Organic Chemistry
4.7(6)
4.7(6)
Card Sorting
1/160
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
161 Terms
1
New cards
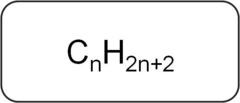
Alkanes
- First four alkanes are methane (CH4), Ethane (C2H6), Propane (C3H8), and Butane (C4H10)
- single Bonded
- single Bonded
2
New cards
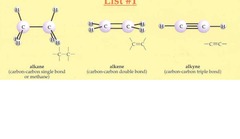
Alkenes and Alkynes
- Contain double and triple bonds respectively.
3
New cards
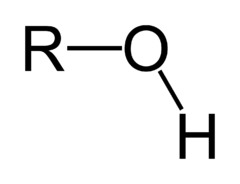
Alcohols
- contain Hydroxyl group (OH)
- suffix ol or hydroxy if a higher priority group is present
- Diols contain two hydroxyl groups.
* Geminal: 2 Hydroxyl groups on the same carbon
* Vicinal: on adjacent carbons
- suffix ol or hydroxy if a higher priority group is present
- Diols contain two hydroxyl groups.
* Geminal: 2 Hydroxyl groups on the same carbon
* Vicinal: on adjacent carbons
4
New cards
Carbonyl group
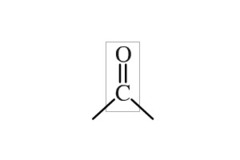
5
New cards
Common Names of Aldehydes
- suffix al
- Common names include
* formaldehyde for methanal (R = H)
* Acetyldehyde for ethanal ( R = CH3)
* Propionaldehyde for propanal (R = CH3CH2)
- Common names include
* formaldehyde for methanal (R = H)
* Acetyldehyde for ethanal ( R = CH3)
* Propionaldehyde for propanal (R = CH3CH2)
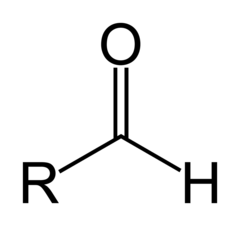
6
New cards
Aldehyde vs. Ketones Terminal group
- An aldehyde has a terminal functional group due to the one hydrogen
- Ketone has two alkyl groups so it's never a terminal group.
- Ketone has two alkyl groups so it's never a terminal group.
7
New cards
Naming cyclic Aldehydes
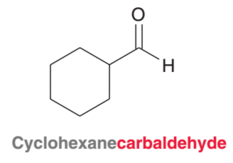
8
New cards
Aldehyde Nomenclature: Methanal
Formaldehyde
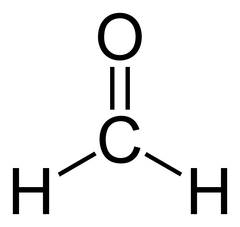
9
New cards
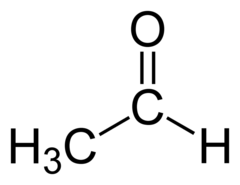
Aldehyde Nomenclature: Ethanal
Acetaldehyde
10
New cards
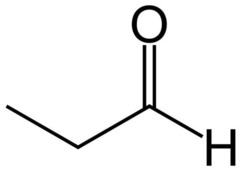
Aldehyde Nomenclature: Propanal
Propionaldehyde
11
New cards
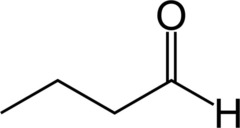
Aldehyde Nomenclature: Butanal
Butyraldehyde
12
New cards
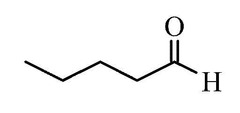
Aldehyde Nomenclature: Pentanal
Valeraldehyde
13
New cards
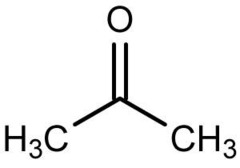
Naming Ketones: 2-propanone
- Dimethyl ketone
- Acetone
- Acetone
14
New cards
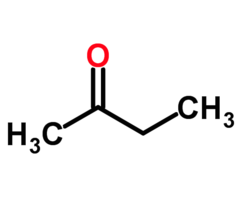
Naming Ketones: 2-butanone
- ethylmethylketone
15
New cards
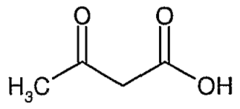
Naming Ketones: 3-oxobutanoic Acid
16
New cards
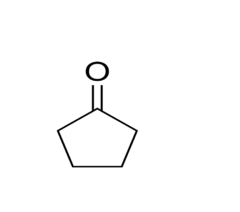
Naming Ketones: Cyclopentanone
17
New cards
Common Names for Ketones
- suffix one
- Acetone (dimethylketone; 2- propanone) ; smallest ketone; similar as the figure
- 2 pentanone (R= CH3CH2CH2)
- Acetone (dimethylketone; 2- propanone) ; smallest ketone; similar as the figure
- 2 pentanone (R= CH3CH2CH2)
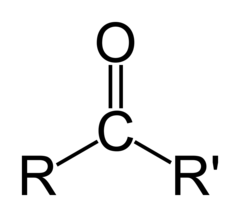
18
New cards
3-butene-2-one
- Naming ketones
- methylvinylketone
- methylvinylketone
19
New cards
Carboxylic Acids and Derivatives
- Contain both carbonyl group C=O and hydroxyl group (OH)
- most oxidized group that appear on the MCAT
- Suffix: Oic acid
- Methanoic acid (Formic Acid)
- Ethanoic acid (acetic acid)
- Propanoic Acid (Propanoic Acid)
- most oxidized group that appear on the MCAT
- Suffix: Oic acid
- Methanoic acid (Formic Acid)
- Ethanoic acid (acetic acid)
- Propanoic Acid (Propanoic Acid)
20
New cards
Ester
- Carboxylic acid derivative
- OH is replaced with OR, an alkoxy group
- OH is replaced with OR, an alkoxy group
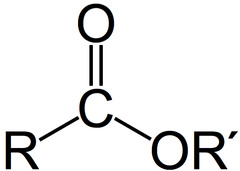
21
New cards
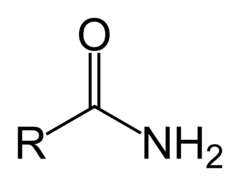
Amides
- Carboxylic acid derivative
- OH is replaced with an amino group
- OH is replaced with an amino group
22
New cards
Anhydrides
- Carboxylic acid derivative
- formed by dehydration of 2 carboxylic acids
* Symmetric = same acid
* asymmetric = two different acids
* cyclic = intramolecular reaction of a dicarboxylic acid
- formed by dehydration of 2 carboxylic acids
* Symmetric = same acid
* asymmetric = two different acids
* cyclic = intramolecular reaction of a dicarboxylic acid

23
New cards
Summary of Functional Groups
Carboxylic acid > anhydride > Ester > Amide > Aldehyde > Ketone > Alcohol > alkene or alkyne > alkane
24
New cards
Structural Isomer
- Share only a molecular formula
- They have different physical and chemical properties
- They have different physical and chemical properties
25
New cards
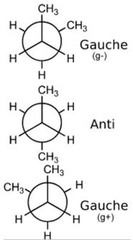
Conformational Isomer
- Same molecule, differ in rotation around single pi bonds.
26
New cards
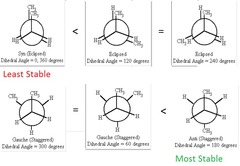
Newman's Projection
- Anti staggered isomer has the lowest energy
- Staggered isomer has the highest energy
- Staggered isomer has the highest energy
27
New cards
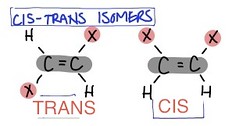
Configurational Isomer
- Can be interconverted only by breaking bonds.
- consist of two categories:
* Enantiomers: nonsuperimposable mirror image and thus have opposite stereochemistry at every chiral carbon.
* Diasteromer: non- mirror image stereoisomers; differ at some but not all chiral centers. Ex) cis - trans isomers
- consist of two categories:
* Enantiomers: nonsuperimposable mirror image and thus have opposite stereochemistry at every chiral carbon.
* Diasteromer: non- mirror image stereoisomers; differ at some but not all chiral centers. Ex) cis - trans isomers
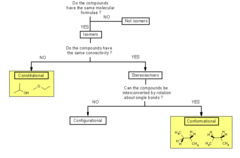
28
New cards
Diastereomers
- non- mirror image configurational isomer.
- differ at some but not all chiral centers. Ex) cis - trans isomers
- differ at some but not all chiral centers. Ex) cis - trans isomers
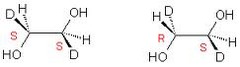
29
New cards
Enantiomer
- Nearly identical physical properties and chemical properties
- They rotate plane polarized light in opposite directions and react differently in chiral environment
- They rotate plane polarized light in opposite directions and react differently in chiral environment
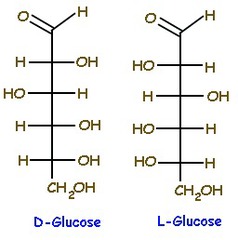
30
New cards
Chiral center
- 4 different group attach to the central carbon
- lack a plane of symmetry
- not superimposable
- lack a plane of symmetry
- not superimposable
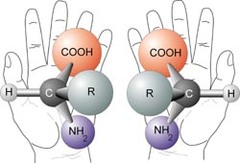
31
New cards
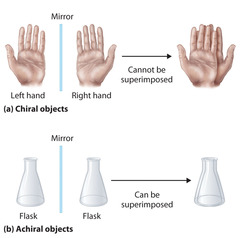
Achiral
- Superimposable
- line of symmetry
- line of symmetry
32
New cards
Racemix Mixture
- Displays no optical activity
- when both (+) and (-) enantiomers are present in equal concentrations.
- Ex) A solution containing 2M (R)-2-butanol and 2 M (S)-2-butanol
- when both (+) and (-) enantiomers are present in equal concentrations.
- Ex) A solution containing 2M (R)-2-butanol and 2 M (S)-2-butanol
33
New cards
Meso Compound
- are essentially the molecular equivalent of a racemic mixture.
- Racemix: when both (+) and (-) enantiomers are present in equal concentrations, no optical activity
- Has a plane of symmetry = no optical activity
- overall achiral ( mirror images that can be superimposed) and will not rotate plane polarized light.
- Racemix: when both (+) and (-) enantiomers are present in equal concentrations, no optical activity
- Has a plane of symmetry = no optical activity
- overall achiral ( mirror images that can be superimposed) and will not rotate plane polarized light.

34
New cards
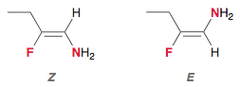
(E) and (Z) Designations of Alkenes
- Z : same side
- E: Opposite side
- used for compounds with polysubstituded double bonds.
- Part of relative configuration
- E: Opposite side
- used for compounds with polysubstituded double bonds.
- Part of relative configuration
35
New cards
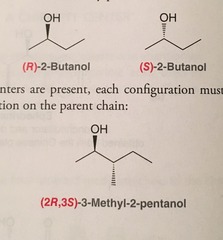
(R) and (S) Nomenclature
- Used for chiral (stereogenic: 4 different groups bound to it in a non superimposable image) centers in molecules.
- (R) rotates to the right; clockwise
- (S) rotates to the left; counterclockwise
- Part of relative configuration
- (R) rotates to the right; clockwise
- (S) rotates to the left; counterclockwise
- Part of relative configuration
36
New cards
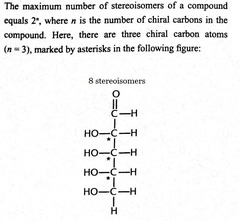
Maximum number of stereoisomer
37
New cards
Equation for specific rotation

38
New cards
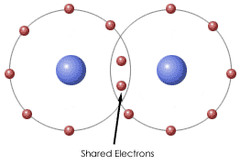
Single Bonds
- Sigma bonds, contains two electrons.
* Permit free rotation
* Permit free rotation
39
New cards
Double Bonds
- Contain one sigma bond and one pi bond.
- Pi bonds are created by sharing of electrons between two unhybridized p-orbitals that align side by side.
- Pi bonds do not permit rotations
- Pi bonds are created by sharing of electrons between two unhybridized p-orbitals that align side by side.
- Pi bonds do not permit rotations
40
New cards
Triple Bonds
- Contain one sigma bond and two pi bonds.
- Pi bonds do not permit rotations
- Pi bonds do not permit rotations
41
New cards
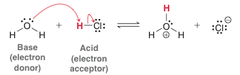
Lewis Acid
- Electron Acceptor in the formation of a covalent bond.
- Tend to be electrophile
- Vacant p-orbitals into which they can accept an electron pair.
- Positively polarized atoms.
- Tend to be electrophile
- Vacant p-orbitals into which they can accept an electron pair.
- Positively polarized atoms.
42
New cards
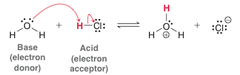
Lewis Base
- Electron Donor in the formation of a covalent bond.
- Nucleophile
- Lone pair of electrons that can be donated, often anions; carrying a negative charge
- Nucleophile
- Lone pair of electrons that can be donated, often anions; carrying a negative charge
43
New cards
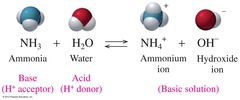
Bronsted Lowry Acid
- Proton Donor
44
New cards
Bronsted Lowry Base
Proton Acceptor
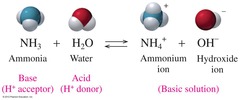
45
New cards
Amphoteric
Ex) water can act as an acid by donating a proton or a base by accepting a proton .
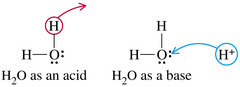
46
New cards
Acid Dissociation constant, Ka
- Measures the strength of an acid in a solution
- pKa can be calculated as -log Ka
* smaller pKa = stronger the acid = below -2
* Weak organic acids have a pKa between -2 and 20.
- pKa can be calculated as -log Ka
* smaller pKa = stronger the acid = below -2
* Weak organic acids have a pKa between -2 and 20.
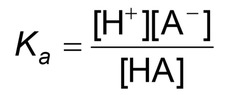
47
New cards
pKa
- lower pKa = stronger Acid = below -2
* -2 to 20 pKa is considered Weak Acid
- Larger pKa = More Basic
* -2 to 20 pKa is considered Weak Acid
- Larger pKa = More Basic
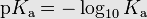
48
New cards
An Acid-Base reaction will proceed when ...
The acid and Base react to form conjugate products that are weaker than the reactants.
49
New cards
Nucleophiles
- Electron Donor; Good Bases
- Tend to have lone pairs or pi bonds that cane be used to form covalent bonds to electrophiles ( electron acceptors)
- (CHON) with a minus sign or lone pairs
- Nucleophile strength is based on relative rates of reaction with a common electrophile; therefore, kinetic property.
- Tend to have lone pairs or pi bonds that cane be used to form covalent bonds to electrophiles ( electron acceptors)
- (CHON) with a minus sign or lone pairs
- Nucleophile strength is based on relative rates of reaction with a common electrophile; therefore, kinetic property.

50
New cards
Nucleophilicity is determined by 4 major factors:
- Charge: Increases with increasing electron density (more negative charge)
- Electronegativity: Decreases as electronegativity increases because these atoms are less likely to share electron density
- Steric Hindrance: Bulkier molecule = less nucleophilic
- Solvent: Protic solvents can hinder nucleophilicity by protonating the nucleophile or through hydrogen bonding.
- Electronegativity: Decreases as electronegativity increases because these atoms are less likely to share electron density
- Steric Hindrance: Bulkier molecule = less nucleophilic
- Solvent: Protic solvents can hinder nucleophilicity by protonating the nucleophile or through hydrogen bonding.
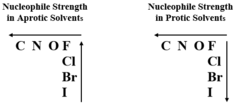
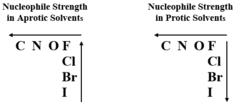
51
New cards
Nucleophile in protic and aprotic solvents
Protic: - I- > Br- > Cl- > F
Aprotic: F- > Cl- > Br-> I-
Aprotic: F- > Cl- > Br-> I-
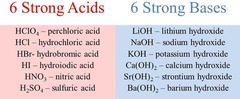
52
New cards
Strong Acids
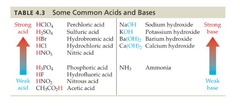
53
New cards
Strong Base
54
New cards
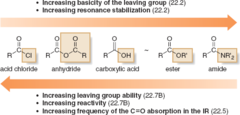
Carboxylic Acid Derivatives
- Often ranked by electrophilicity.
- Anhydrides are the most reactive according to Kaplan.
- Higher reactivity can form derivatives of lower reactivity but not vice versa.
- Anhydrides are the most reactive according to Kaplan.
- Higher reactivity can form derivatives of lower reactivity but not vice versa.
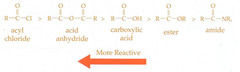
55
New cards
Heterolytic Reactions
- A bond is broken and both electrons are given to one of the two products.
- The best leaving group is able to stabilize the extra electrons.
* Weak bases (conjugate bases of strong acids such as I-, Br-, and Cl-) make good leaving groups
- The best leaving group is able to stabilize the extra electrons.
* Weak bases (conjugate bases of strong acids such as I-, Br-, and Cl-) make good leaving groups
56
New cards
Mechanism of SN1 Reaction
1) rate-limiting step in which the leaving group leaves, generating a positively charge carbocation.
2) The nucleophile attacks the carbocation resulting in the substitution product.
3) Product will usually be a racemic mixture.
4) rate = k [R-L] ; [R-L] is an alkyl group containing a leaving group
2) The nucleophile attacks the carbocation resulting in the substitution product.
3) Product will usually be a racemic mixture.
4) rate = k [R-L] ; [R-L] is an alkyl group containing a leaving group
![1) rate-limiting step in which the leaving group leaves, generating a positively charge carbocation.
2) The nucleophile attacks the carbocation resulting in the substitution product.
3) Product will usually be a racemic mixture.
4) rate = k [R-L] ; [R-L] is an alkyl group containing a leaving group](https://knowt-user-attachments.s3.amazonaws.com/905b458a289a48038bf6183b2e29f115.jpg)
57
New cards
Mechanism of SN2 Reaction
- Contains only one step
1) Nucleophile (Backside attack; must be strong and substrate cannot be statically hindered) attacks the compound at the same time as the leaving group leaves
2) Substrate will often be alkyl halides, Tosylate, or mesylate
3) Rate = k [Nu:] [R-L]; [R-L] is an alkyl group containing a leaving group
4) Inversion of relative configuration will correspond to change in absolute configuration from (R) to (S) or vice versa.
1) Nucleophile (Backside attack; must be strong and substrate cannot be statically hindered) attacks the compound at the same time as the leaving group leaves
2) Substrate will often be alkyl halides, Tosylate, or mesylate
3) Rate = k [Nu:] [R-L]; [R-L] is an alkyl group containing a leaving group
4) Inversion of relative configuration will correspond to change in absolute configuration from (R) to (S) or vice versa.
![- Contains only one step
1) Nucleophile (Backside attack; must be strong and substrate cannot be statically hindered) attacks the compound at the same time as the leaving group leaves
2) Substrate will often be alkyl halides, Tosylate, or mesylate
3) Rate = k [Nu:] [R-L]; [R-L] is an alkyl group containing a leaving group
4) Inversion of relative configuration will correspond to change in absolute configuration from (R) to (S) or vice versa.](https://knowt-user-attachments.s3.amazonaws.com/33902302dd4a4ae194b05c43486add2d.png)
58
New cards
How do the definitions of nucleophile and electrophile differ from those of Lewis Acids and Lewis Base?
- Nucleophilicity and electrophilicity are based on relative rates of reactions therefore are kinetic properties.
- Acidity and Basicity are measured by the position of equilibrium in a protonation or deprotonation reaction and are therefore thermodynamic properties.
- Acidity and Basicity are measured by the position of equilibrium in a protonation or deprotonation reaction and are therefore thermodynamic properties.
59
New cards
How must the nucleophile and leaving group be related in order substitution reaction to proceed?
- It will proceed when the nucleophile is a strong base (more reactive) than the leaving group.
- Acid catalyst is required if the leaving group is a hydroxide ( bad leaving group) so it can get protonated to water (good leaving group) .
- Acid catalyst is required if the leaving group is a hydroxide ( bad leaving group) so it can get protonated to water (good leaving group) .
60
New cards
What trends increase electrophilicity?
- Greater positive charge increases electrophilicity, and better leaving groups increase it by making the reaction to proceed.
61
New cards
What are some features of good leaving groups?
- Good leaving groups can stabilize the extra electrons that result from heterolysis.
- Weak bases (conjugate bases of strong acids such as I-, Br-, and Cl-) are good leaving groups.
- Resonance stabilization also improve leaving groups
- Weak bases (conjugate bases of strong acids such as I-, Br-, and Cl-) are good leaving groups.
- Resonance stabilization also improve leaving groups
62
New cards
Oxidation State Order of Increase
- Carboxylic Acid > Aldehydes, Ketones, and imines, which in turn are more oxidized than alcohols, alkyl halides, and amines.
- Oxidation increases as the number of bonds to oxygen increases or other heteroatom (atoms besides carbon and oxygen)
- Oxidation increases as the number of bonds to oxygen increases or other heteroatom (atoms besides carbon and oxygen)
63
New cards
Oxidizing Agents and Reactions
- Accepts an electron from another species.
- High Affinity for electrons such as O2, O3, and Cl2 or unusually high oxidation states (like Mn7+ in permanganate, MnO4-, and Cr6+ in chromate, CrO_4 ^2-)
- Oxidation reactions: inc. # of bonds to oxygen
- Oxidizing Agents: Metals bonded to large number of oxygen atoms.
- High Affinity for electrons such as O2, O3, and Cl2 or unusually high oxidation states (like Mn7+ in permanganate, MnO4-, and Cr6+ in chromate, CrO_4 ^2-)
- Oxidation reactions: inc. # of bonds to oxygen
- Oxidizing Agents: Metals bonded to large number of oxygen atoms.
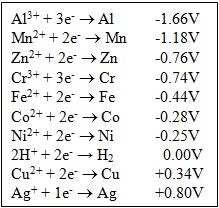
64
New cards
Reduction of Carbon
- Gains Electrons
- When an atom that is more electronegative than carbon is replaced with less electronegative than carbon
- Means increasing the number of bonds to Hydrogen and decreasing the number of bonds to carbons, nitrogen, oxygen and halides
- When an atom that is more electronegative than carbon is replaced with less electronegative than carbon
- Means increasing the number of bonds to Hydrogen and decreasing the number of bonds to carbons, nitrogen, oxygen and halides
65
New cards
Good Oxidizing Agents
Metals bonded to a large number of Oxygen atoms.
66
New cards
Good Reducing Agents
Metals bonded to a large number of Hydrides.

67
New cards
Bond Strength decreases down the periodic table
- acidity increases.
- Also Higher electronegative an atom, higher the acidity.
- Also Higher electronegative an atom, higher the acidity.
68
New cards
Oxidation of Primary Alcohol
-Primary Alcohol Forms an aldehyde by Pyridinium Chlorochromate (PCC)
- Primary Alcohol is oxidized to a carboxylic acid by CrO3 (Jones Oxidation)
- Primary Alcohol is oxidized to a carboxylic acid by CrO3 (Jones Oxidation)
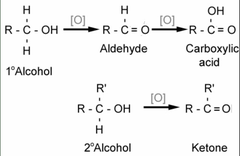
69
New cards
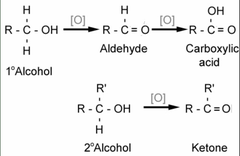
Oxidation of a Secondary Alcohol
- Forms a Ketone by a Dichromate salt (Na_2Cr_2O_7 & K_2Cr_2O_7)
70
New cards
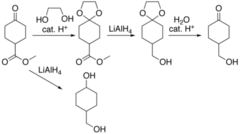
Protecting Groups of Acetals and Ketals
- Acetals: primary carbon with 1 OR and an OH atom
- Ketals: Secondary carbons with two OR groups.
- Forms in the presence of a strong oxidizing agents.
- Ketals: Secondary carbons with two OR groups.
- Forms in the presence of a strong oxidizing agents.
71
New cards
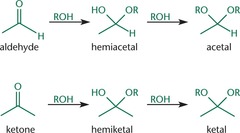
Acetals and Ketal Formation
- Acetals and Ketals are comparatively inert, are frequently used as protecting groups for carbonyl functionalities.
- once a hemiacetal and hemiketal is formed, the hydroxyl group is protonated and released as a molecule of water; alcohol then attacks, forming an acetal or ketal.
- once a hemiacetal and hemiketal is formed, the hydroxyl group is protonated and released as a molecule of water; alcohol then attacks, forming an acetal or ketal.
72
New cards
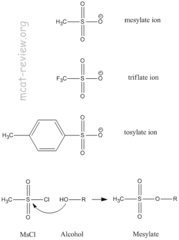
Protecting Groups for alcohols
- Make hydroxyl groups a better leaving groups for nucleophilic substitution
- They can act as a protecting group when we do not want alcohol to react.
- They can act as a protecting group when we do not want alcohol to react.
73
New cards
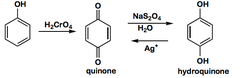
Quinone
- Serve as electron acceptor biochemically; Electron Transport Chain in both photosynthesis and aerobic respiration.
- Phylloquinone (Vitamin K1): important for photosynthesis and the carboxylation of some of the clotting factors in blood.
- Menanquinones (Vitamin K2)
- Phylloquinone (Vitamin K1): important for photosynthesis and the carboxylation of some of the clotting factors in blood.
- Menanquinones (Vitamin K2)
74
New cards
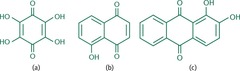
Three Examples of Hydroxyquinones
- Share the same ring and carbonyl backbone as quinones but differ by the addition of one or more hydroxyl groups
- a) Tetrahydroxybenzoquinone; b) 5-hyroxynaphthoquinone; c) 1,2-dihydroxyanthraquinone
- a) Tetrahydroxybenzoquinone; b) 5-hyroxynaphthoquinone; c) 1,2-dihydroxyanthraquinone
75
New cards
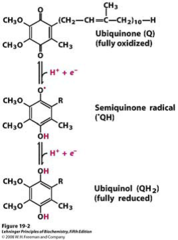
Ubiquinone
- Biologically active quinone (electron acceptor in photosynthesis and aerobic respiration)
- Reduced to ubiquinol upon the acceptance of electrons.
- Long alkyl chain = lipid soluble = act as an electron carrier within the phospholipid bilayer.
- Reduced to ubiquinol upon the acceptance of electrons.
- Long alkyl chain = lipid soluble = act as an electron carrier within the phospholipid bilayer.
76
New cards
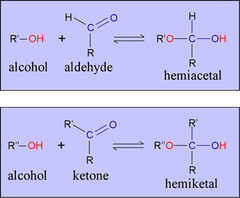
Hemiacetal Formation
- The oxygen in the alcohol functions as a nucleophile, attacking the carbonyl carbon, and generating a hemiacetal.
- Hemiacetals are unstable and the hydroxyl group is rapidly protonated and lost as water under acidic conditions, leaving behind a reactive carbocation.
- Hemiacetals are unstable and the hydroxyl group is rapidly protonated and lost as water under acidic conditions, leaving behind a reactive carbocation.
77
New cards
Imine Formation
- Ammonia (NH3) is added to the carbonyl, resulting in the elimination of water, and generating an imine.
- Imine can undergo tautomerization and form enamine
- Example of condensation reaction since a small molecule is lost during the formation of a bond between two molecules.
- Imine can undergo tautomerization and form enamine
- Example of condensation reaction since a small molecule is lost during the formation of a bond between two molecules.
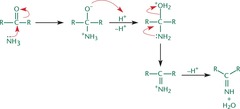
78
New cards
Imines and Enamines
79
New cards
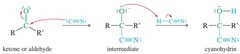
Cyanohydrin or Hydrogen Cyanide (HCN)
Cyanide functions as a nucleophile, attacking the carbonyl carbon and generating a cyanohydrin.
80
New cards
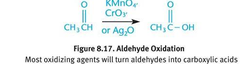
Aldehyde oxidation
- Also H2O2
81
New cards
Reduction by Hydride Reagents
- Lithium aluminum hydride (LiAlH4)
- Sodium borohydride (NaBH4)
- Sodium borohydride (NaBH4)
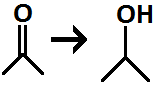
82
New cards
Geminal Diol
- A compound with two hydroxyl groups on the same carbon due to a hydration reaction, water adds to a carbonyl.
-
-
83
New cards
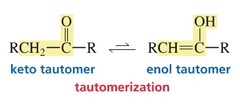
Tautomers
- Two isomers, which differ in the placement of a proton and the double bond
84
New cards
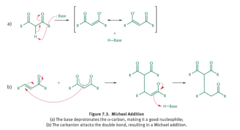
Michael Addition
85
New cards
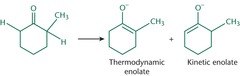
Kinetic and Thermodynamic Enolates
- The kinetic enolate forms more quickly, irreversible, low temp, sterically hindered base,
and is less stable than the thermodynamic enblate.
- Thermodynamic forms more slowly, reversible, weaker or smaller bases, higher tempreture
and is less stable than the thermodynamic enblate.
- Thermodynamic forms more slowly, reversible, weaker or smaller bases, higher tempreture
86
New cards
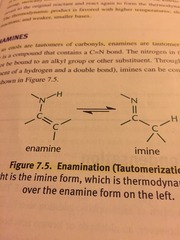
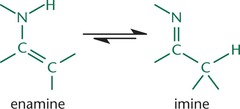
Enamination
Imine from is the thermodynamically favored over the enamine form on the left.
87
New cards
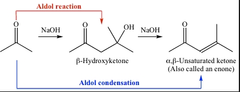
Aldol Condensation Reaction
- The aldol condensation involves two reaction series, the aldol addition reaction and the condensation reaction. Dehydration occurs through an elimination (technically, E1cB) mechanism to form an α, β-unsaturated aldehyde (enal) or ketone (enone), not the aldol.
* The first step of aldol condensation involves a strong base like hydroxide abstracting a proton from the α-carbon (not the β-carbon) of a carbonyl compound (aldehyde or ketone) to form the enolate.
* The second step of aldol condensation involves the enolate attacking the aldehyde or ketone through a nucleophilic acyl addition mechanism (not substitution). Only carboxylic acid and its derivative can undergo nucleophilic acyl substitution.
- Examples are dehydration, nucleophilic addition , and aldol reaction.
* The first step of aldol condensation involves a strong base like hydroxide abstracting a proton from the α-carbon (not the β-carbon) of a carbonyl compound (aldehyde or ketone) to form the enolate.
* The second step of aldol condensation involves the enolate attacking the aldehyde or ketone through a nucleophilic acyl addition mechanism (not substitution). Only carboxylic acid and its derivative can undergo nucleophilic acyl substitution.
- Examples are dehydration, nucleophilic addition , and aldol reaction.
88
New cards
Dehydration Reaction
A molecule of water is lost
89
New cards
Nucleophile-electrophile Reaction
A nucleophile pushes an electron pair to form a bond with an electrophile.
90
New cards
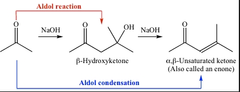
Aldol reaction
Contains both aldehyde and alcohol functional groups.
91
New cards
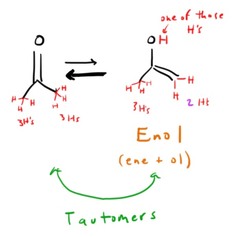
Enol Form
ene + ol = double bond + hydroxyl group
92
New cards
Esterification reaction
Formation of esters from carboxylic acids and alcohols.
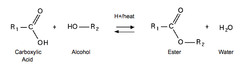
93
New cards
Elimination reaction
A reaction in which a part of a reactant is removed and a new multiple bond is introduced.
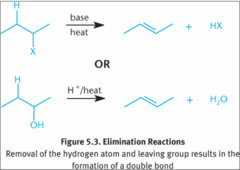
94
New cards
Dehydration reaction
A reaction in which a molecule of water is eliminated
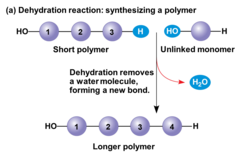
95
New cards
When an aldehyde or ketone with a alpha hydrogen is treated with a strong base such as LDA
It forms the more nucleophilic enblate carboanion.
96
New cards
Common Names of Dicarboxylic Acid
- suffix: dioic acid
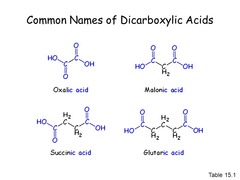
97
New cards
Carboxylic Acid characteristics
- polar and can form hydrogen bonds.
- Acidity is due to resonance stabilization and can be enhanced by the addition of electronegative groups or a greater ability to delocalize charge.
- pKa: 4.8
- Acidity is due to resonance stabilization and can be enhanced by the addition of electronegative groups or a greater ability to delocalize charge.
- pKa: 4.8
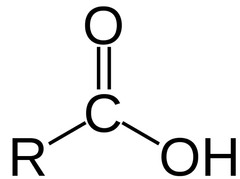
98
New cards
Nucleophilic Acyl Substitution
Step 1: Nucleophilic Addition
Step 2: Elimination of the leaving group and reformation of the carbonyl.
Step 2: Elimination of the leaving group and reformation of the carbonyl.
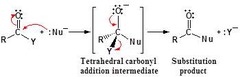
99
New cards
Formation of an Amide by Nucleophilic Acyl Substitution
- Carboxylic acid can be converted into amides if the incoming nucleophile is ammonia (NH3).
- Can be carried out in acidic or basic solution.
- Amides are named by replacing the oic acid with amide in the name of the parent carboxylic acid.
- Can be carried out in acidic or basic solution.
- Amides are named by replacing the oic acid with amide in the name of the parent carboxylic acid.
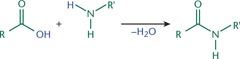
100
New cards
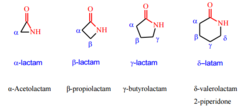
Amides that are cyclic are known as
- Lactam
- replacing oic acid with lactam
- replacing oic acid with lactam