Ch.1 Atomic Structure
1/59
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
What is Chemistry?
Study of Matter
What is Matter?
Anything that occupies space (volume) and has mass
Everything around us (solid, liquid, gas)
What is the fundamental unit of Matter?
An atom
What is an Atom?
fundamental unit of matter
smallest unit of matter to keep its identity
smallest identifiable unit of an element
from ancient Greek word: Atomos: meaning “uncuttable” or “indivisible” (cannot be further divided)
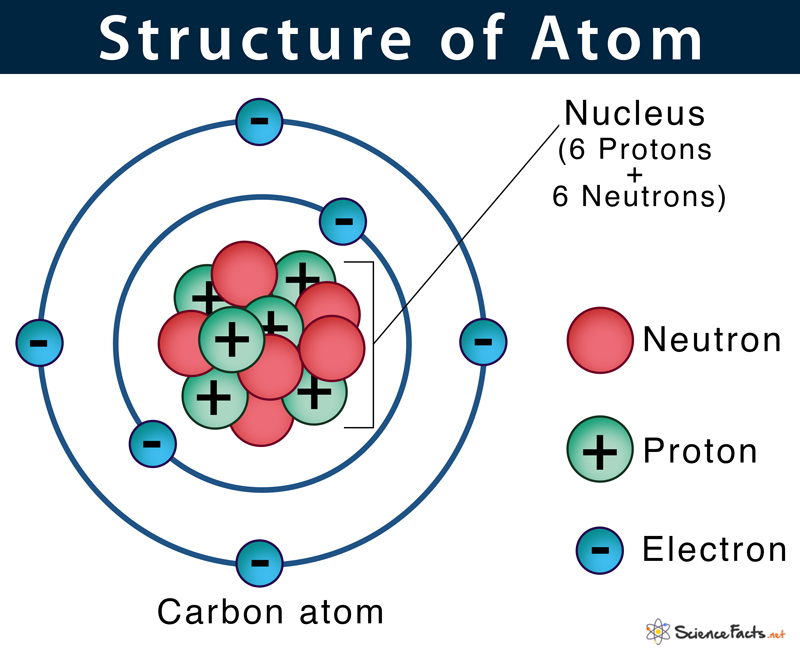
What is an atom composed of?
subatomic particles: protons, neutrons, electrons
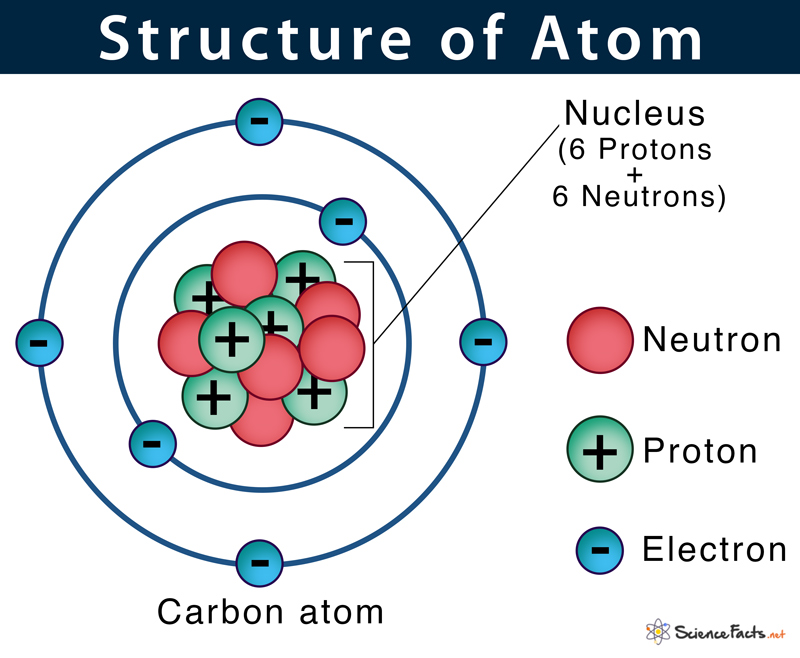
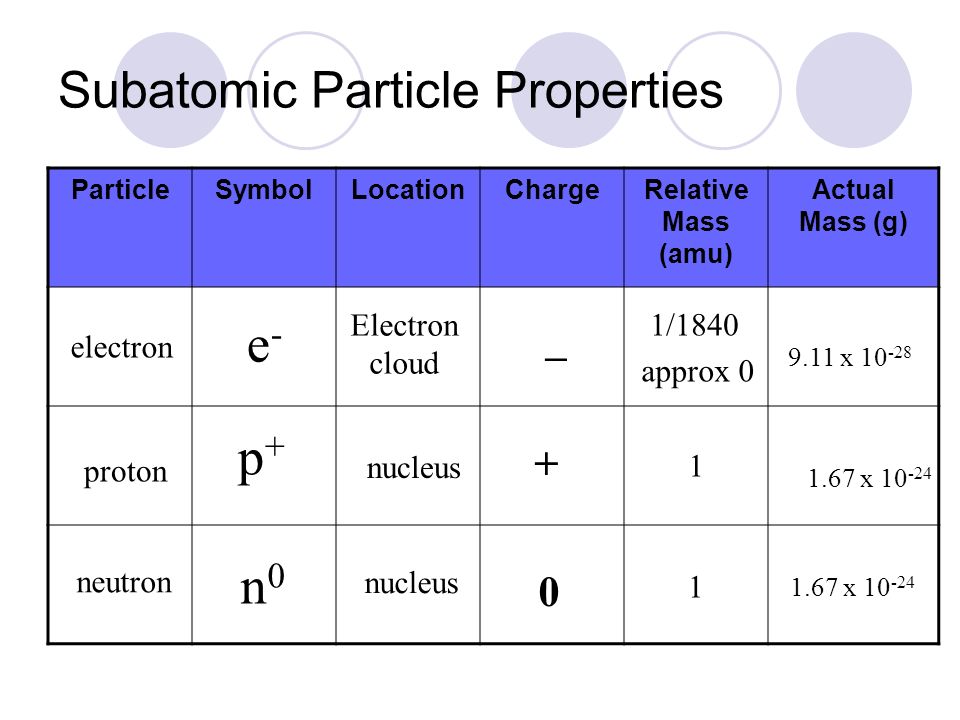
Proton (p+)
Found in the nucleus of an atom
Have a positive electrical charge (+1)
Mass of 1 amu (atomic mass unit)
The atomic number (Z) of an element is equal to the number of protons found in an atom of an element
Neutron
are neutral (no charge)
mass is slightly larger than protons
found in the nucleus of an atom
mass of 1 amu
What is the nucleus of an atom composed of?
Protons and Neutrons
What makes up the mass of an atom?
Protons and Neutrons make up almost the entire mass of an atom
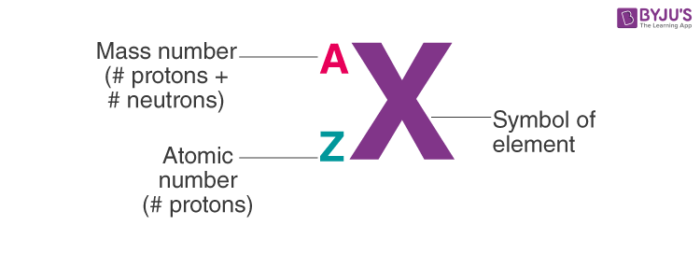
Mass Number
represented by the letter (A)
sum of protons & neutrons
Atomic Number
equal to the number of protons
represented by the letter (Z)
unique identifier of each element because elements are defined by the number of protons they contain
What is an Isotope?
Atoms that have the same number of protons (atomic number) but different number of neutrons (mass number)
Almost all elements in nature exist as two or more isotopes
What notation is used to show A and Z?
Where are the electrons found?
move through the space surrounding the nucleus
associated with varying levels of energy
Electron (e-)
found in space surrounding nucleus
-1 electromagnetic charge (a charge equal in magnitude to that of a proton but with opposite sign (-))
mass of an e- is ~1/2000 of a proton
Why are the electrostatic forces between protons and electrons far greater than gravitational force?
Because subatomic particles’ masses are so small
Electrons levels of energy
Electron shells (this concept came from Bohr which was later discovered to be an electron cloud)
Electrons move around nucleus at varying distances, which corresponds to varying levels of electrical potential energy
electrons closer to nucleus at lower energy levels
electrons further out have higher energy
Electrons furthest from nucleus interact strongest with surrounding environment because weakest interaction with nucleus
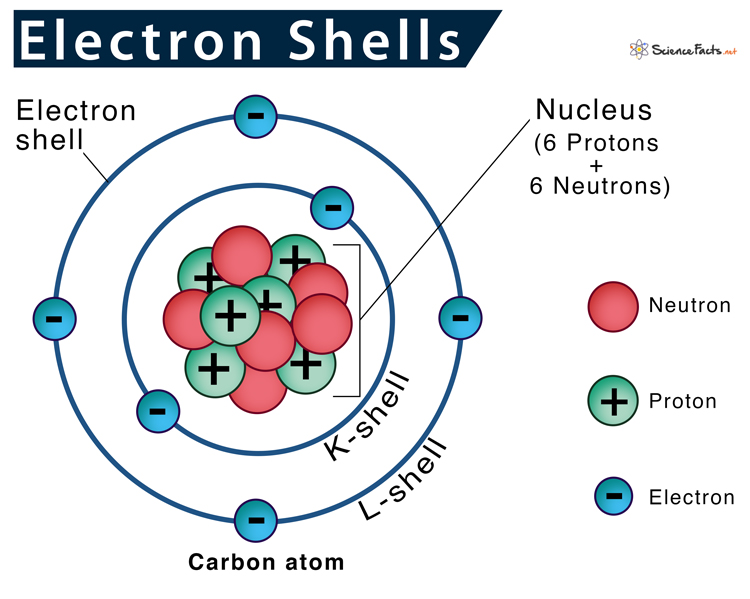
What are Valence electrons?
electrons FURTHEST from nucleus
much more likely to become involved in bonds with other atoms because they experience least electrostatic pull from their own nucleus
can determine an atoms chemical properties
What is the neutral state of an atom?
equal number of protons and electrons
What is a CATION?
an atom that has lost an electron and become POSITIVELY charged
cats have paws PAW-sitive
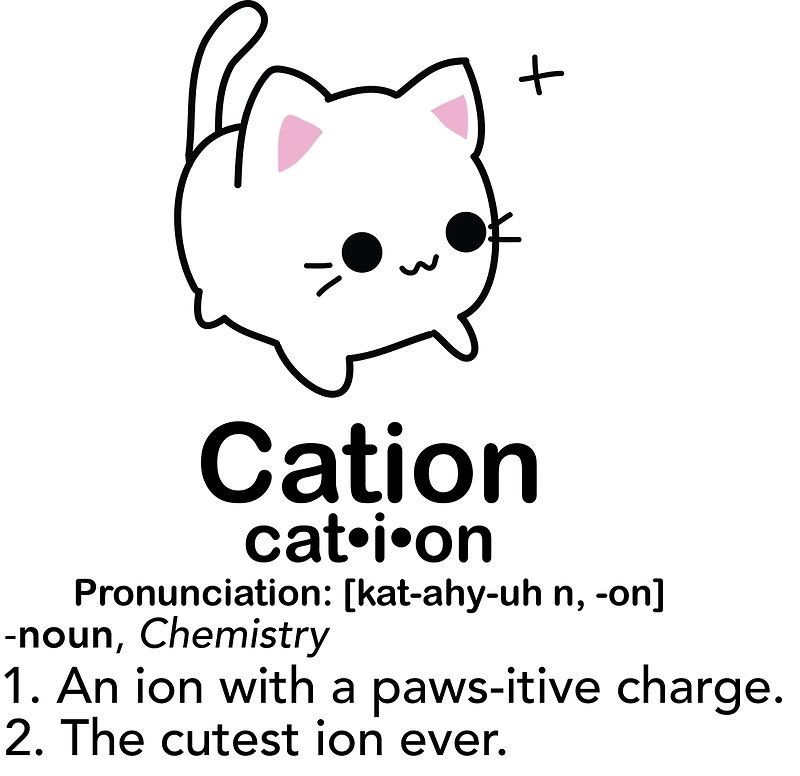
What is an ANION?
an atom that has gained an electron and become NEGATIVELY charged
Properties of subatomic particles
Define atomic mass unit (amu)
1/12th the mass of a Carbon-12 atom
1.66 X 10^-24g
Carbon-12 has 6 protons and 6 neutrons so 1 amu is equal to the ~mass of a proton or a neutron (proton and neutrons almost the same mass, very smallll difference)
Atomic Mass
Slightly less than the mass of protons plus mass of neutrons
Do chemical properties of isotopes vary?
isotopes have the same number of protons and electrons, so they generally exhibit similar chemical properties
Atomic Weight
in nature almost all elements exist as 2 or more isotopes
isotopes are usually present in same proportions in any sample of naturally occurring element
the weighted average of these different isotopes is referred to as atomic weight and is the number reported on the periodic table
Is the atomic mass of an isotope exactly equal to an element’s atomic weight?
No, e.g. Bromine is listed on periodic table as 79.9 amu, which is the average weight of the naturally occurring Bromine isotopes (79, 81), which occur in almost equal proportions. No bromine atom with an actual mass of 79.9 amu (naturally occurring Bromine atoms have a mass of 79 amu or 81 amu)
How to calculate atomic weight of given isotopes?
[(Naturally Occurring %/100) x (Atomic mass)] + [(Naturally Occurring %/100) x (Atomic mass)] + …
![<p>[(Naturally Occurring %/100) x (Atomic mass)] + [(Naturally Occurring %/100) x (Atomic mass)] + …</p>](https://knowt-user-attachments.s3.amazonaws.com/5516692f-ac72-4008-8209-eca384926e43.jpg)
What does atomic weight represent?
weighted average of naturally occurring isotopes of an element
mass of 1 mole of an element in grams
What is a mole?
a mole is a number of “things” (atoms, ions, molecules)
that number is 6.02 ×10²³ (Avogadro’s number)
(like a dozen is 12 number of “things”)
mass of one mole of an element = atomic weight in grams
What is Avogadro’s number?
6.02 x 10²³
Overview of History of Chemistry
500 BCE Leucippus and Democritus propose matter is made up of indivisible particles called “atomos”
1789 Antoine Lavoisier: Law of Conservation of Mass
1794 Joseph Proust: Law of Constant Proportions
1803 John Dalton: Atomic Theory
1897 Discovery of Electron: Cathode Ray Experiment (JJ Thomson)
1898 Discovery of Proton: Anode Ray Experiment (E Goldstein)
1904 JJ Thomson: Plum Pudding Model
1911 Ernest Rutherford: Nuclear Model
1913 Niels Bohr: Planetary Model (Bohr Model)
1926 Erwin Schrodinger: Quantum Model
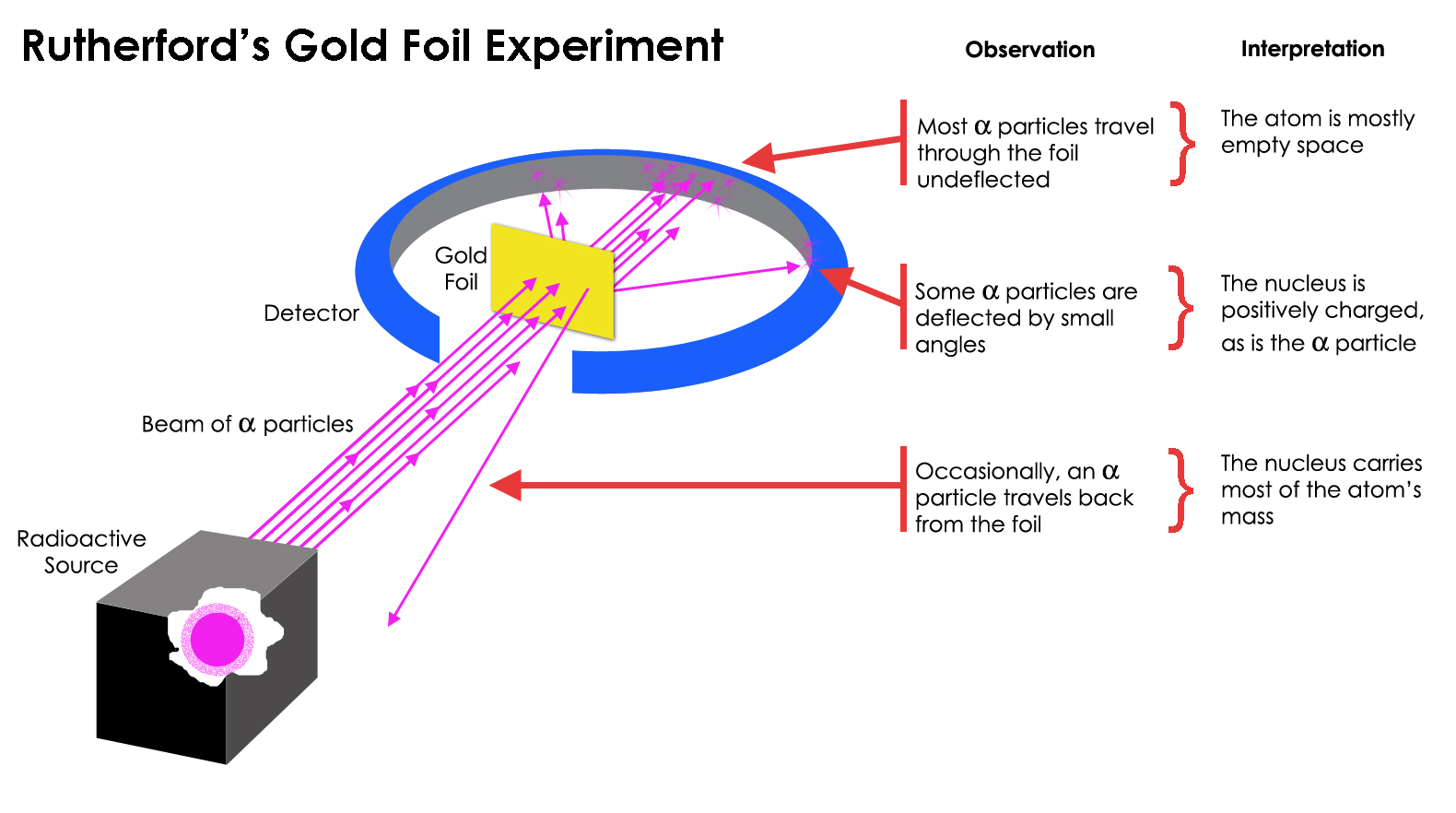
Ernest Rutherford (1910)
Gold Foil Experiment
Concluded:
an atom has a dense positively charged center
most of the space in atom is empty
electrons orbit or revolve around dense positive center (but Rutherford could not explain how, classical physics states that charged particles (e-) during acceleration in circular motion would continuously lose energy and ultimately spiral and collapse into nucleus, Bohr was able to explain and define this using the work of Planck)
Max Planck (1900)
energy is absorbed or emitted in a discontinuous manner
as discrete wave packets (aka quanta/ photons)
energy of a photon is proportional to frequency (f)
E=hf
h is Planck’s constant and equal to 6.626×10-34 Js
J= Joules (unit of energy), s = second
synonyms: distinct/discrete/defined/quantized
Light (Electromagnetic Radiation)
Dual Nature
Wave nature: when light moves through space and time
Particle nature (photon): when light interacts with matter
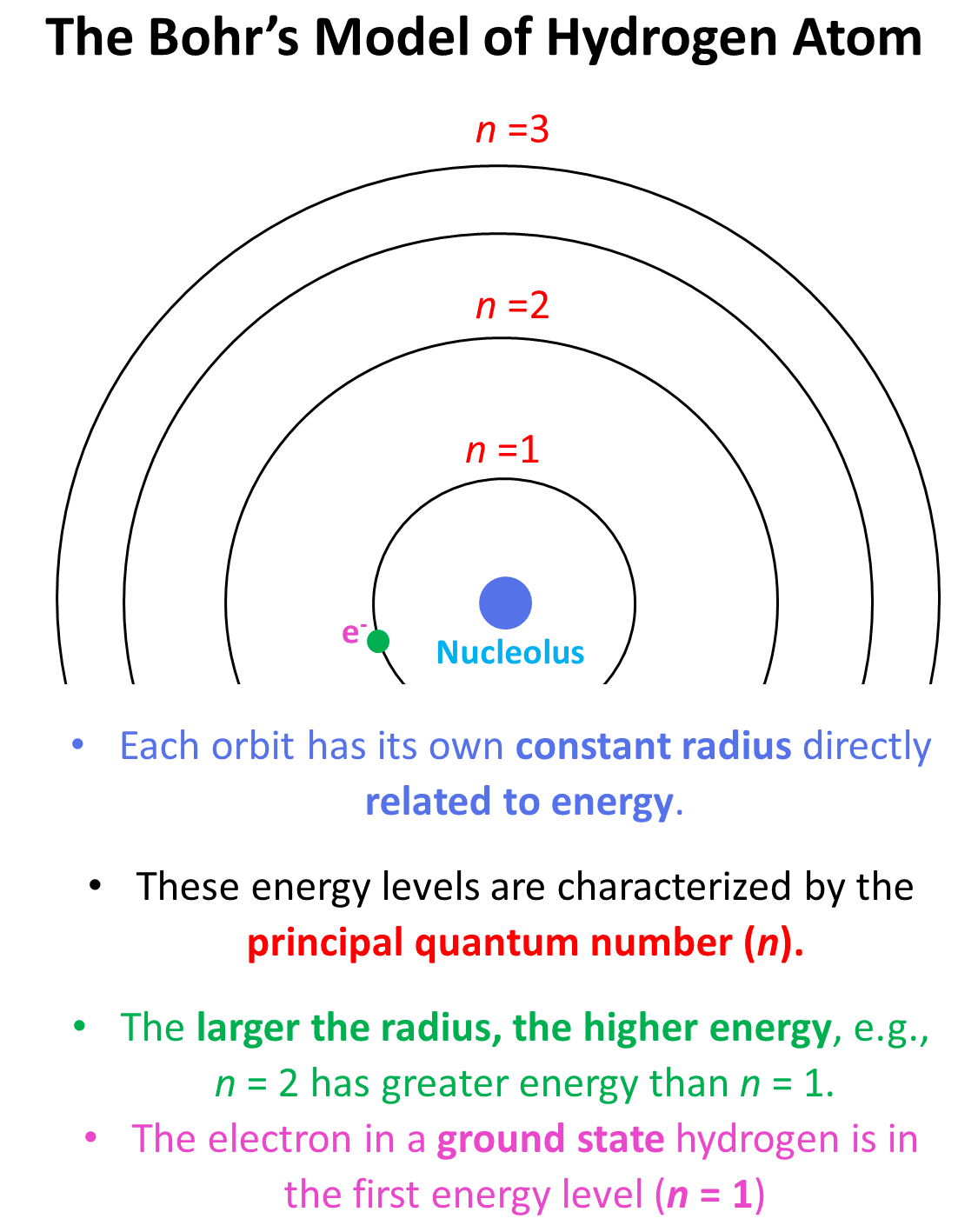
Niels Bohr explanation of how an electron can orbit nucleus
stated that electrons orbit could not take on any value for radius but rather the radii could only take on some distinct/discrete values (energy shells n =1,2,3…)
Energy of an electron is directly proportional to n
E = -RH/n² (n=principal quantum number) RH= 2.18X10-18 J/electron (Rydberg unit of energy)
as n becomes larger the overall value becomes less negative (moving closer to 0), thus energy increases the further you move out from nucleus
Bohr Model
Described the model of Hydrogen atom
Dense positive core with 1 proton, around which 1 electron revolved in a distinct/discrete/defined pathway (orbit)
orbit = energy of electron, which is directly proportional to principle quantum number (n)
Bohr model useful for explaining atomic emission and absorption spectra of atoms
Ground State of an atom
Lowest energy state of an atom
when all the electrons in an atom are in there lowest possible energy levels
Very stable (normal state)
Excited State of an atom
When at least one electron of an atom has moved to a higher energy state or “jumped” up to another orbital
Very Unstable, in this state for a very brief period
How does an atom go in Excited State?
If an electron absorbs energy exactly equal to the difference between one orbit and another, it jumps to an orbit with a larger radius (more energy)
Limitation of Bohr Model
Electrons aren’t in fixed pathways (orbits) but rather localized in certain regions of space (electron cloud later discovered)
Can only explain atomic spectrum of Hydrogen atom and 1 electron systems e.g. He+, Li2+
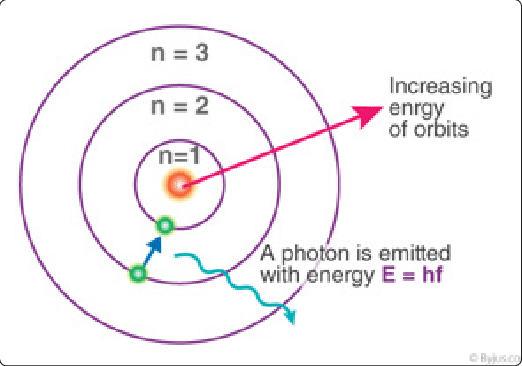
Atomic Emission Spectra
e- always want to remain in lowest energy level
when electron returns back to ground state from excited state, energy is emitted in form of radiation (Electromagnetic Waves, fluorescence)
The energy of emitted photon equals to the difference in energy of electron between the higher energy orbit (initial state) and ground state (final state)
ΔE= Ef - Ei= -RH (1/nf2 - 1/ni2)
The wavelength of the photon emitted (color) will be characteristic of the specific energy transition it undergoes
e.g. from n=2 to n=1 or n=3 to n=1 or n=3 to n=2 or n=4 to n=2 or etc.
Atomic emission spectrum is like a fingerprint for an element
because each element can have it’s electron excited to a different set of distinct energy levels
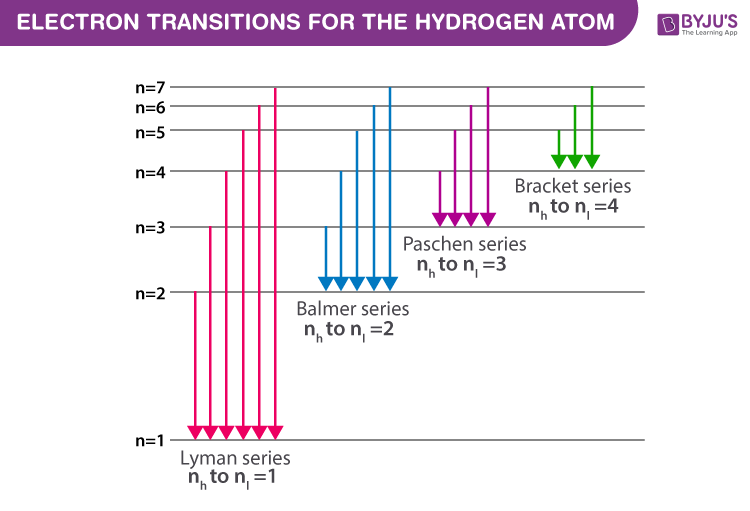
Emission Lines
emission lines named after scientists who discovered them
Lyman Series: an electron returning to ground state n=1
Balmer Series: an electron returning to ground state n=2
Paschen Series: an electron returning to ground state n=3
Difference between Bohr model and modern quantum mechanical model?
Bohr postulated electrons follow a clearly defined circular pathway around nucleus, but that’s not the case. Now it’s understood that electrons move rapidly and are localized within regions of space around nucleus: Orbitals
Bohr model did not take into account the repulsion forces within atoms with multiple electrons
Heisenberg Uncertainty Principle
It is impossible to pinpoint where an electron is
It is impossible to exactly determine with full confidence, with perfect accuracy, the momentum and the position of an electron at the same time
The higher the certainty of momentum of an electron higher the uncertainty of it’s position and vice versa
For position the electron would have to stop (no momentum)
If we want to assess the momentum then electron has to be moving thus the position changes (very very very very fasttt)
Orbitals
A region of space around the nucleus defined by the probability of finding an electron in that region of space
Quantum Numbers
Four quantum number (n, l, ml, ms)
Give information about electron (like an address)
Pauli Exclusion Principle
no two electrons in a given atom can have same set of four quantum numbers
i.e. no two electrons in a given atoms can have the same address (makes sense)
Principal Quantum Number
first quantum number denoted by letter n
n can be any positive integer
larger the value of n, the larger the radius and energy level of the electron’s shell
gives information about the size of electron’s shell
Equation for maximum number of e- within a shell?
2n²
n = principal quantum number
*electrons do not travel in clearly defined orbits, this just simplifies visual representation of the motion of an e-
Is the energy difference between shells further away from nucleus (e.g. n=5 and n=6) less or more than the energy difference between shells closer to nucleus (e.g. n=1 and n=2)
less, can plug into delta E to observe
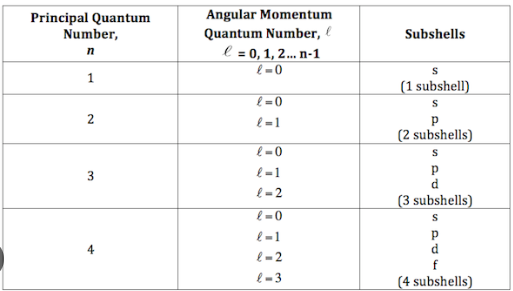
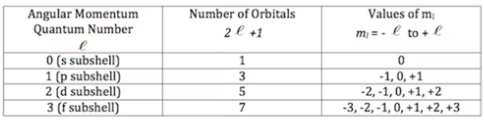
Azimuthal (angular momentum) Quantum Number
second quantum number denoted by letter l
l is 0 to (n-1) (n = principal quantum number)
gives information about the shape and number of subshells
n also gives information about number of subshells
e.g. n=2, means 2 subshells, the subshells being l=0,1
energy of subshells increases as l increases but energy of subshells from different principal energy levels can overlap
4s<3d
Equation for energy of subshell
n+l
if n+l same value, then the subshell with the larger principal number has more energy
e.g. 2p (2+1 =3) and 3s (3+0 =3); 3s has higher energy than 2p
Spectroscopic Notation
e.g. 4d (4 = principal quantum number (n), d = azimuthal quantum number (l=2))
l | subshell | shape |
|---|---|---|
0 | s | sphere |
1 | p | dumbbell |
2 | d | double dumbbell or 4 leaf clover |
3 | f | complicated |
Maximum number of electrons a given subshell can hold?
4l+2
subshell (l) | maximum # of electrons |
|---|---|
0 (s) | 2 |
1 (p) | 6 |
2 (d) | 10 |
3 (f) | 14 |
Magnetic Quantum Number
third quantum number denoted by ml
gives information about number of orbitals and the particular orbital within a subshell where the e- is most likely to be
ml = integers from -l to +l
To calculate number of orbitals per subshell, can count the values of ml or equation 2l+1
s=1 orbital
p=3 orbitals
d=5 orbitals
f=7 orbitals
Spin Quantum Number
Relating l, ml and ms
4l+2 = maximum number of electrons per subshell
p=1 → 4l+2 → 4(1)+2= maximum 6e-
each orbital can have maximum 2 electrons with opposite spins
subshell p has 3 orbitals, 3×2= maximum 6e-
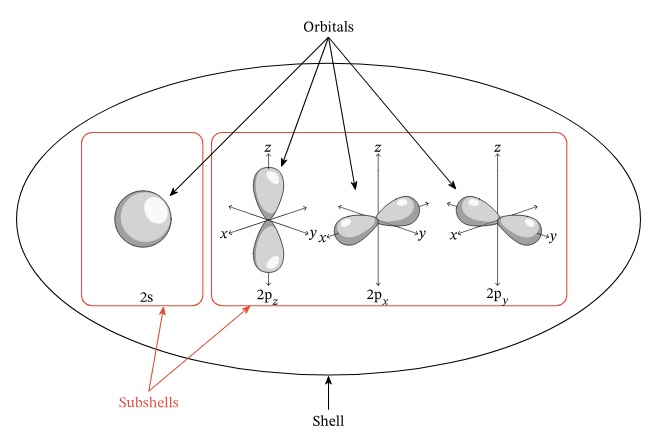
Visualizing Shells, Subshells and Orbitals
n → shell
l → subshell
s subshell → 1 orbital (s orbital)
p subshell → 3 orbitals (pz, px, py orbital)
d subshell → 5 orbitals
f subshell → 7 orbitals