Strengthening Mechanisms in Engineering Materials made by ai
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
67 Terms
Grain Size Control
A mechanism for controlling strength in metals by altering the size of the grains.
Phase Balance
A mechanism for controlling strength in metals that involves the distribution of different phases in the material.
Solid Solution Strengthening
A mechanism that increases the strength of a metal by adding solute atoms to the base metal.
Precipitate Strengthening
A mechanism that enhances the strength of a material by forming small particles within the metal matrix.
Work Hardening
A process that increases the strength of a metal by deforming it, which introduces dislocations.
Plastic Deformation
The process by which a metal deforms permanently due to the movement of dislocations.
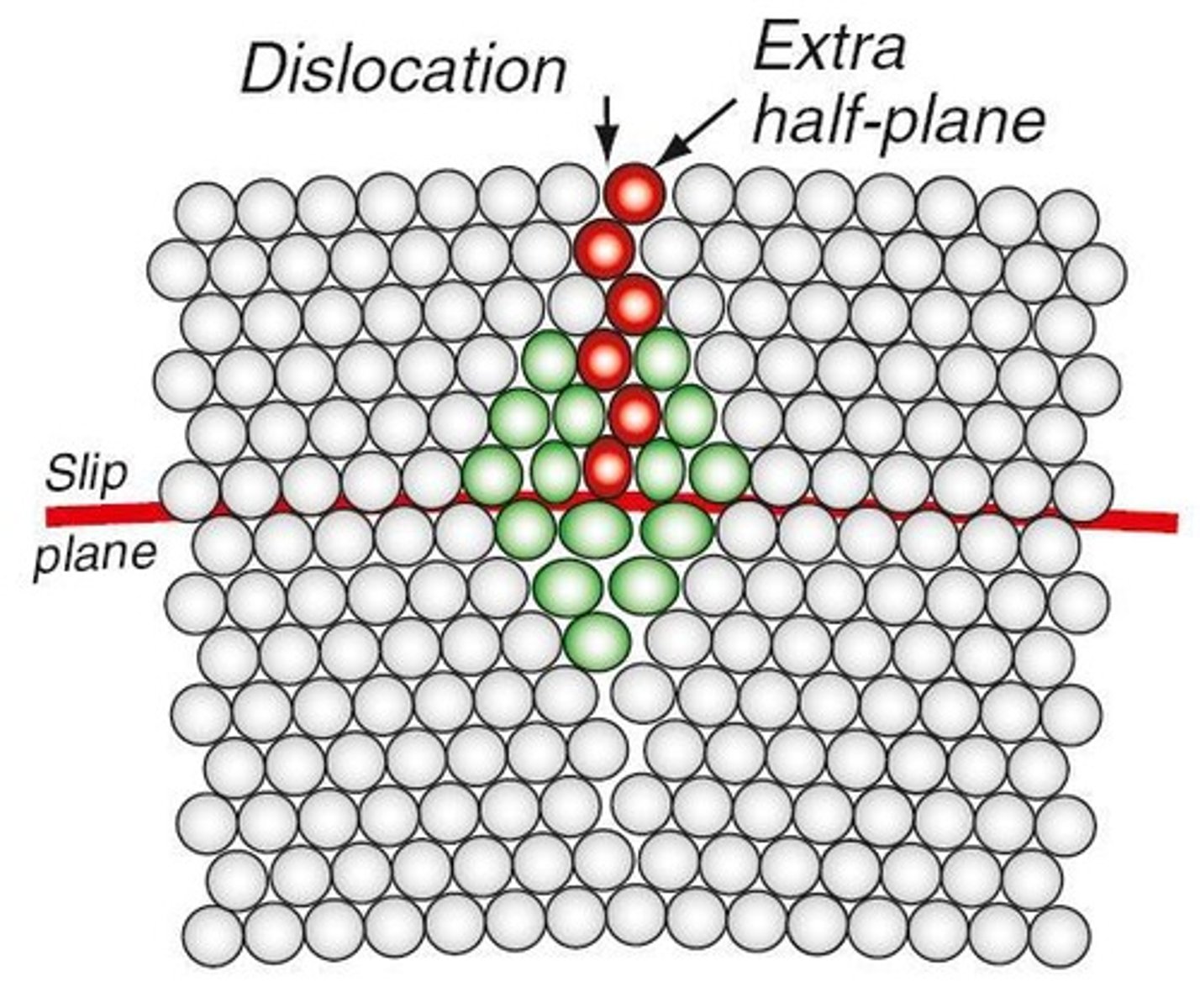
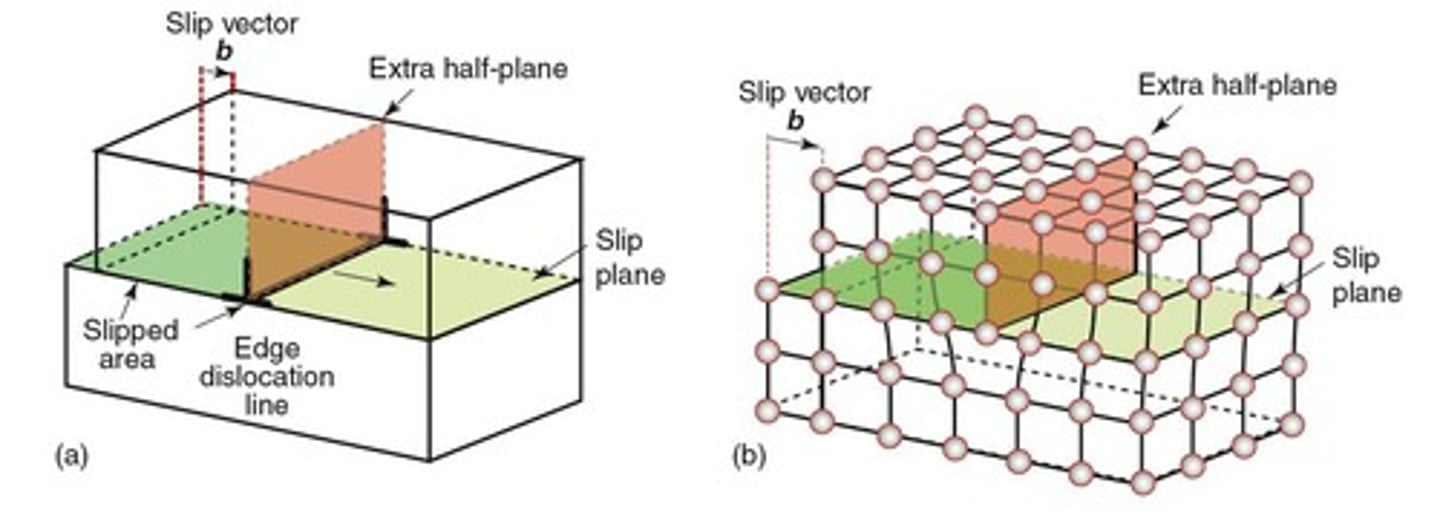
Dislocation
An extra half-plane of atoms in a crystal that distorts the lattice and affects material properties.
Grain Boundaries
The interfaces where differently oriented crystals meet in a material.
Edge Dislocation
A type of dislocation characterized by an extra half-plane of atoms that causes distortion in the crystal.
Slip Plane
The preferred plane along which dislocations move in a crystal.
Slip Direction
The preferred direction along which dislocations move within a slip plane.
FCC Crystals
Face-Centered Cubic crystals that have more slip planes than Body-Centered Cubic or Hexagonal Close-Packed crystals.
Vacancies
Defects in a crystal structure where an atom is missing from its lattice site.
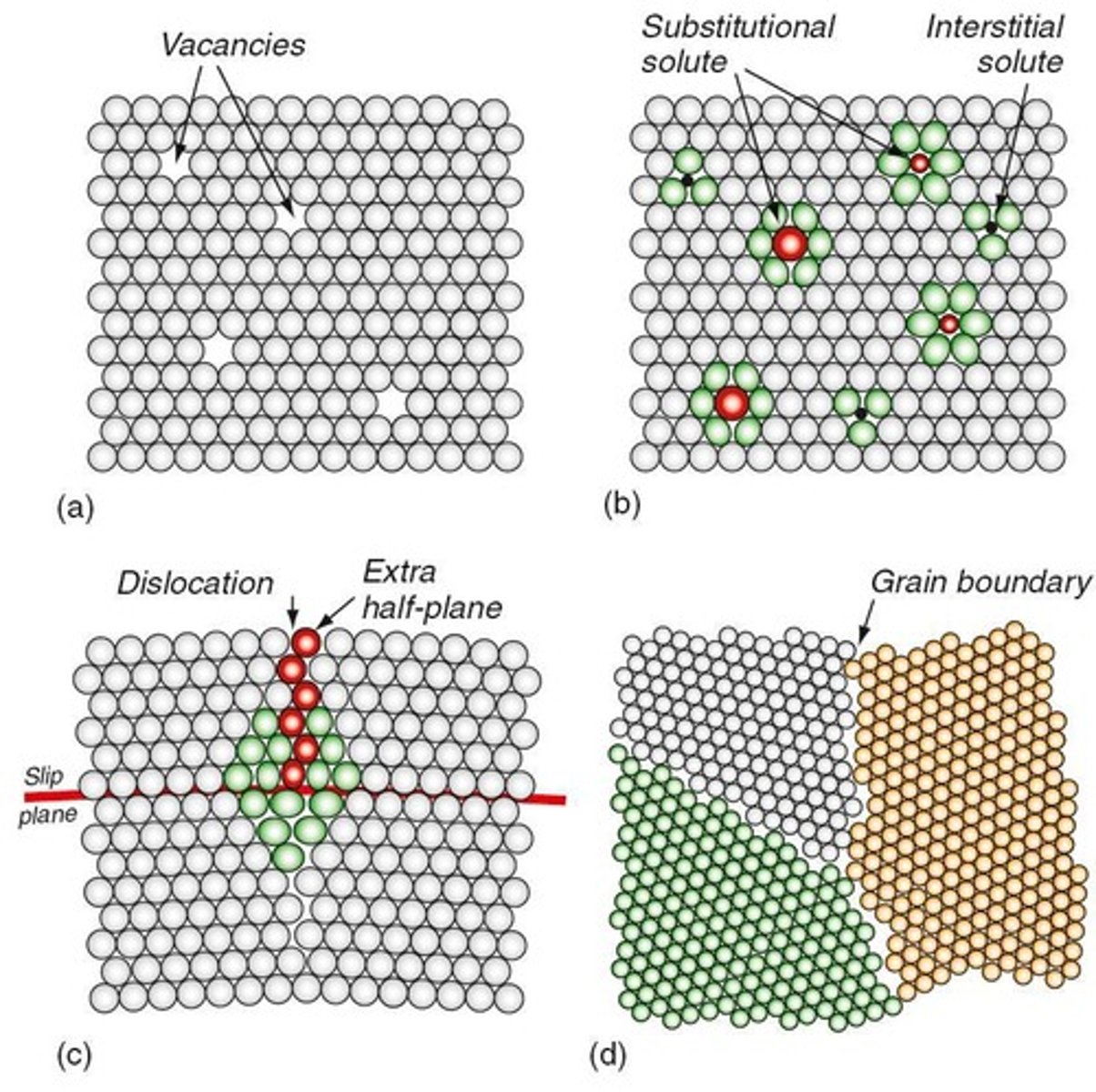
Solute Atoms
Atoms that are added to a base metal to form a solid solution, affecting its properties.
Fracture in Ceramics
The primary mode of failure in ceramics, which dominates their strength in tension.
Yield Strength
The stress at which a material begins to deform plastically.
Tensile Strength
The maximum stress a material can withstand while being stretched or pulled before failing.
Macroscopic Deformation
The overall change in shape or size of a material as a result of dislocation movement.
Dislocation Movement
The process by which dislocations move through a crystal lattice, leading to plastic deformation.
Defects in Metals
Imperfections such as vacancies, dislocations, and grain boundaries that prevent materials from achieving ideal strength.
Shear Strain
The deformation of a material that occurs when a dislocation moves across a slip plane.
Caterpillar Locomotion Analogy
A comparison used to describe dislocation motion, likening it to the movement of a caterpillar's legs.
Metal strength
Depends on the microstructure and on the alloy composition and processing history.
Grain size control
The size of the grains of our material can have an influence on the strength of the material.
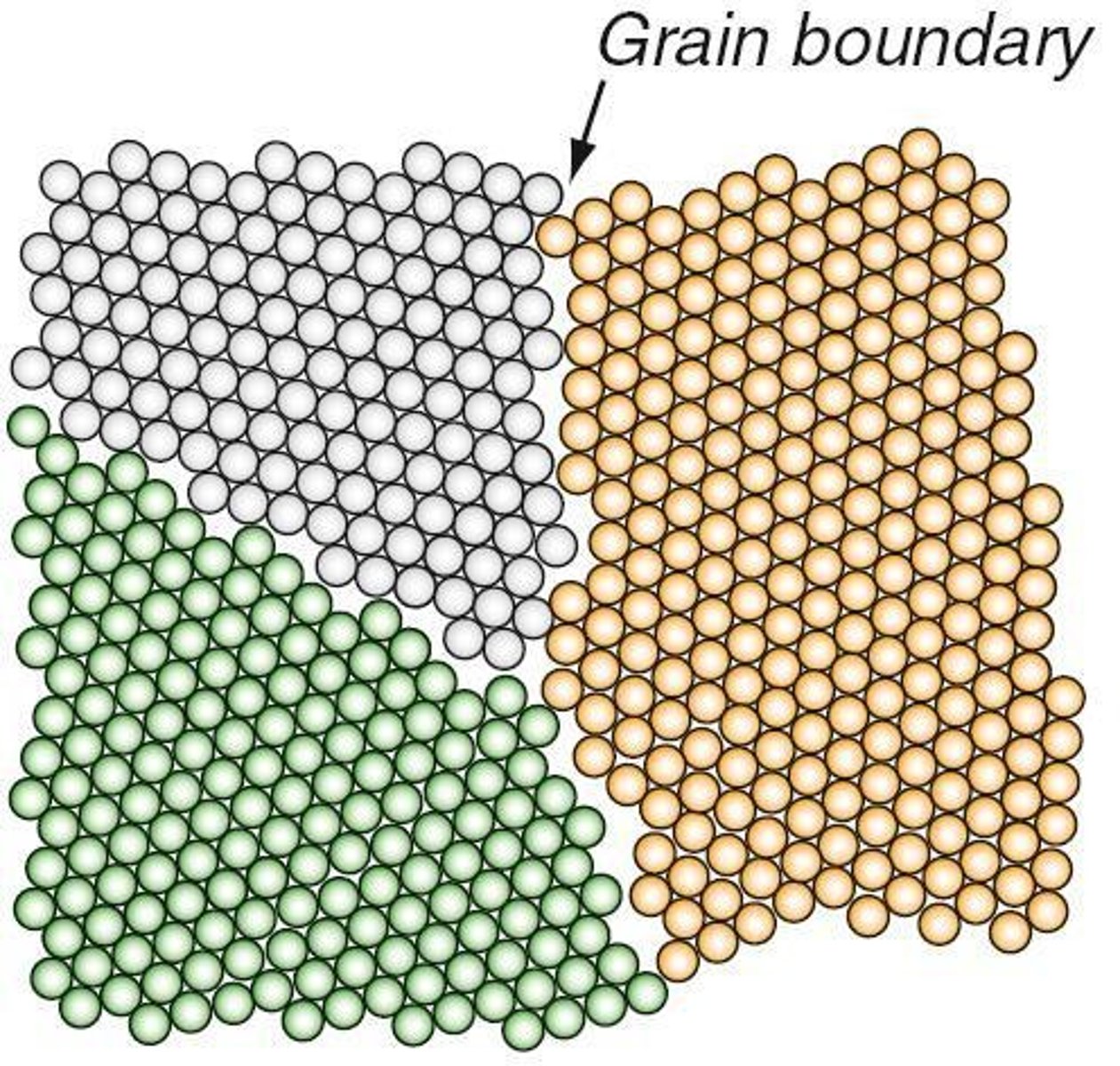
Grain
An individual crystal in a polycrystalline metal or ceramic.
Grain boundary
The interface separating two adjoining grains with different crystallographic orientations.
Grain size
The average grain diameter as determined from a random cross section.
Hall-Petch equation
σy = σ0 + kyd, where σy is yield strength, ky is a constant, and d is grain size.
Fine grained material
Stronger than one with a coarse grain structure due to a larger area of boundaries to impede dislocation motion.
Solid solution strengthening
Occurs when substitutional atoms create tensile or compressive fields, affecting dislocation motion.
FCC
Face-centered cubic structure, an example is Aluminium.
Substitutional atoms
Atoms that replace host atoms in a crystal lattice, affecting material strength.
Phase balance control
Changing the phase balance in metal alloys can change the strength level.
Carbon steels
Consist of two phases - ferrite and pearlite; increasing pearlite increases tensile strength.
Cooling paths on phase diagrams
Illustrate how different cooling rates affect the phases present in alloys.
Work hardening
A method of strengthening metals by plastic deformation.
Precipitate strengthening
A method of strengthening metals by forming small particles within the metal matrix.
Phase
A distinct state of matter in a material, characterized by uniform physical and chemical properties.
Dislocations
Defects in the crystal structure that affect the mechanical properties of materials.
Tensile fields
Fields created by smaller substitutional atoms that can lead to strengthening.
Compressive fields
Fields created by larger substitutional atoms that can lead to strengthening.
Strain energy
The energy stored in a material due to deformation.
Alloying element
An element added to a base metal to improve its properties.
Limit of solid solution strengthening
The maximum amount of alloying element that can be added before it no longer goes into solution.
Pearlite
A microstructure in steel that increases tensile strength when its amount is increased by raising the carbon content.
Phase balance control
The method of adjusting the proportions of different phases in a metal alloy to change its properties.
Pure metals
Metals that consist of only one phase.
Metal alloys
Materials that can consist of more than one phase.
α-brass
A type of brass that consists of 70% copper and 30% zinc.
α + β brass
A type of brass that consists of 60% copper and 40% zinc.
Ferrite
One of the two phases in carbon steels.
Precipitate strengthening
A strengthening mechanism where discrete particles form in an alloy that enhance its strength.
CuAl2 particles
Particles formed when copper is added to aluminum alloys, contributing to strengthening.
TiN particles
Particles formed when nitrogen is added to steels containing small amounts of titanium, contributing to strengthening.
Dislocations
Defects in a crystal structure that can move and are responsible for plastic deformation.
Work hardening
The process by which a material becomes stronger and harder through plastic deformation.
Ductility
The ability of a material to deform plastically before fracture.
Dislocation density
The number of dislocations in a given volume of material, which affects its strength.
Copper water pipes
Pipes sold in various conditions (soft, half hard, fully hard) that demonstrate the effects of work hardening.
Car body panel
A component that work hardens during press forming, increasing its yield strength and dent resistance.
Heat-treatable Al alloys
Aluminum alloys that can be strengthened through age hardening.
Carbon & alloy steels
Steels that can be strengthened through quenching and tempering.
Grain size control
A method of controlling strength in metals by adjusting the size of grains in the microstructure.
Solid solution strengthening
A strengthening mechanism that occurs when solute atoms are added to a solvent metal.
Normalised steel
Steel that has undergone a process to achieve a uniform microstructure of ferrite and pearlite.
As-quenched steel
Steel that has been rapidly cooled to form martensite, a hard microstructure.
Electrical properties of copper alloys
Properties that can be influenced by composition and processing trajectories in copper alloys.