3.2.1 - Fractional Distillation Of Crude Oil
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
Alkanes
Saturated (single Carbon bonds) hydrocarbon (only made up of carbon and hydrogen)
General Formula : CₙH₂ₙ₊₂
Comes from the fractionation of crude oil
They aren’t polar and only weak van der waals forces occur as carbon and hydrogen have similar electronegativity
BP of alkane increases with chain length due to a larger SA and ↑ no. Of e- ∴ stronger van der waals forces
The more branched the alkane is the SA ↓ ∴ there are weaker van der waals forces resulting in ↓ BP
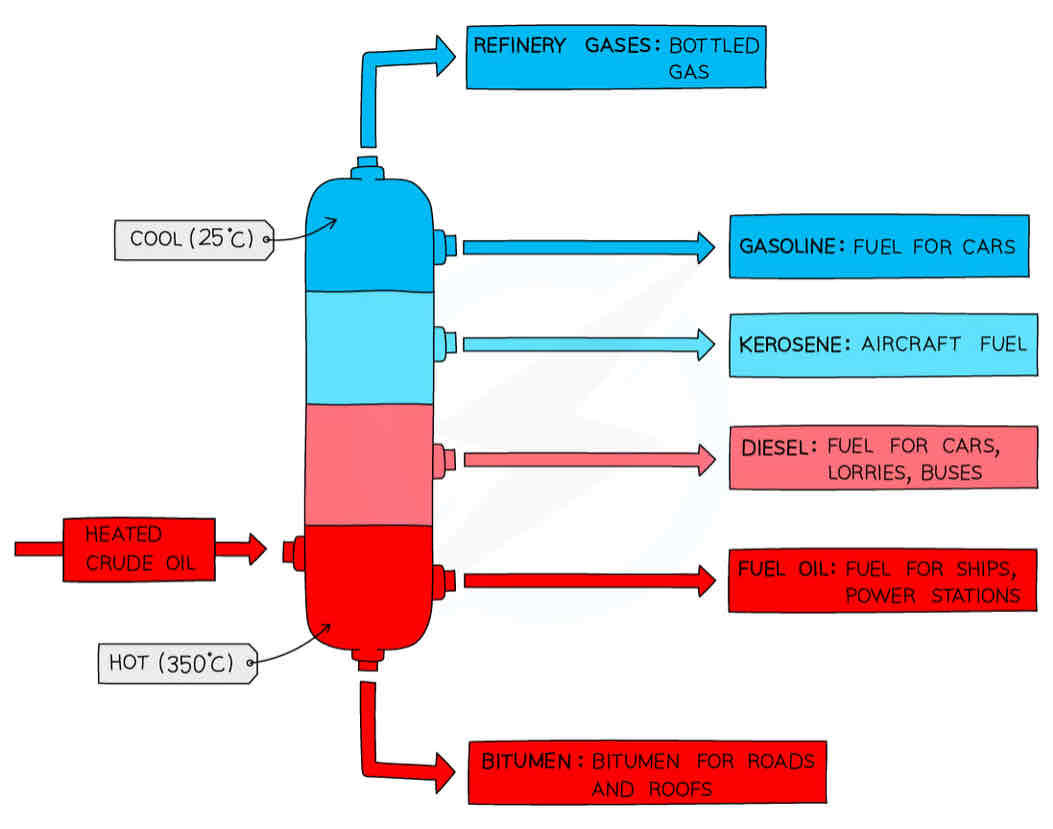
Fractional Distillation
Crude oil = fossil fuel formed from break down of human and plant remains which has been subjected to high pressure
separated crude oil into different fractions based on their BP (carbon chain)
Each fraction has a group of hydrocarbons which have a similar BP
How does fractional distillation work
Crude oil enters fractionating column and is hated so vapour rises
Vapour of hydrocarbons with a ↑ BP and a longer hydrocarbon chain will immediately condense into liquid in higher temperatures (found at the bottom of the column)
Vapours of hydrocarbons with a ↓ BP And shorter hydrocarbon chain will rise to the top of the column and condense at the top due to its low temp as gases
crude oil contains small amounts of different other compounds - some may contain Sulfur and when burned Sulfur dioxide is produced → acid rain
Petroleum
Mixture consisting mainly of alkane hydrocarbons that can be separated by fractional distillation