the Arrhenius equation
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
1
New cards
What does the Arrhenius equation help us understand?
How k is affected by temperature + presence of a catalyst
2
New cards
What is the Arrhenius equation?
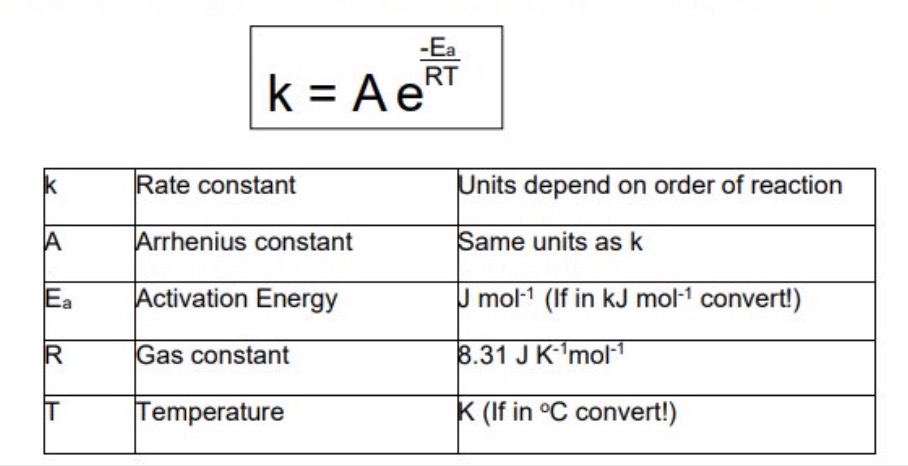
3
New cards
What is e?
A mathematical quantity + is the base of the natural log
4
New cards

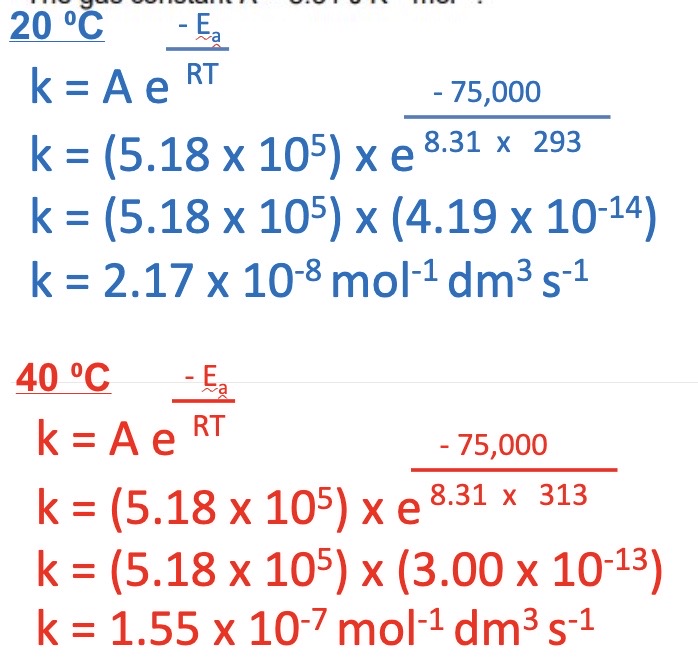
5
New cards
What is the equation to calculate the activation energy or temperature?
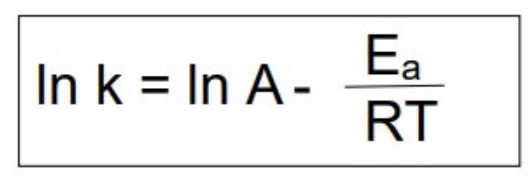
6
New cards


7
New cards
Rearrange the equation to find temperature from logarithmic form
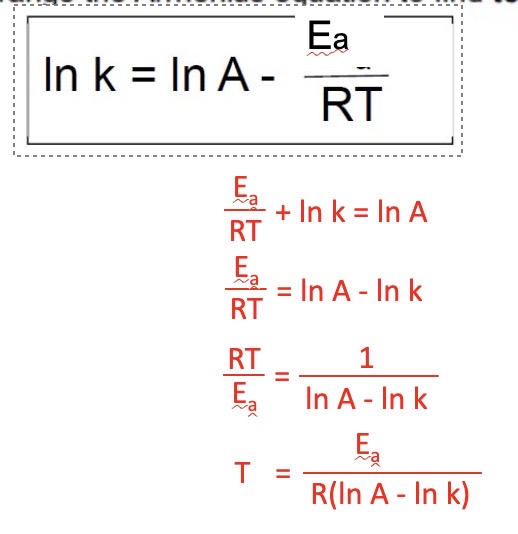
8
New cards
How can the logarithmic form of the equation follow the equation of a straight line?
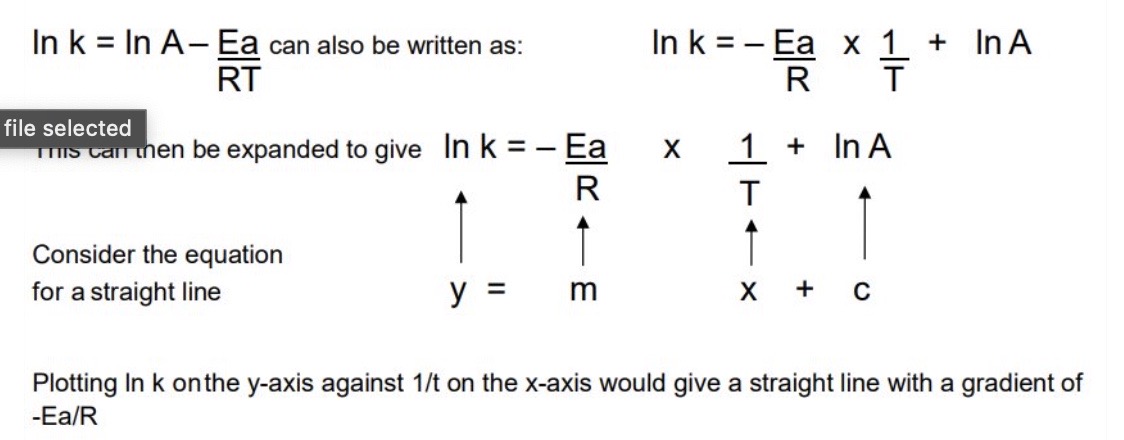
9
New cards
How to calculate activation energy from gradient?