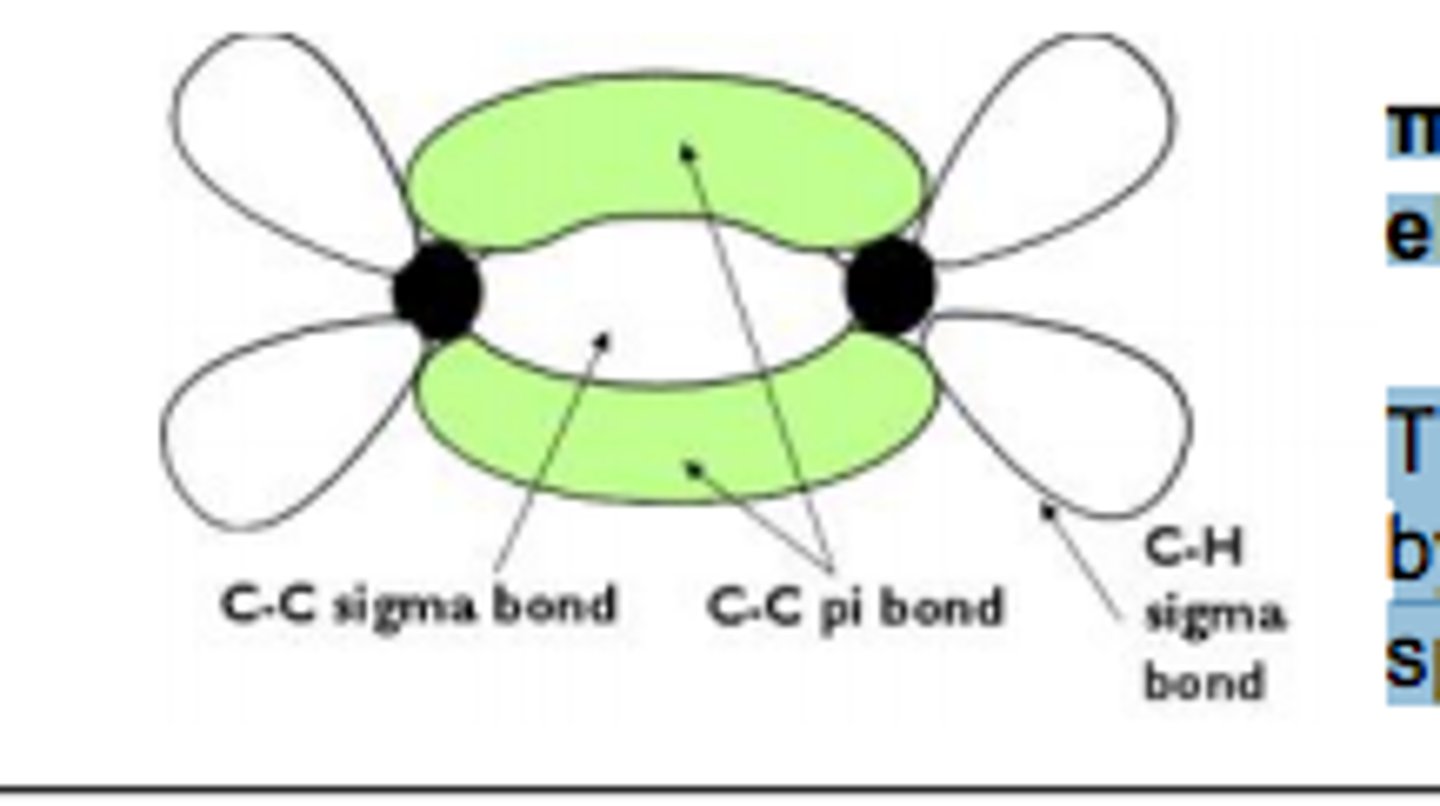
which area of double bond is vulnerable and why
the pi bond. They are therefore vulnerable to attack
by species which 'like' electrons: these
species are called electrophiles.
π bonds are exposed and have high electron density
what shape is the double bond
The arrangement of bonds around the
>C=C< is planar and has the bond angle 120o
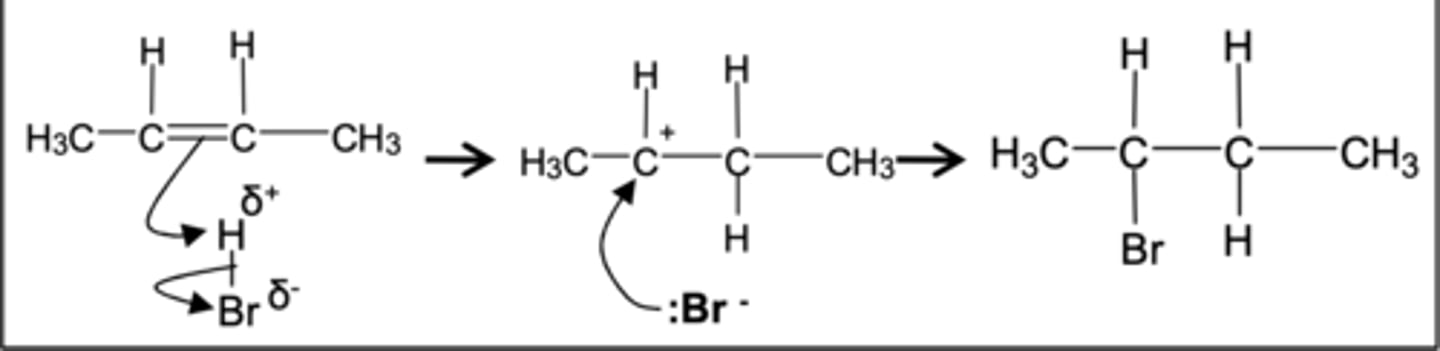
in reaction with hydrogen bromide with alkenes, which is the electrophile
Electrophile, H(delta)+
which is attracted to who in reaction with hydrogen bromide with alkenes
The H δ + is
attracted to the
electron-rich pi bond
what sort of molecule is HBr
is a polar
molecule because
Br is more electronegative than H.
what is the intermediate formed in reaction alkene --> halogenoalkene
carbocation
What becomes a nucleophile alkene --> halogenoalkene
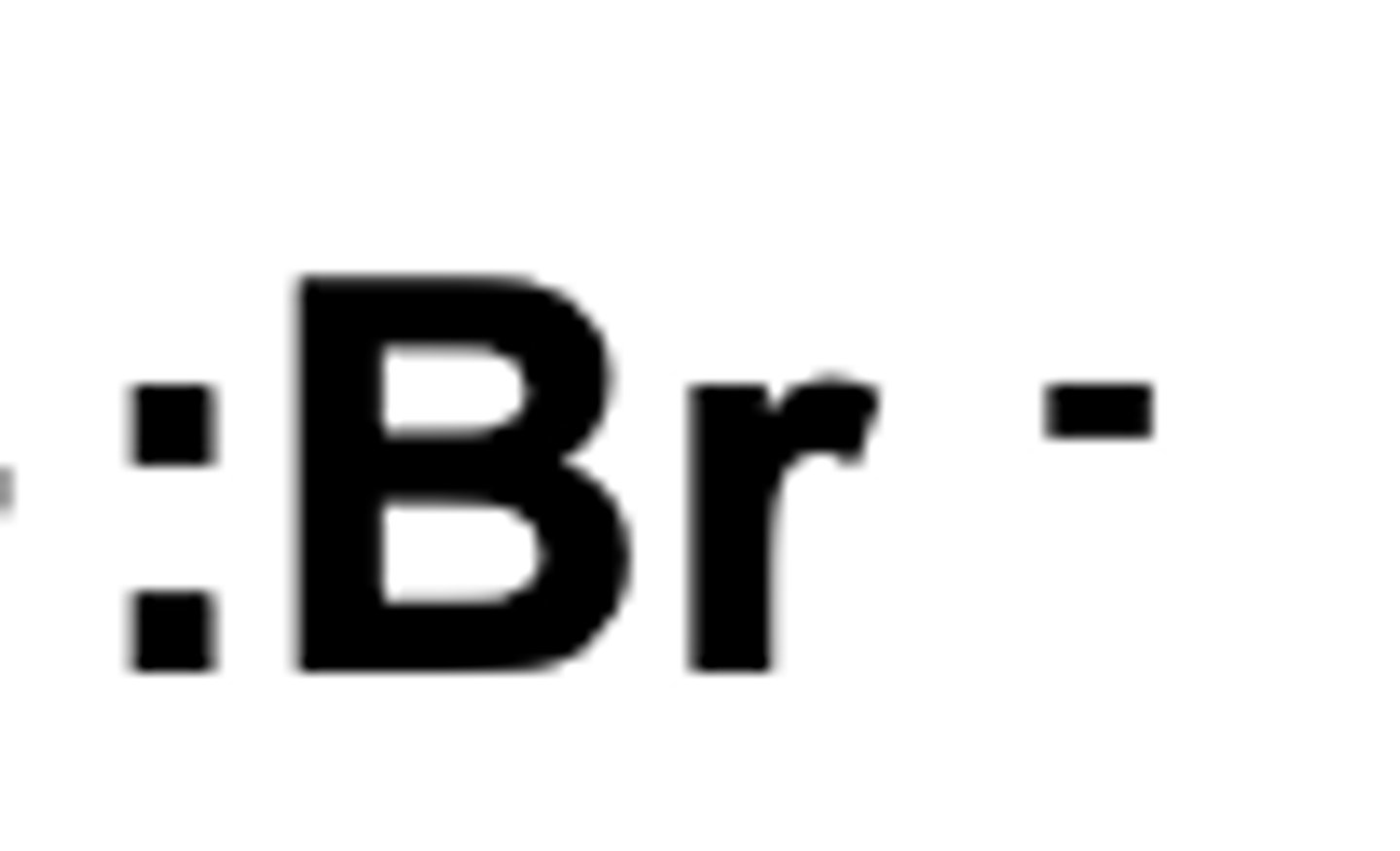
reaction of hydrogen bromide with alkenes
what will form when alkene is assymetrical (reaction of hydrogen bromide with alkenes)
This reaction can lead to two products when the alkene is unsymmetrical, 90% major product and 10% minor product
order of stability of carbocations
tertiary > secondary > primary
mechanism of markovnikov's rule
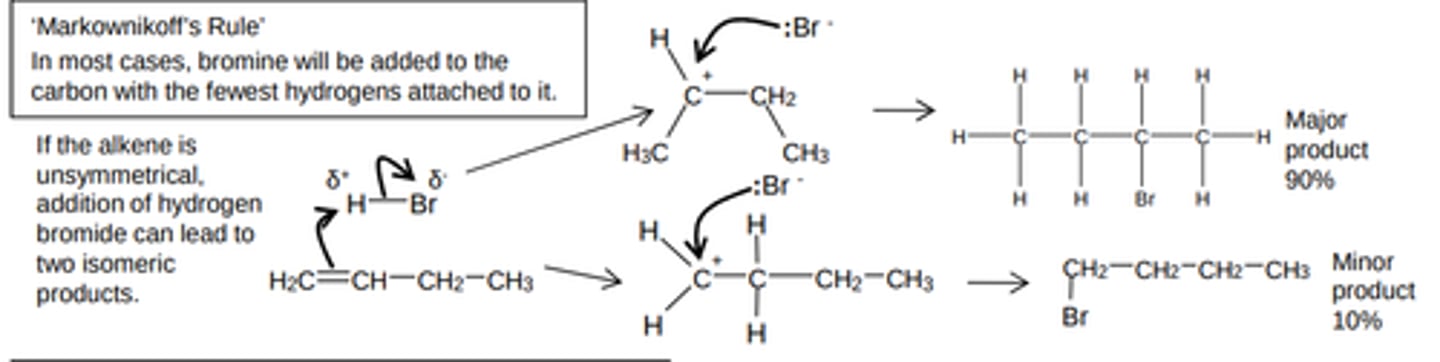
how is a product formed in electrophilic addition to alkenes
In electrophilic addition to alkenes, the major product is formed via the more stable carbocation intermediate
why is secondary carbocation more stable
Because it has two methyl groups releasing electrons towards the positive carbon
functional group
Functional group is an atom or group of atoms which when present in different molecules
causes them to have similar chemical properties
cycloalkanes and alkenes
aldehydes and propane
addition polymerisation
Addition polymers are formed from alkenes
Example of addition polymerisation
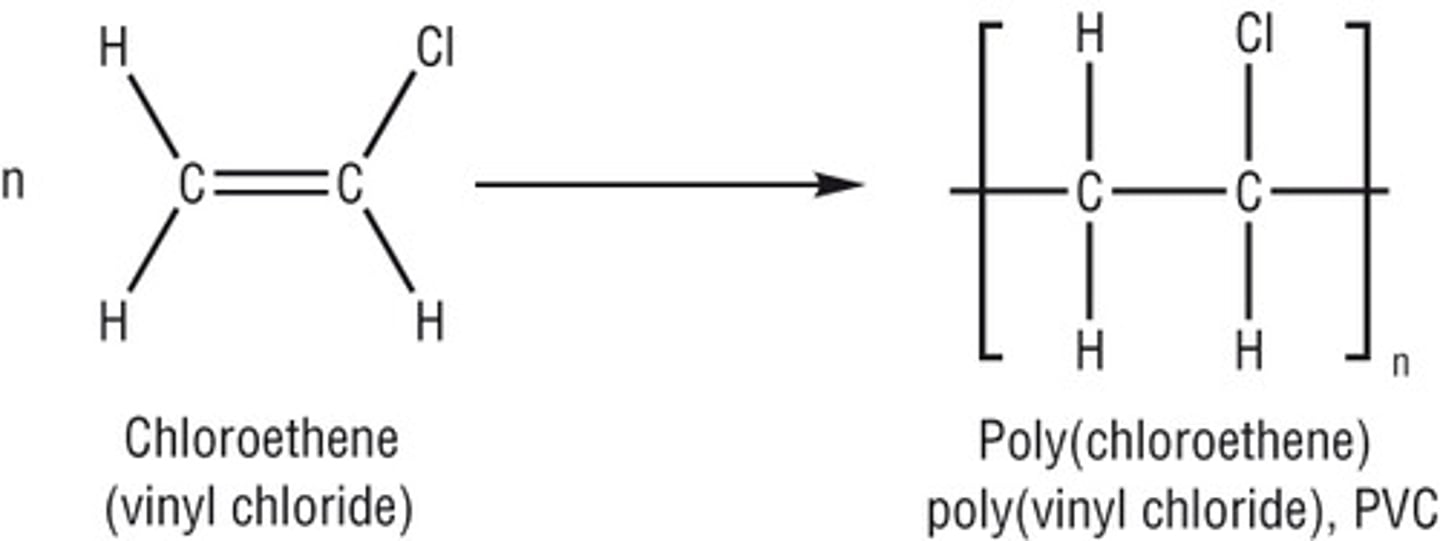
why are polymers unreactive
they are saturated
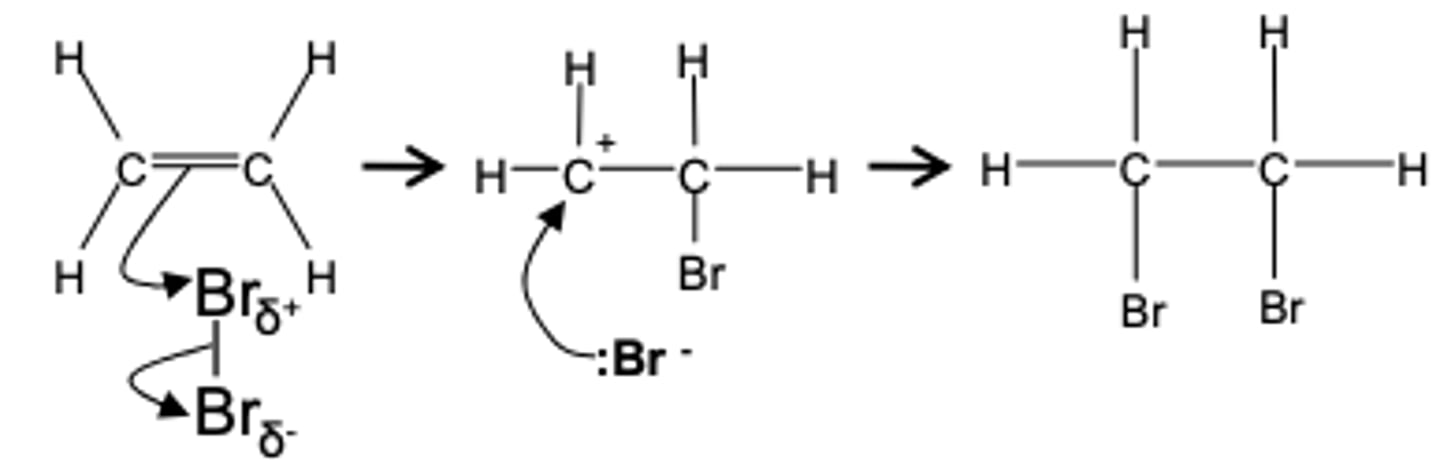
reaction of bromine with alkenes
Reagent: Bromine
Conditions: Room temperature (not in UV light)
Mechanism: Electrophilic addition
Type of reagent: Electrophile, Br+
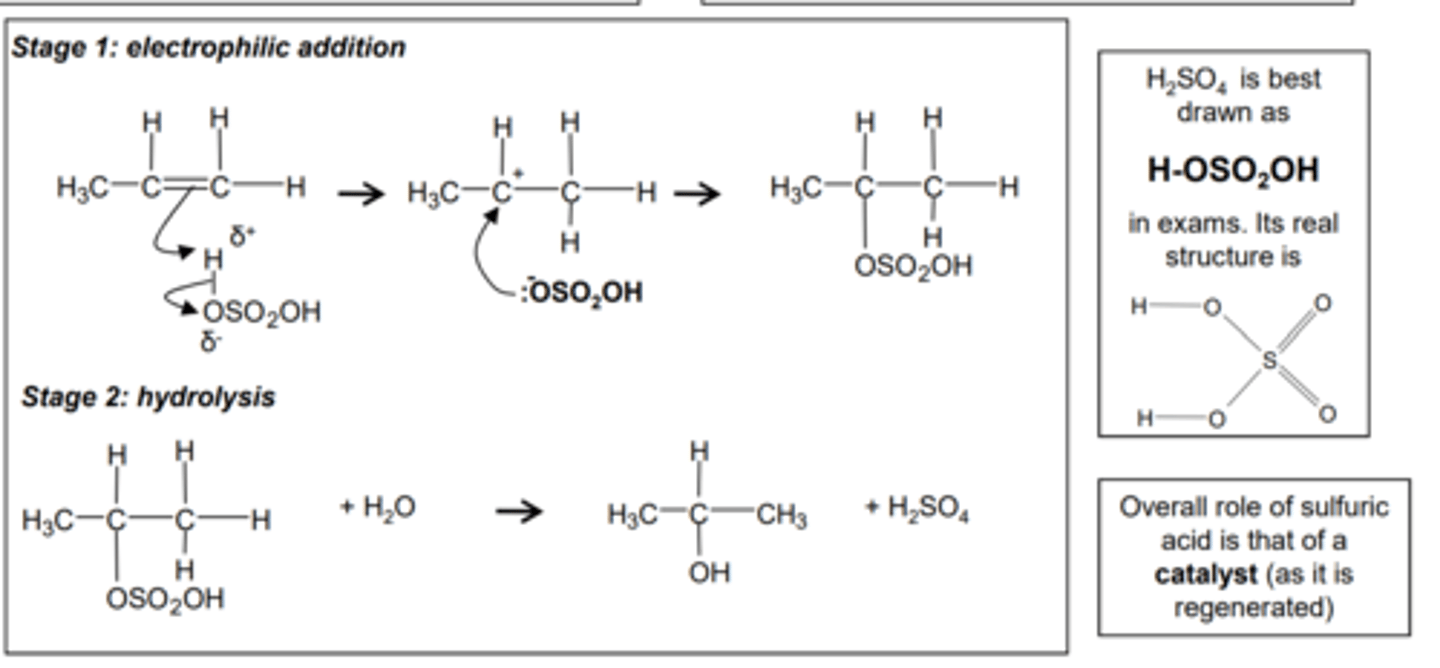
3. Reaction of sulfuric acid with alkenes
Change in functional group
alkene alkyl hydrogensulfate
Reagents: concentrated H2SO4
Conditions: room temperature
Mechanism: Electrophilic addition
Type of reagent: Electrophile, H2SO4
CH2=CH2 + H2SO4 CH3CH2OSO2OH
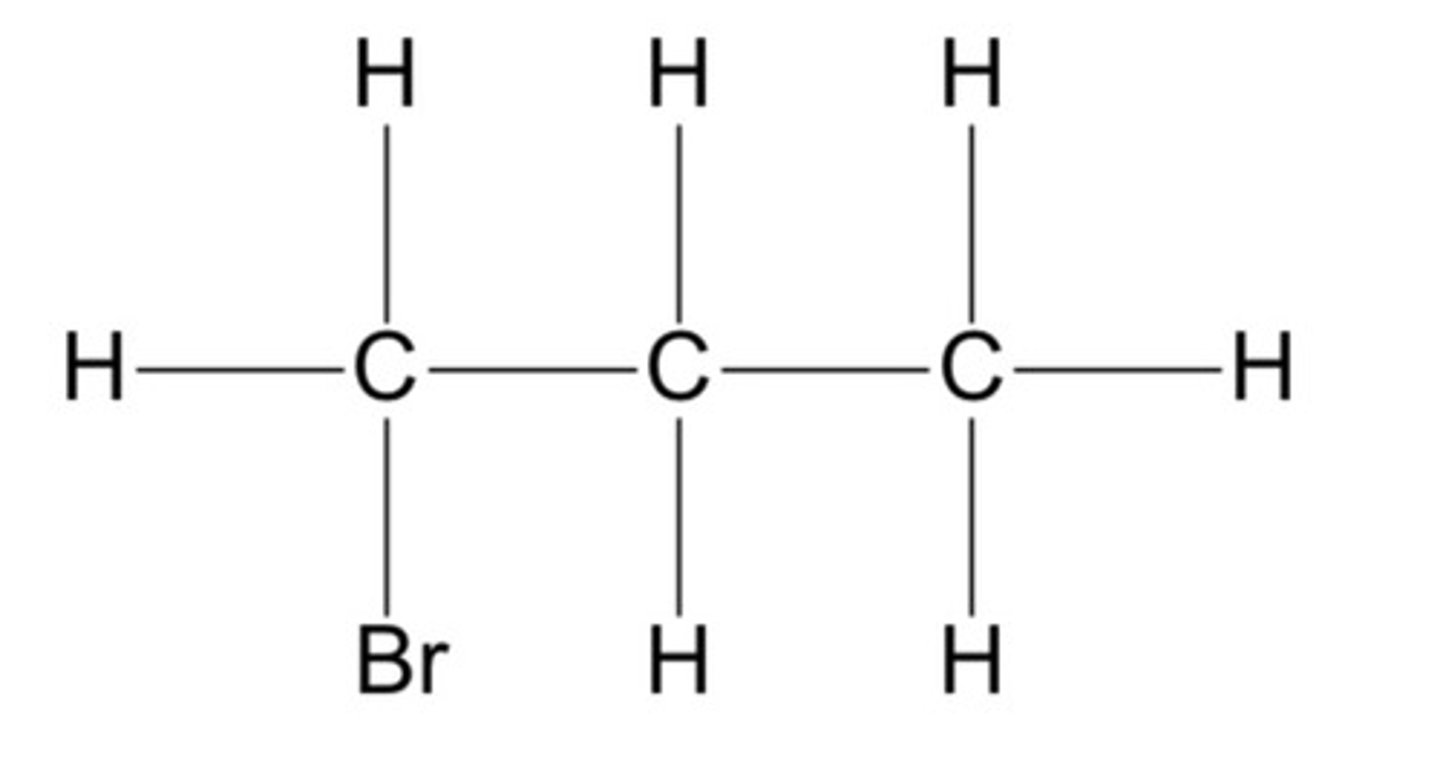
Primary halogenoalkane
One carbon attached to the carbon atom adjoining the halogen
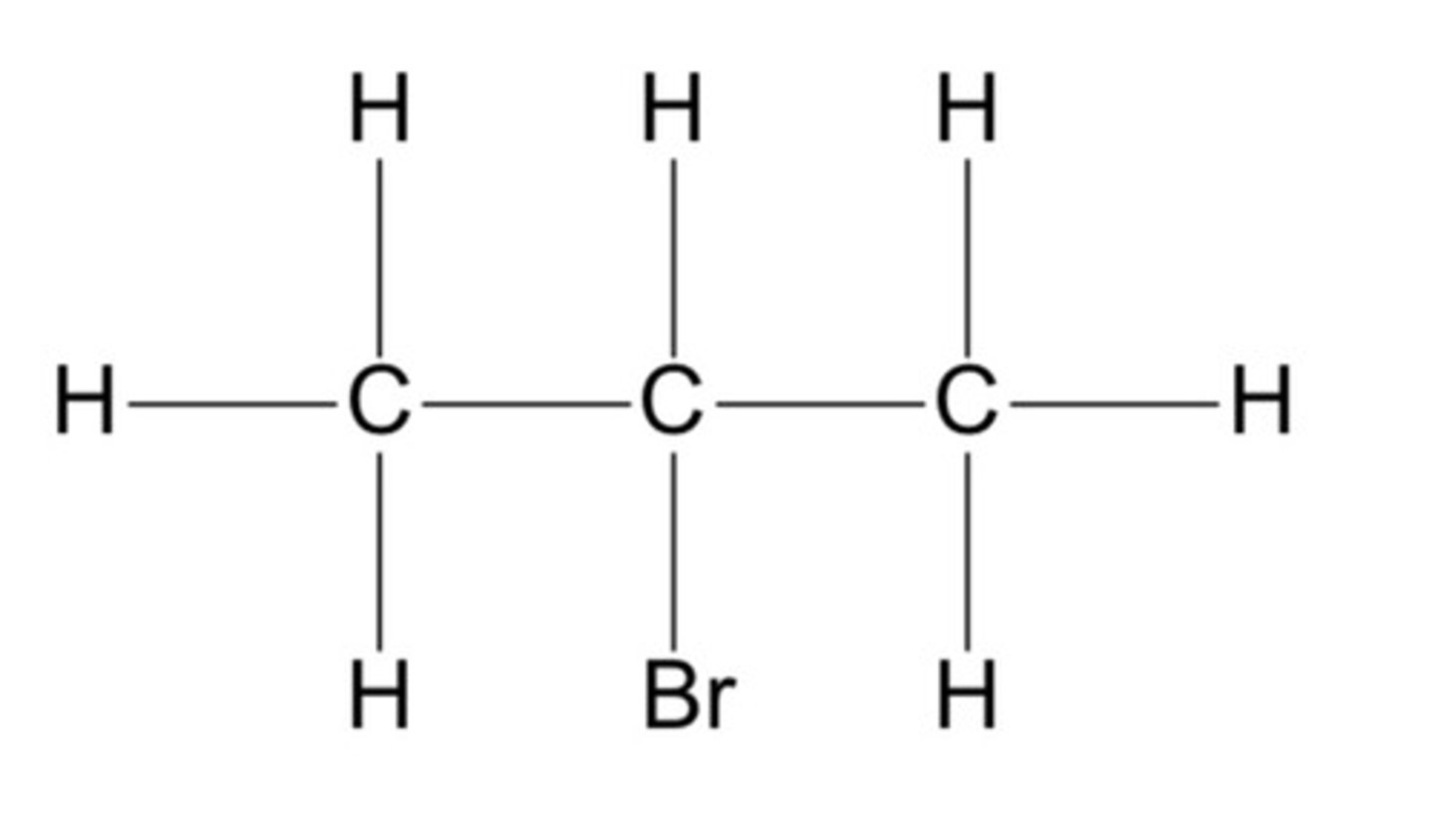
Secondary halogenoalkane
A halogenoalkane which has two carbon atoms directly bonded to the carbon atom that is bonded to the halogen.
Tertiary halogenoalkane
A halogenoalkane which has three carbon atoms directly bonded to the carbon atom that is bonded to the halogen.
Direct hydration of ethene
Ethene reacts with steam at a temperature of 300°C, a pressure of 65 atm (6500kPa) and in
the presence of a catalyst of either concentrated phosphoric acid or concentrated
sulphuric acid.
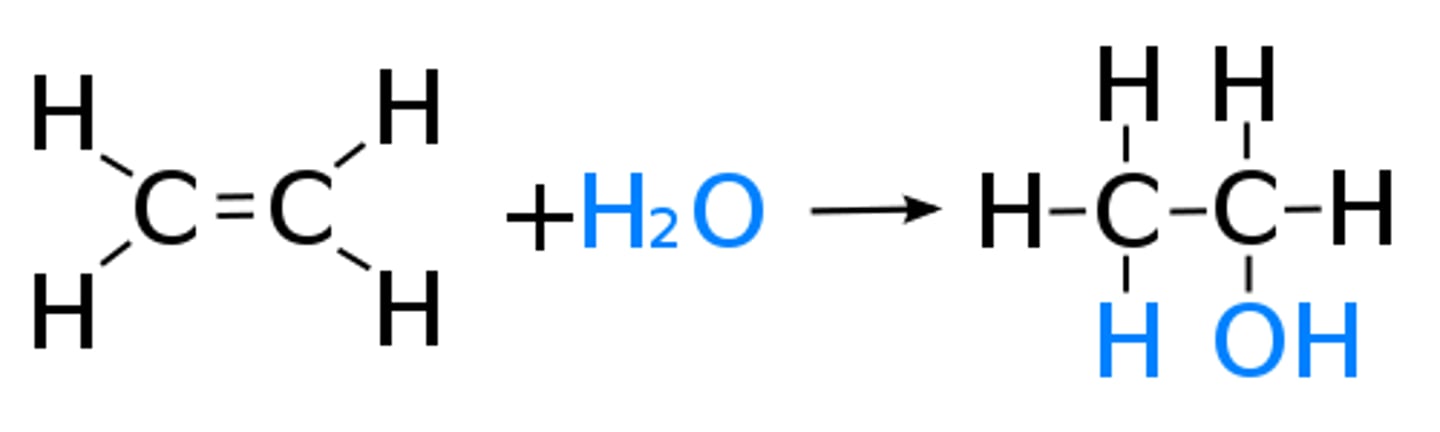
(CH2)2CHCH2CH3, structural formula, name
2-methylbut-1-ane
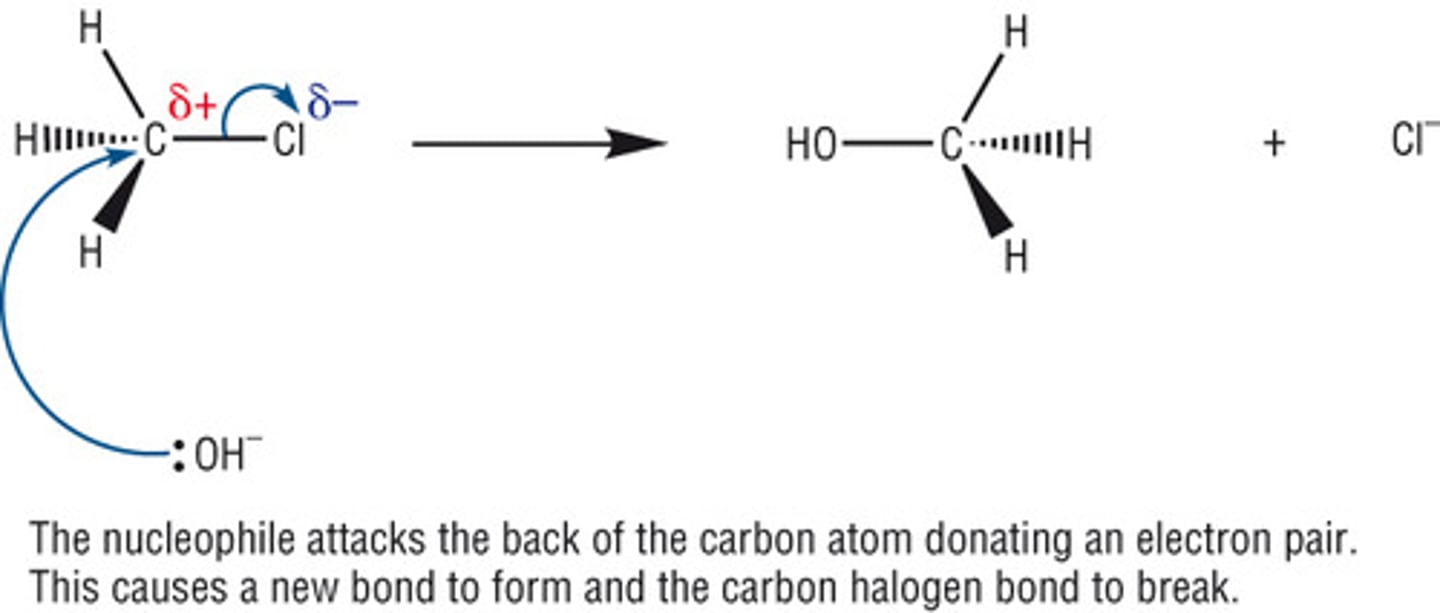
nucleophilic substitution reaction hydroxide ions
NaOH (AQ) + Heat.
Haloalkane + NaOH mixed in ethanol to make miscible
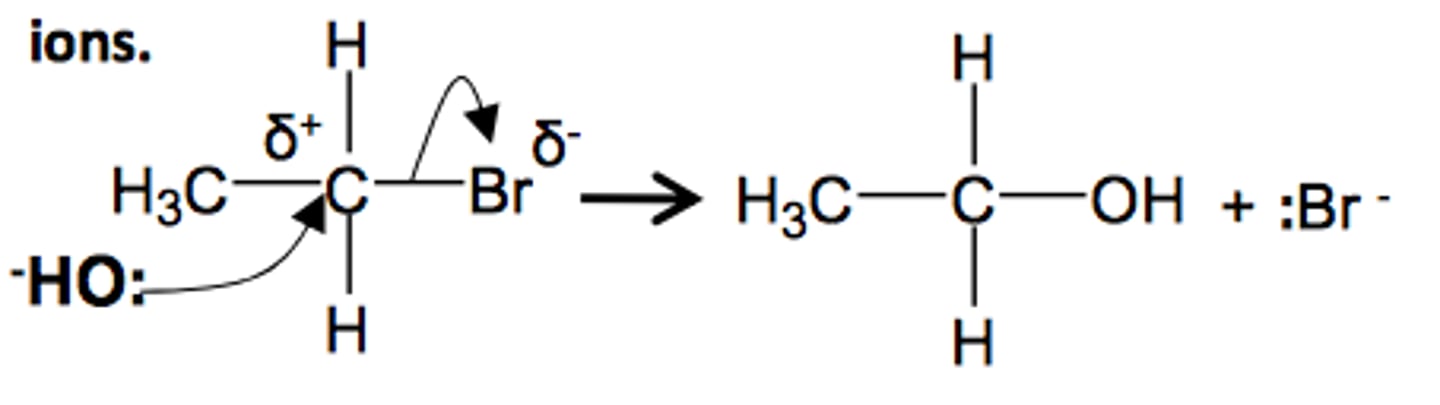
nucleophilic substitution cyanide ions
KCN + Heat
This reaction increases the length of the carbon chain (which is reflected in the name) In the above example butanenitrile includes the C in the nitrile group
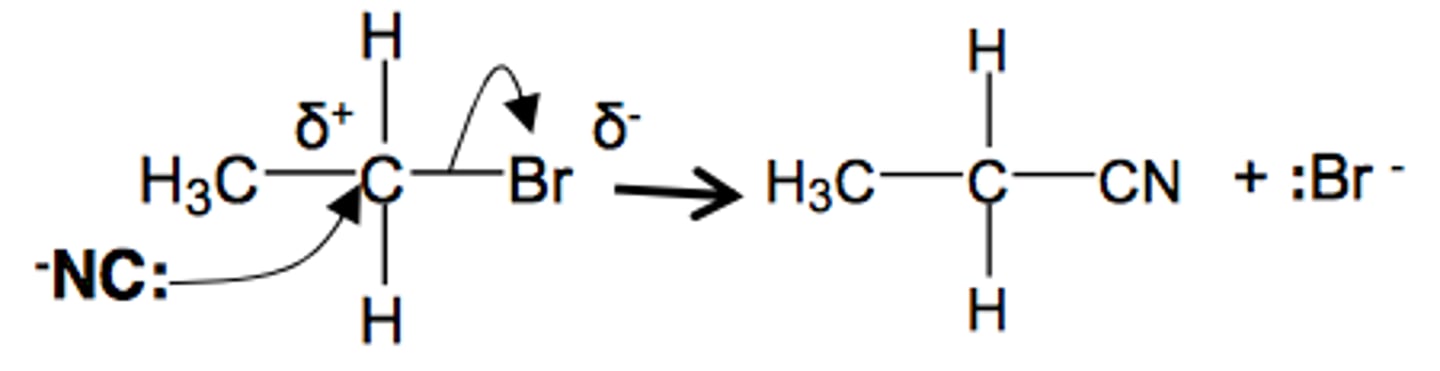
which halogenoalkane is unreactive
The iodoalkanes are the fastest to substitute and the fluoroalkanes are the slowest. The strength of the C-F bond is such that fluoroalkanes are very unreactive
The rate of these substitution reactions depends on the strength of the C-X bond The weaker the bond, the easier it is to break and the faster the reaction
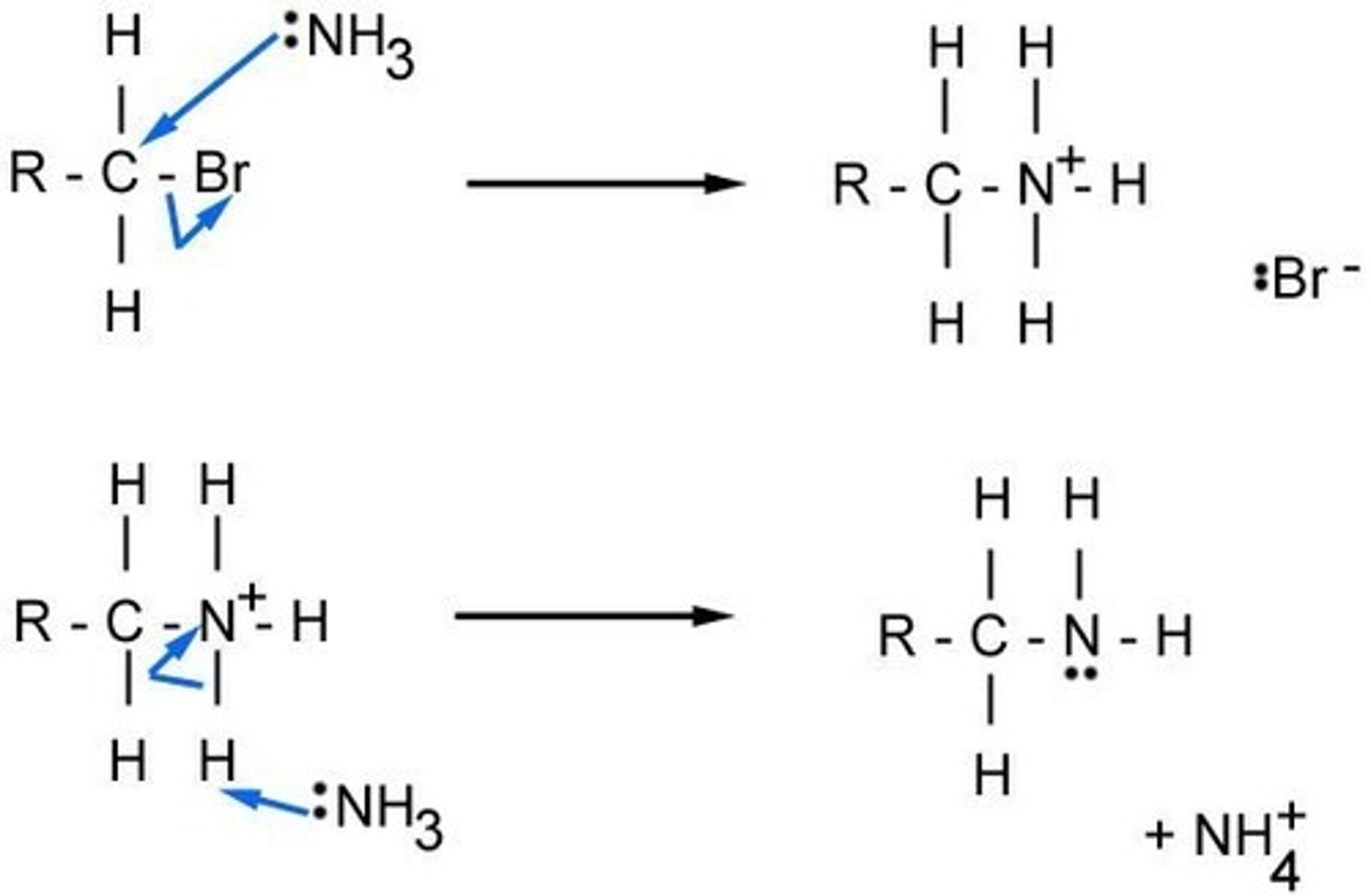
nucleophilic substitution with ammonia
HALOGENOALKANE to AMINE
Reagent: NH3 dissolved in ethanol
Conditions: heat under pressure
Mechanism: nucleophilic substitution
Type of reagent: Nucleophile, :NH3
CH3CH2Br + 2NH3 → CH3CH2NH2 + NH4Br
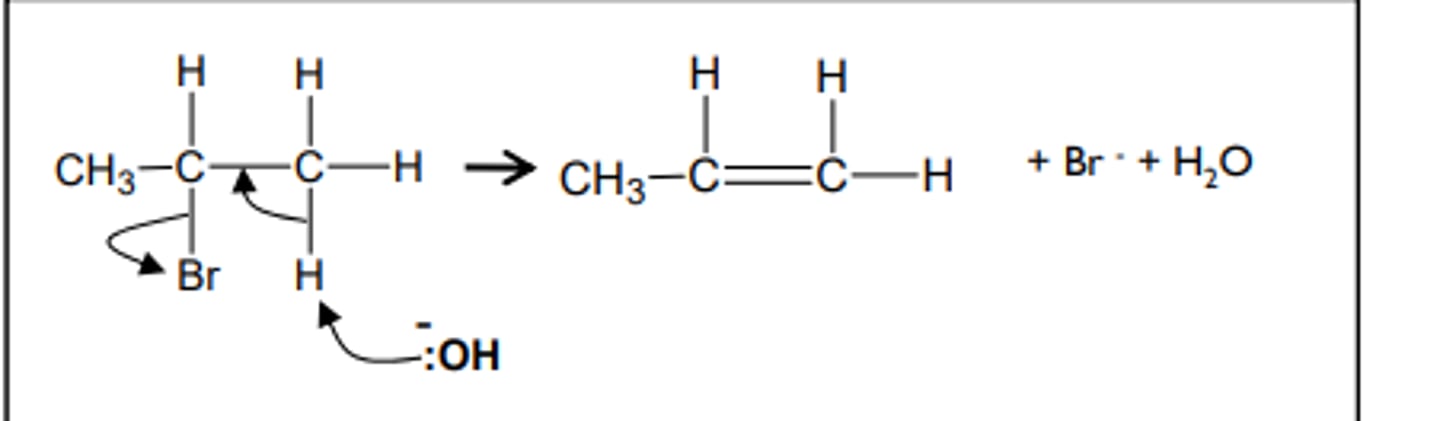
nucleophilic elimination
The formation of alkenes from haloalkanes happens when the same sodium or potassium hydroxide is used as before, but this time the conditions are alcoholic (dissolved in ethanol). This means an elimination mechanism occurs rather than the nucleophilic substitution seen previously. e.g. the formation of propene from 2-bromopropane
Chlorination of methane
Methane does not react with chlorine at room temperature, or in the dark. In the presence of
ultraviolet light, however, a mixture of methane and chlorine will explode forming hydrogen
chloride and a mixture of chlorinated methane's.
Free radical substitution mechanism's take place in several steps.
Initiation
The ultraviolet light (sunlight) provides the energy needed to break the Cl-Cl bond, splitting
some chlorine molecules into two atoms (radicals). This bond breaks as it is weaker than the
alternative C-H bonds
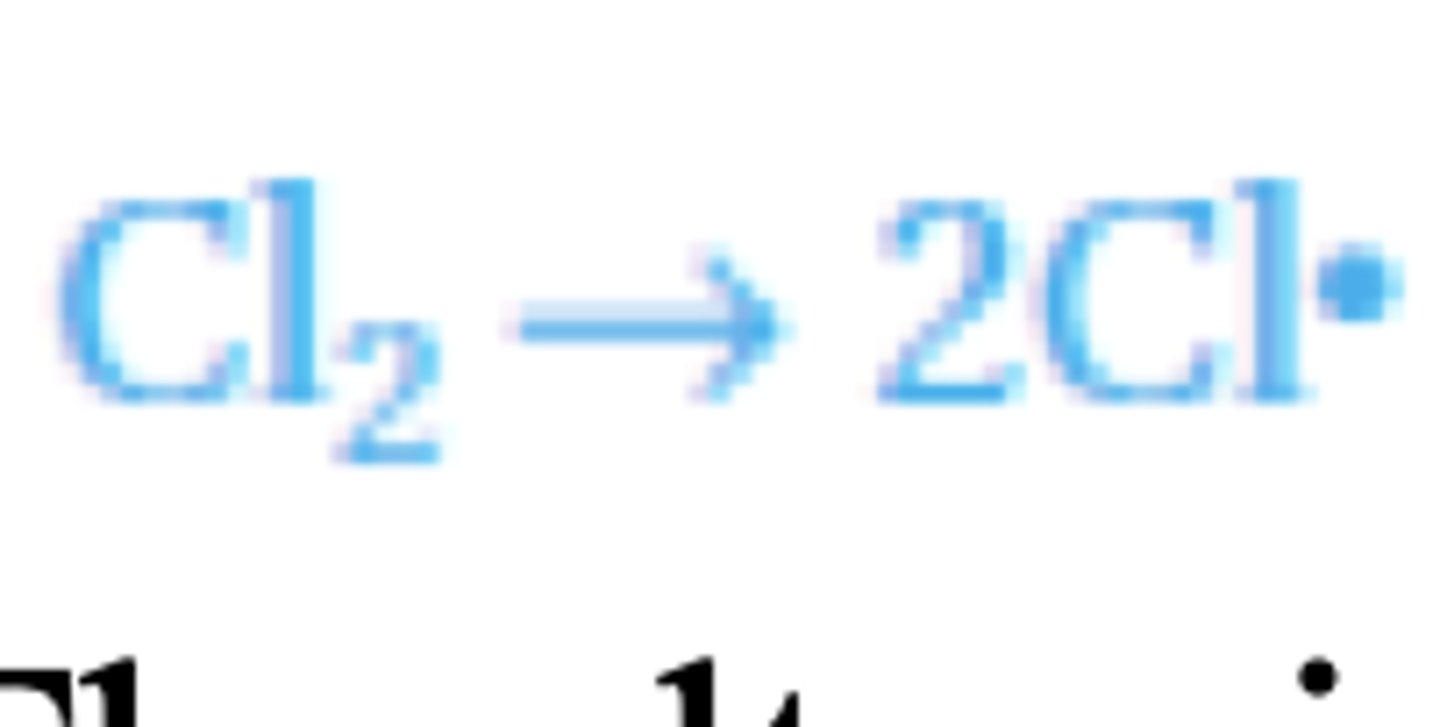
Propagation
In each step above, a radical is used and a new radical is formed, this leads to a chain
reaction as the process continues. Each step is exothermic so the chain reaction produces an
explosion.
The overall reaction is the sum of these two propagation steps.

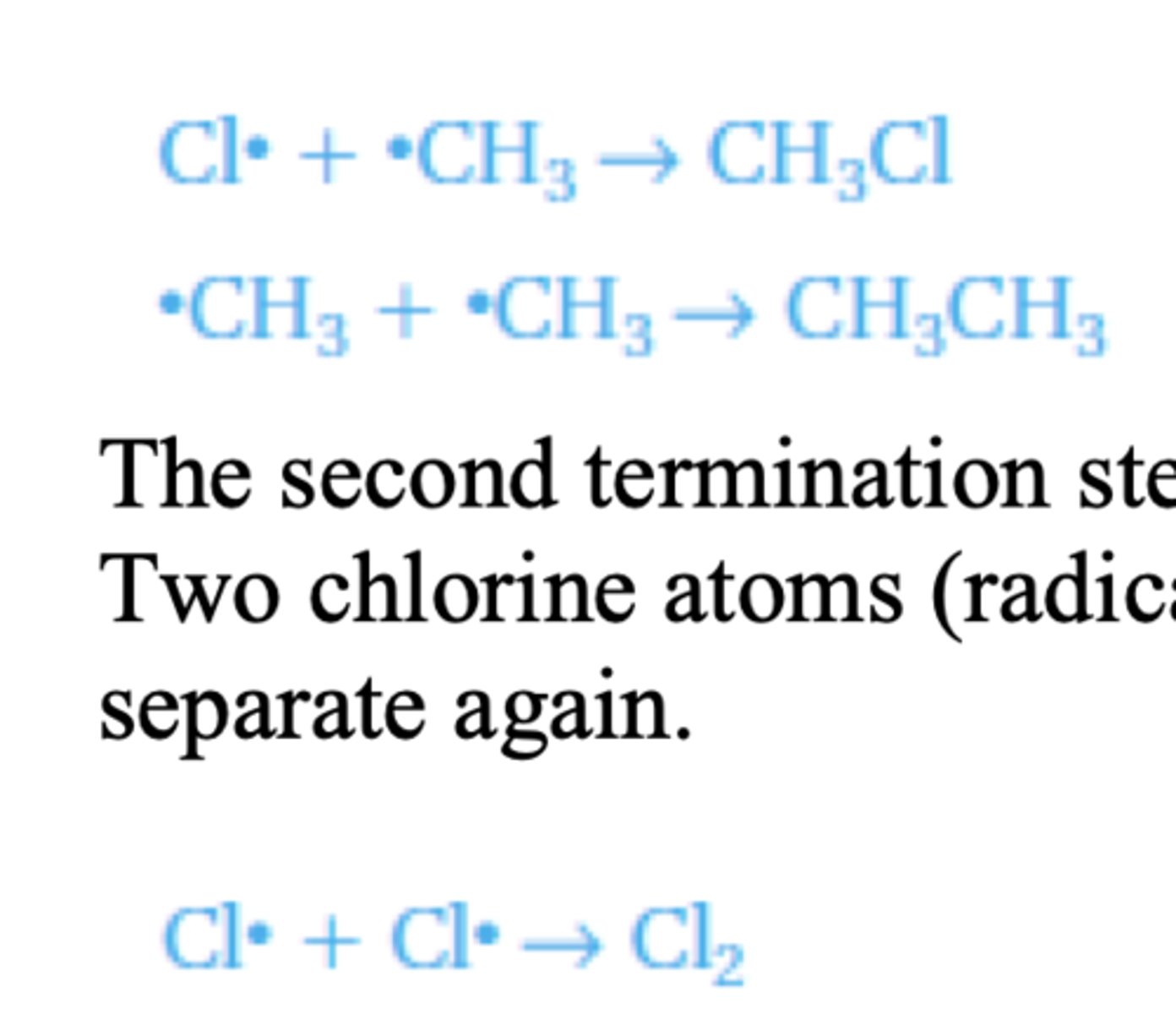
Termination
When two radicals combine (the two individual electrons form a covalent bond) they form a
stable molecule and the reaction stops. Two possible termination steps are:
The second termination step forms an impurity of ethane that can be formed in this reaction.
Two chlorine atoms (radicals) could recombine but this is not likely as they would just
separate again
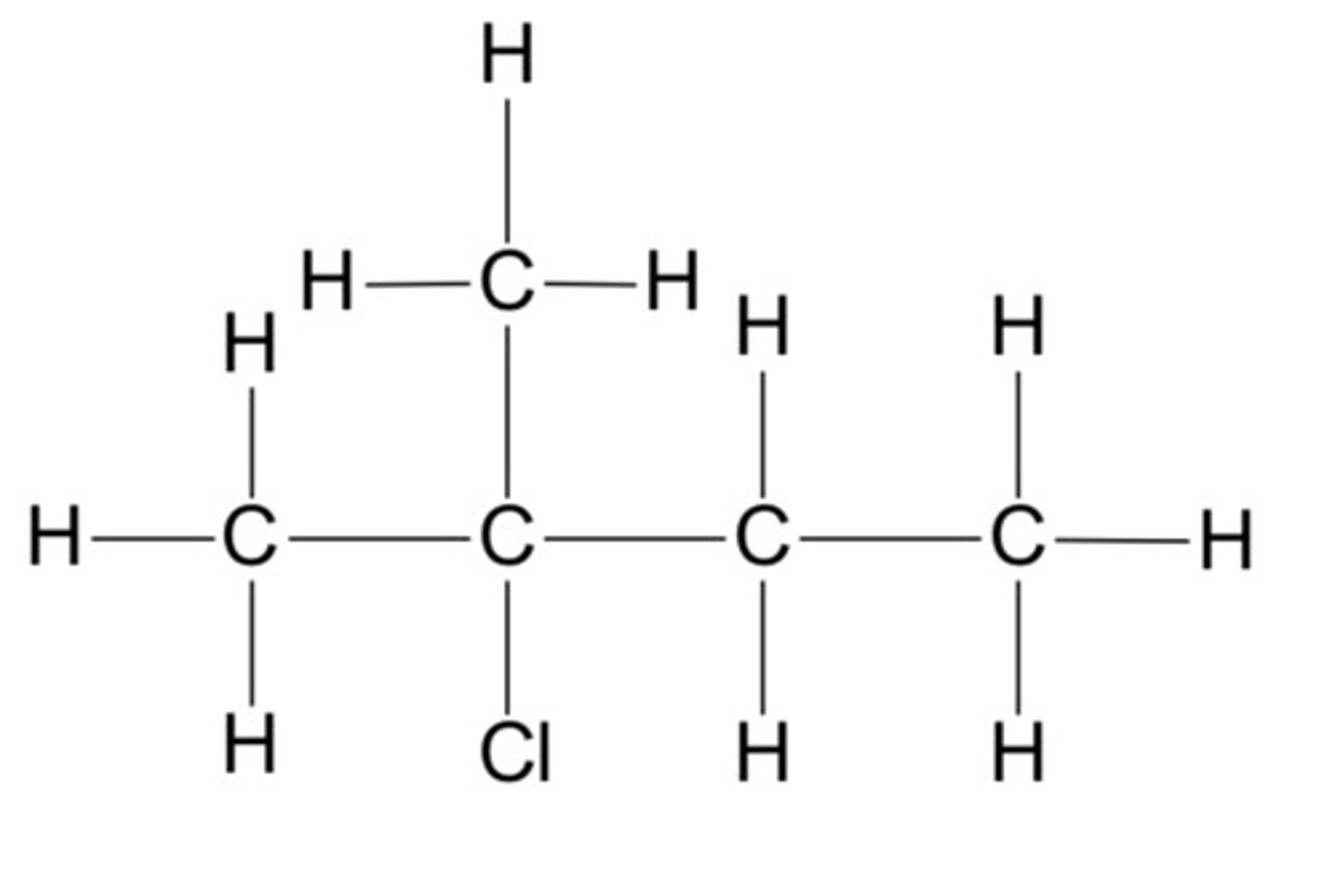
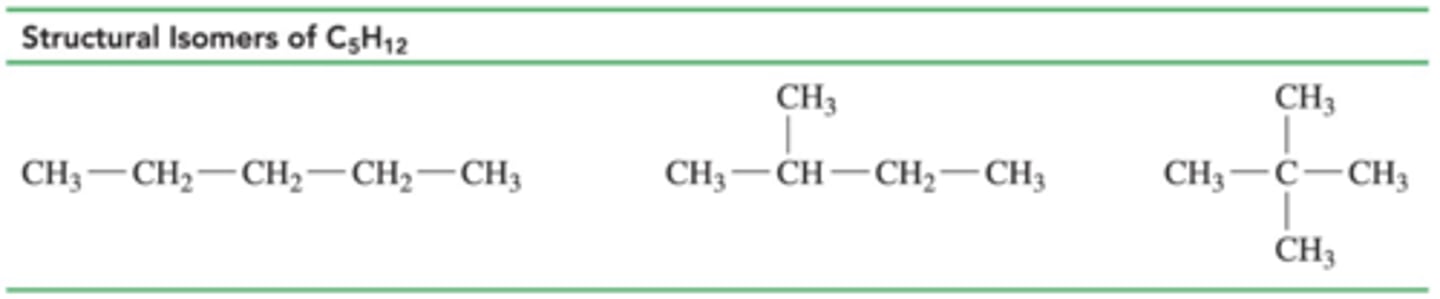
draw the possible isomers for C5H12 (6)
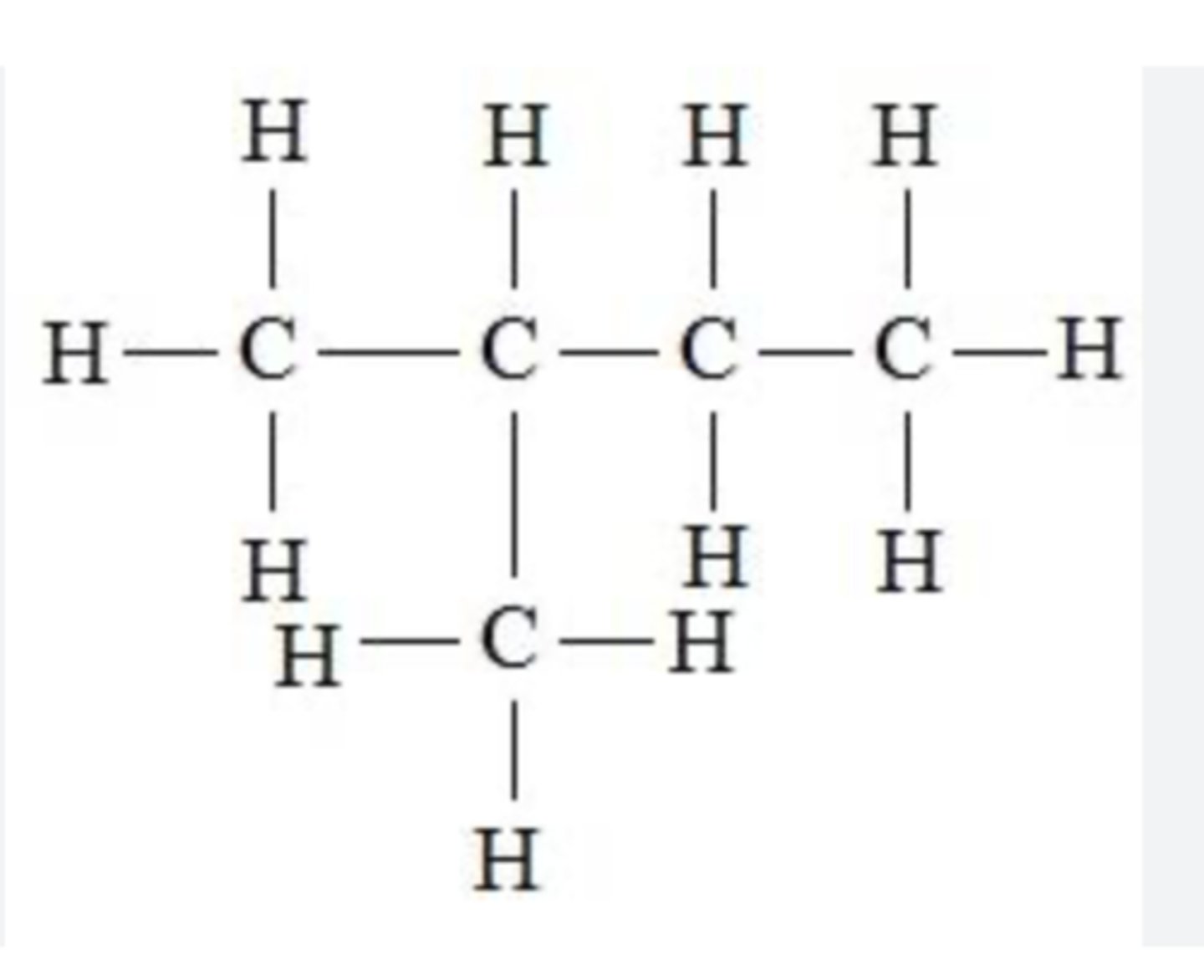
draw chain isomers for c4h10
relative ease of substitution
the ease of substitution depends on bond enthalpy
Bond Enthalpy Table
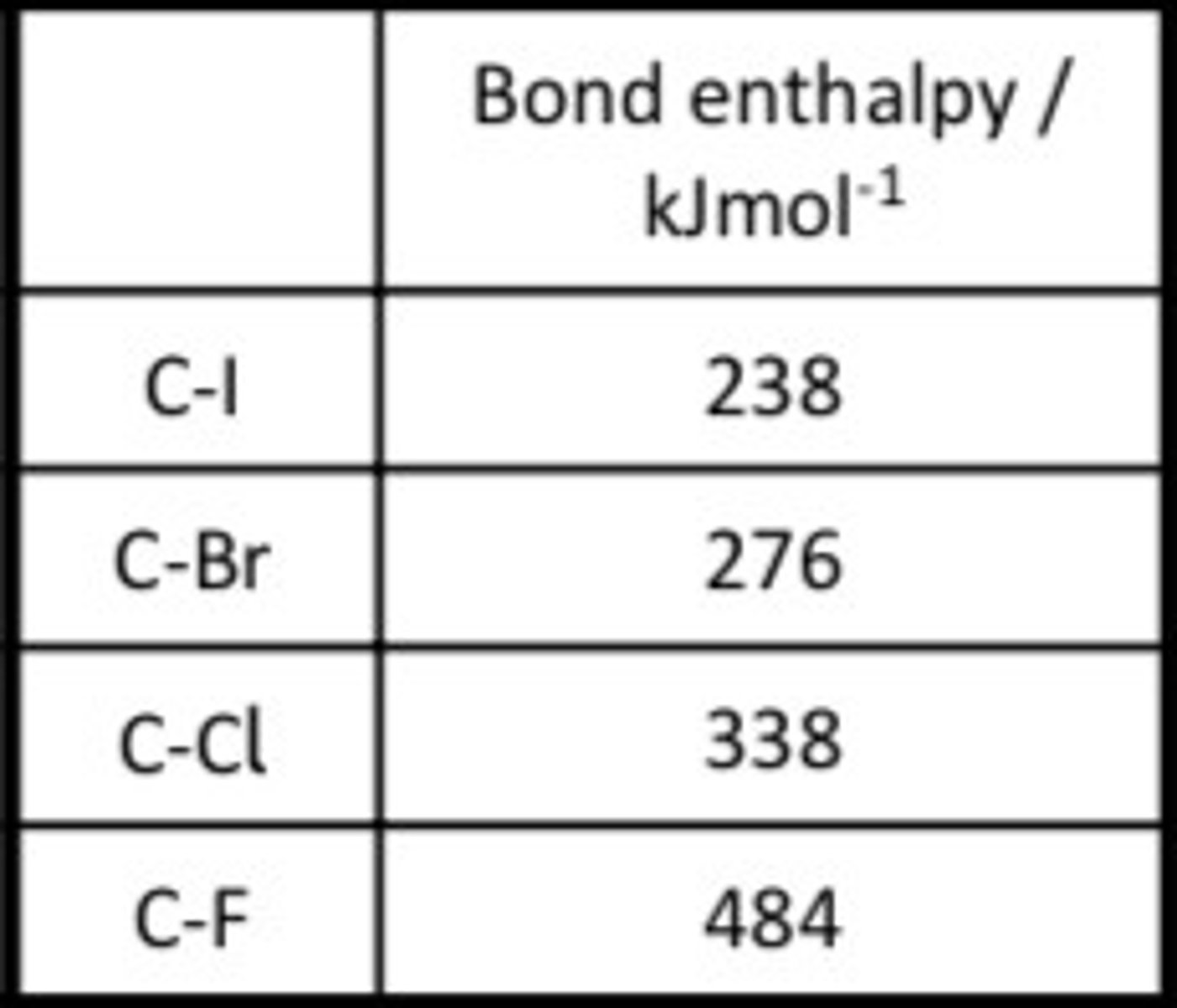
bond enthalpy and bond strength
the lower the C-X bond enthalpy, the weaker the bond, the more reactive it is, the easier it is to substitute
uses of chloromethane
Halo alkanes have been used as anaesthetics, refrigerants, aerosol propellants, insecticides
and solvents. Their use is now no longer encouraged or in some cases allowed as it has been
discovered that haloalkanes are toxic and some have negative effects on the atmosphere.
Toxicity of haloalkanes
1,1,1-trichloroethane, CH3CCl3, was used as a dry cleaning solvent to remove grease on
clothes and also as the thinner in tippex. Tetrachloromethane (carbon tetrachloride), CCl4,
was also used by dry cleaners and as a solvent. Both of these compounds are now considered
so toxic that their use is banned.
what are CFCs
A chlorofluorocarbon or CFC is a compound where all of the hydrogen atoms have been
replaced with either a chlorine or fluorine atom as in the examples below.
trichlorofluoromethane, CCl3F.
dichlorodifluoromethane, CCl2F2
why are CFCs unreactive
CFCs are very unreactive compounds due to the large amount of energy needed to break the
C-Cl or C-F bonds, (338kJmol-1 and 484kJmol-1 respectively). Their inertness and volatility
made them really useful as refrigerants and aerosol propellants. However it was eventually
discovered that their use caused a hole in the ozone layer in the upper atmosphere so their use
is now banned.
ozone
Ozone, O3, is an allotrope of oxygen. It is formed when ultraviolet radiation from the sun
breaks down oxygen molecules, O2, into two oxygen atoms (radicals). These oxygen radicals
then react with more oxygen to form ozone.
what will UV do to ozone
Ultraviolet radiation will decompose ozone into an oxygen molecule and oxygen radical. This
radical then further reacts with another ozone molecule reforming O2 molecules. This natural
cycle of reactions above results in harmful UV radiation from the sun being absorbed by the
ozone layer and hence much less of it reaches the Earth's surface.
However it was discovered, in the 1970s, that the ozone layer was much thinner over
Antarctica than expected due to the reaction of ozone with chlorine radicals in the upperatmosphere. These chlorine radicals were formed in the atmosphere as a result of the
breaking of C-Cl bonds in CFCs when exposed to UV radiation:

chlorines reaction with ozone
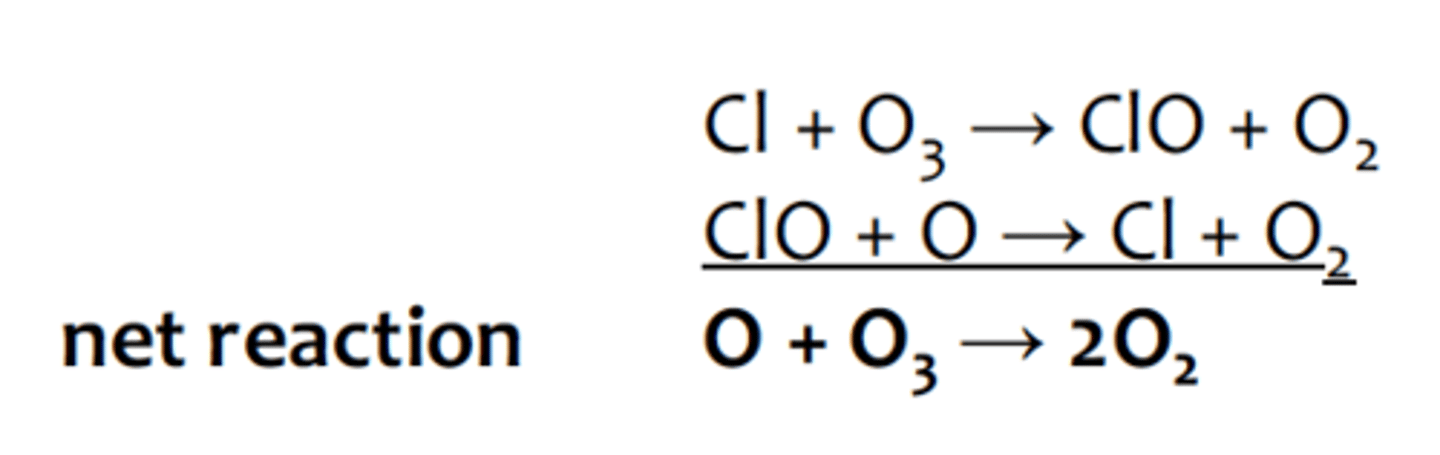
alcohol general formula
CnH2n+1OH
examples and properties of alcohols (SOME BONDING)
Methanol, ethanol, propanol, butanol
PERMANENT DIPOLE ACROS O-H BOND
VSHAPED, 104.5, water solubility
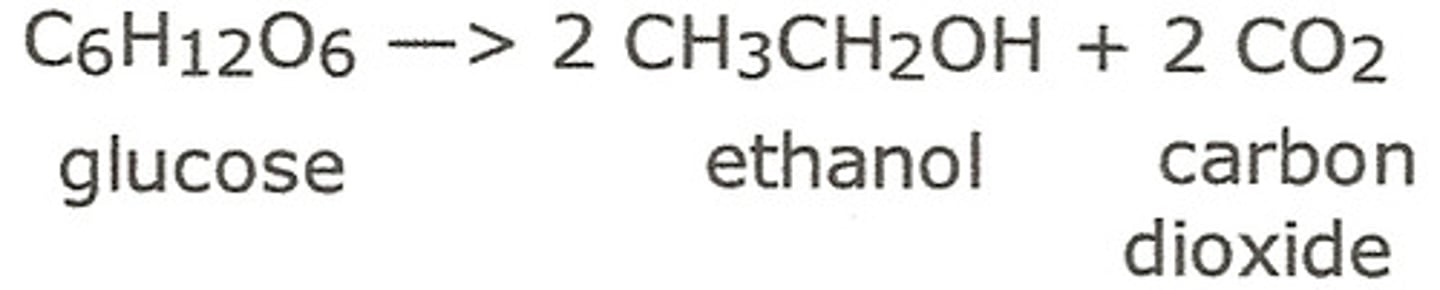
Fermentation
Ethanol is produced by fermentation which converts glucose into ethanol and carbon dioxide
by using yeast as a catalyst.
This reaction is carried out between 30-40°C (optimum temperature for the yeast) in an
oxygen free environment, anaerobic conditions, and in neutral pH.