Metabolism
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
84 Terms
If Ken ask: what do you think my metabolism is?…
people don’t really know what it is, just like to have a feeling about it
Ken: Information in food found on campus…
-Hickory Nuts
-Cornelian Cherry (+Ken’s purpose of showing)
-Hickory Nuts: $, eat=eat CO2 someone breathed out or stored in tree
-Cornelian Cherry: Natural iridoids and Liver/Cardiovascular System functions
*PPAR: enzyme pathway making mito; what people who go running try to activate for adaptations
***we don’t have to know all these details; need some knowledge though

“Most Astounding fact” video
-astrophysicist Neil Tyson
-H and He from stars smashed and make heavier elements
-”universe is us; our atoms came from stars”
-people want to feel like they mean something in this world
-all connected, and want to feel connected
Review of Metabolism from EXPH
-can’t store ATP, so constantly modulating to meet energy needs at given moment
-can’t directly burn carbs and fats, need to convert to ATP first (store lots kcals)
-”Metabolism”: rate burn carbs and fats to make ATP
-1 kcal/min @ rest
-RER 0.7 - 1.0 (fat → carb; what burn aerobically)
-can’t see metabolism
-no perfect human diet; animals often find balance
How should we rephrase: “You are what you eat?”
-The phrase “You are what you eat” does not stand up to the reality of the digestive system.
-To be more accurate, the phrase should be “You are what you absorb in conference with whom you host.”
-So what exactly becomes of what you absorb?…
how many kcal do you burn to run a mile?
~100 kcal/mile
Metabolism definition
-is the sum total of all of the chemical reactions in living cells.
-More specifically, energy metabolism includes all of the reactions by which the body obtains and expends the energy from food.
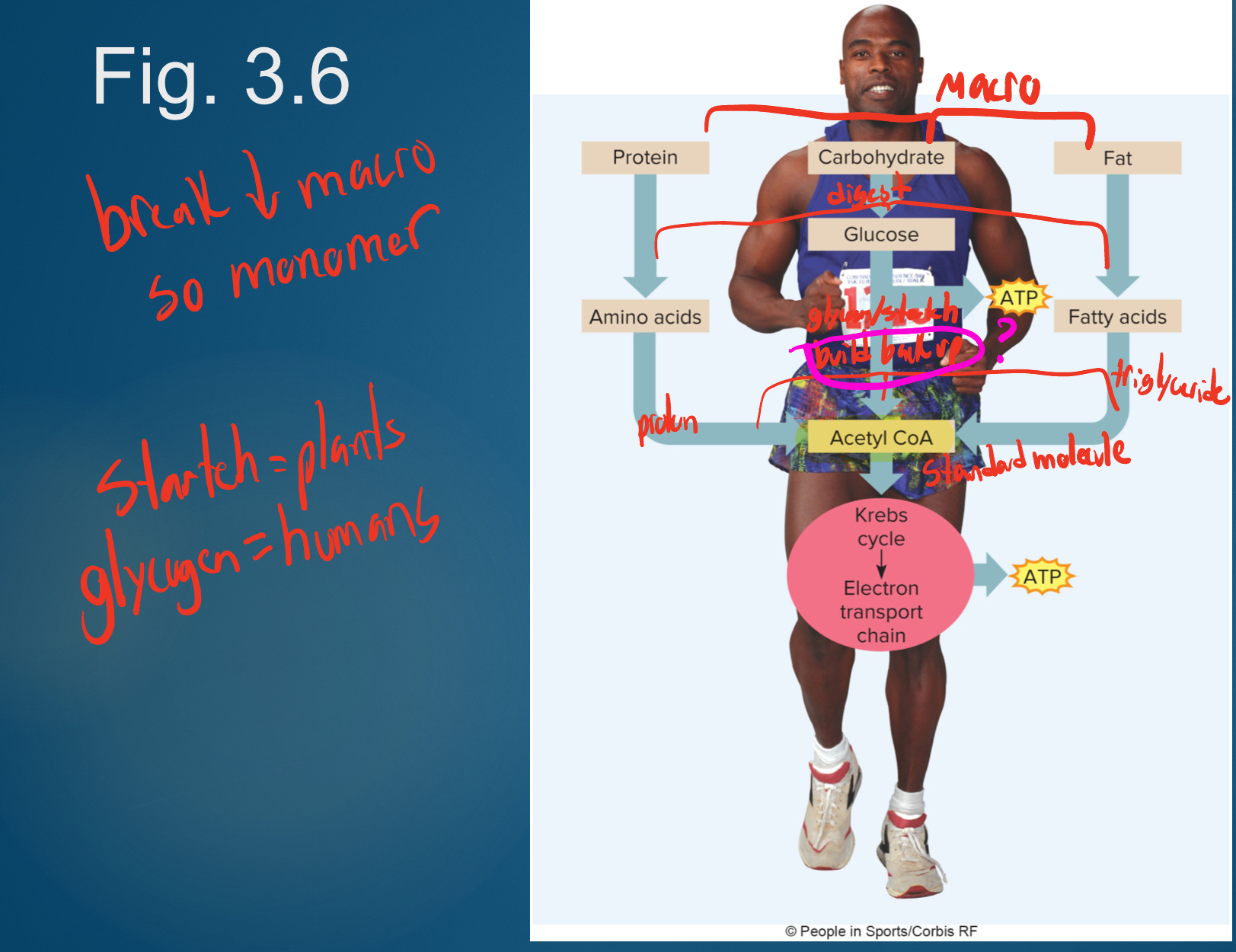
Major metabolic destinations for macronutrients
-Energy for active processes
*most of what’s happening
-Synthesis into structural or functional molecules
-Storage as fat or glycogen for later use as energy
*burn storage between meals; same weight= burnt all kcals (unless lose weight or gain weight=store fat and protein on body)
Without net change in storage or synthesis, macronutrients are essentially for __
energy
primary means by which all metabolic reactions are controlled
Enzyme regulation
metabolism: anabolic or catabolic
-Organic metabolic reactions can be classified into either catabolism or anabolism.
-The coupling of energy-requiring (anabolic) and energy-releasing (catabolic) reactions is achieved through ATP (adenosine triphosphate). It provides the energy for the energy requiring reaction.
-However, ATP cannot be stored, so it must constantly be resynthesized. Therefore the rate of this resynthesis is your rate of metabolism.
*overall goal: make ATP
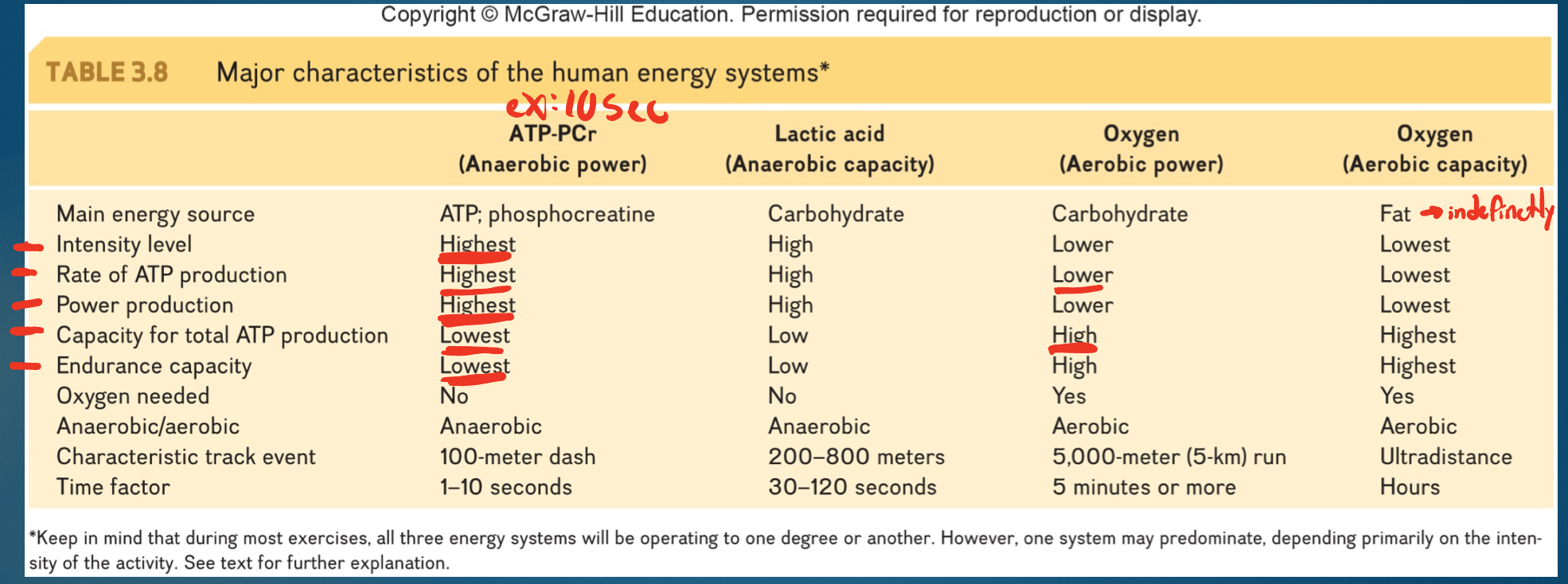
3 energy systems in ATP resynthesis (list + overview)
*resynthesis: ADP + Pi → ATP
1. Immediate energy system: reactions catalyzed by creatine kinase
*1 step so quick; creatine kinase: takes P off PCr and puts on ATP
2. Glycolysis
*10 steps; invest 2 prod 4= 2 ATP
^1 & 2= anaerobic
3. Aerobic metabolism
*products from glycolysis (or fat and protein breakdown) → Krebs → ETC where get 28-30 ATP
Advantages and Disadvantages of 3 energy systems
-PCR: + make ATP fast; - run out fast
-Glycolysis: + make ATP next fastest; - lactate decrease pH and leads to fatigue
-Aerobic: + unlimited supply (could walk to Boston!); - ATP slow (even at max)
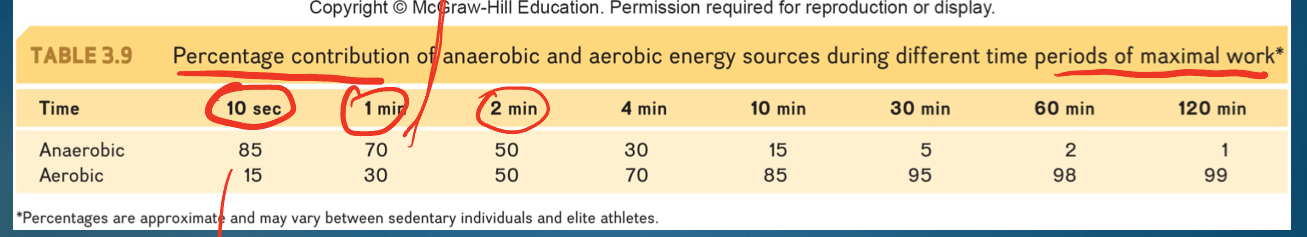
timing for when each energy system is dominant
-10 sec: majority PCR (not glycolysis though)
-1 min: ana dominant but aerobic bigger
->2.5 min: aerobic dominant
*increase time= increase dominance aerobic
*not just about how fast; are they going max for certain time?
true or false: athletes can make ATP aerobically faster than us anerobically?
true!
true or false: All nutrient molecules have energy stored in their structure
true!
Oxidation (+ in context of metabolism)
-Oxidation is the removal of electrons from a molecule and results in a decrease in the energy content of the molecule
-and because most biological oxidations involve the loss of hydrogen atoms (that includes an electron), they are called dehydrogenation reactions (take H off).
(e- + H+ = H)
*H has energy!
Coenzymes
-When a substance is oxidized, the liberated hydrogen atoms do not remain free in the cell but are transferred immediately by coenzymes to another compound.
*grab on to H; B-vitamins
-Two coenzymes are commonly used by living cells to carry hydrogen atoms:
1. Nicotinamide adenine dinucleotide (NAD+ ) (from niacin)
2. Flavin adenine dinucleotide (FAD) (from riboflavin)
*get H=reduced
Reduction
Reduction is the opposite of oxidation, that is, the addition of electrons to a molecule, usually accompanied by a proton.
Oxidation and Reduction reactions are always ___
coupled
• Whenever a substance is oxidized, another is almost simultaneously reduced.
• This coupling of reactions is simply referred to as oxidation-reduction (redox) reactions
Carb Metabolism: what gets the first crack at it?
-During digestion, polysaccharides and disaccharides (bc amylase) are converted to monosaccharides (primarily glucose; bc BBE), which are absorbed into capillaries in intestinal villi and transported to the liver via the hepatic portal vein.
-Liver cells convert much of the remaining fructose and practically all of the galactose to glucose, so carbohydrate metabolism is primarily concerned with glucose metabolism.
Fate of Carbs
-the fate of absorbed glucose depends on the energy needs of body cells.
1. Catabolism including oxidation to reform ATP (ADP + Pi = ATP) (*breakdown 100% over time; for energy)
2. Glycogenesis (*store as glycogen; ex: if eat)
3. Amino acid synthesis
4. Lipogenesis
^3 & 4: certain circumstances/if excess calories so convert to AA and fat; more likely convert to fat but that takes energy; so stop fat burn, burn carbs, and store fat normally burn
when is glucose the body's preferred source for synthesizing ATP? why glucose?
-when it makes up the bulk of nutrient intake AND also during high intensity exercise
-because it yields more energy per liter of VO2
true or false: Complete oxidation of glucose to reform ATP occurs in every cell of the body
true!
beginning in the cytoplasm w/ glycolysis and finishing in the mitochondria (except red blood cells, which lack mitochondria)
*glycolysis in cytoplasm bc where actin and myosin are
*ATP in mito has to get out
Glucose Catabolism: Cellular Respiration (overview/steps)
-Complete oxidation of glucose (or other food monomers) to CO2 and H2O (in the presence of O2) produces large amounts of energy and is called cellular respiration.
-Complete glucose oxidation occurs in four successive stages
1. Glycolysis (occurs in the cytoplasm)
2. Formation of acetyl coenzyme A (acetyl CoA)
3. Krebs cycle
4. Oxidative phosphorylation (the electron transport chain)
*^2-4: in mito
Glycolysis
-Anaerobic breakdown of glucose (6-C molecule) into two pyruvate molecules (3-C)
Under “anaerobic” conditions (when ATP demand is high), pyruvate is reduced to lactate. (if rate fast)
Under aerobic conditions, pyruvate is converted to acetyl CoA and enters the Krebs cycle. (if rate slow)
-After glycolysis, there is a net production of 2 molecules of ATP.
*No CO2 produced so no C lost; no O2 used
*22222222302222222222230 (but if only 1 glucose molecule would want to do aerobic)
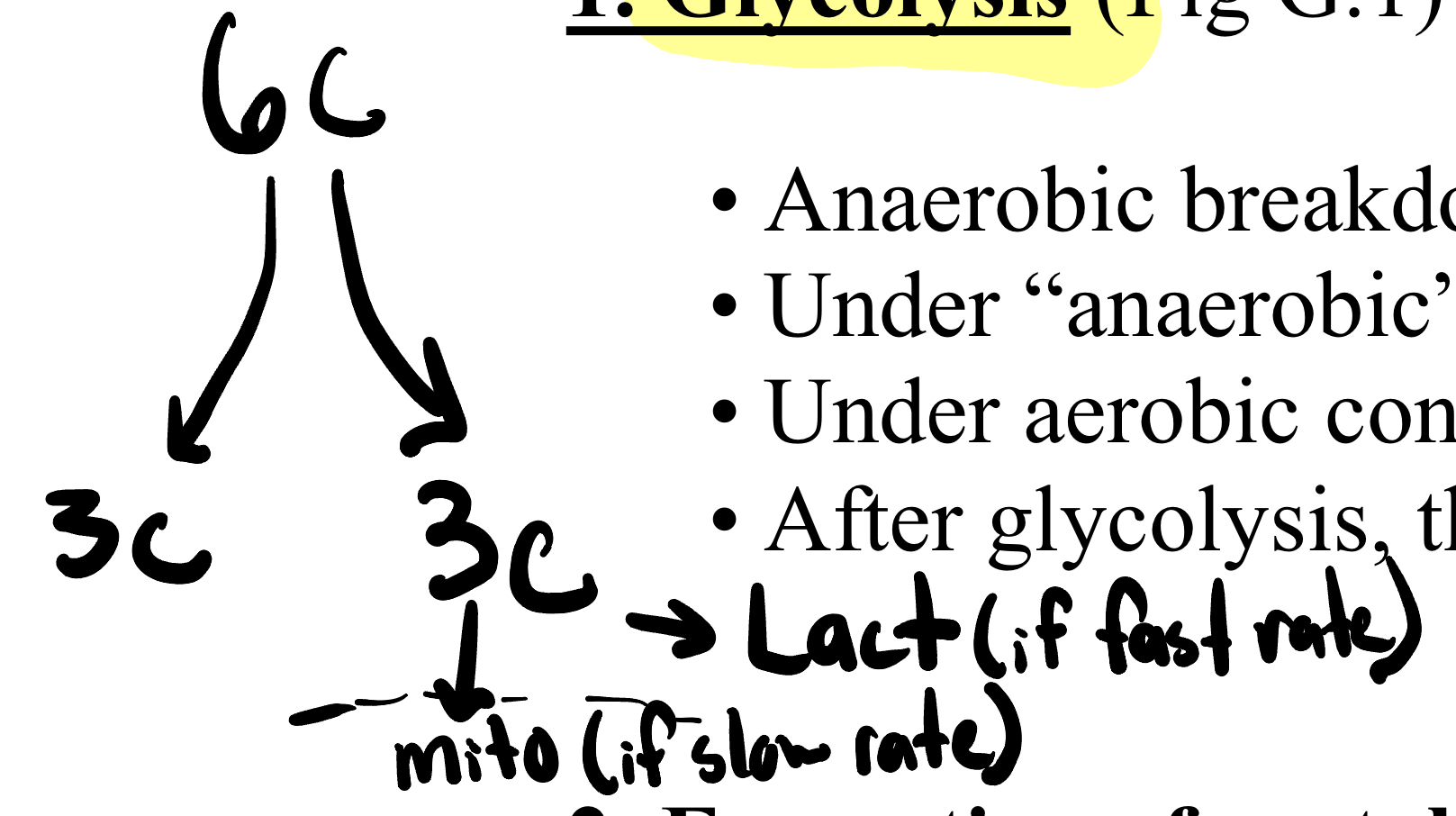
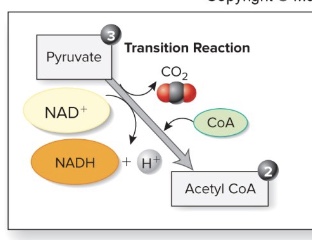
Formation of acetyl CoA
-Each pyruvate is transported into the mitochondria and prepared for entrance into the Krebs cycle by conversion to a two-carbon compound (acetyl group) followed by the addition of coenzyme A (CoA) to form acetyl CoA.
-Thus, our first CO2 molecule is released.
-Coenzyme A is derived from pantothenic acid, a B vitamin (*no known UL; ushers molecule to next step/Krebs)
-The decarboxylation (remove C) of pyruvate requires thiamin (B vit) and magnesium.
*could be glucose, protein, or fat
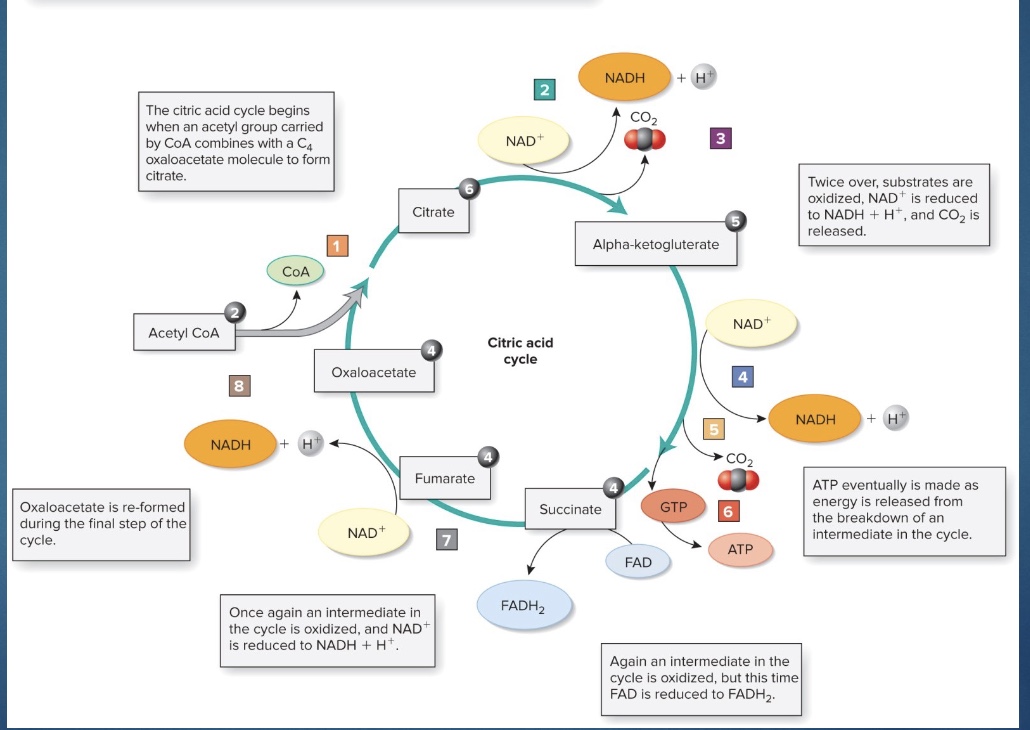
Krebs Cycle (/citric acid cycle/TCA clyce)
*yield H; no C left
-This process involves a series of biochemical reactions that occur in the matrix of mitochondria and acts to harness chemical energy primarily in the form of H atoms (protons and electrons).
-Thus, the formation of acetyl CoA and the subsequent Krebs cycle produce:
•CO2; NADH+; H+; FADH2; GTP (guanosine triphosphate, the equivalent of ATP)
-The energy originally in glucose and then each pyruvate is primarily in the reduced coenzymes NADH + H+ and FADH2, because they have the H atoms (and go to the ETC)
true or false: every reaction in metabolism releases heat?
true! (otherwise would freeze!)
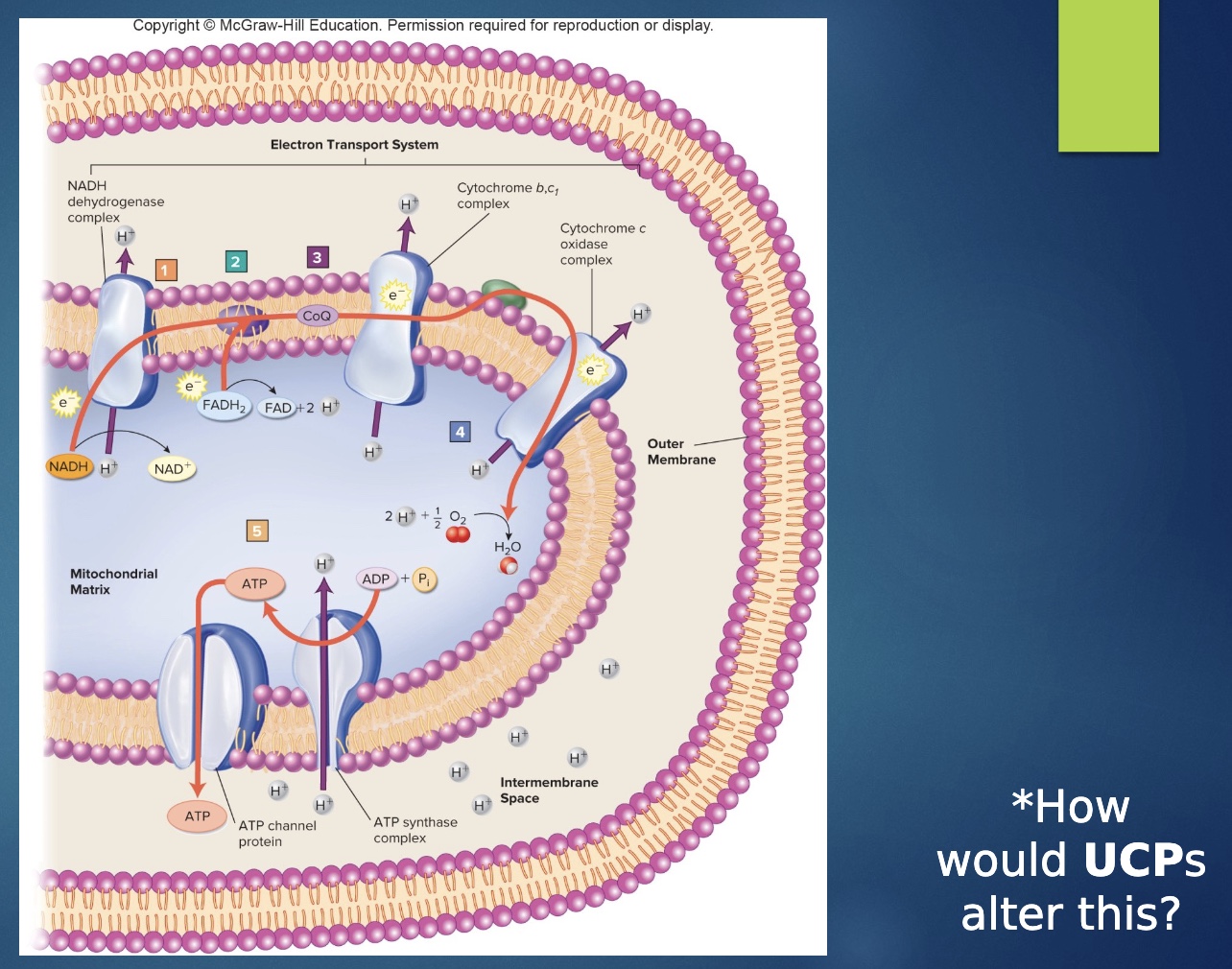
Oxidative Phosphorylation (ETC)
-A sequence of electron carrier molecules on the inner mitochondrial membrane capable of undergoing a series of oxidation-reduction reactions that liberate the energy in NADH + H+ and FADH2 and use it to reform ATP.
• Within the inner mitochondrial membrane, the carriers of the electron transport chain cluster into three complexes, each of which acts as a proton pump that expels H+ from the mitochondrial matrix and helps create an electrochemical gradient for H + .
• As electrons are passed through the chain, there is a stepwise release of energy from the electrons for the pumping of H+ . Iron and copper are involved in this process.
*in mito and muscle myoglobin (ex: animal meat, well water)
• ATP resynthesis occurs as H+ diffuses back into the mitochondrial matrix through a special inner membrane H+ channel called an ATP synthase. These protons come back into the matrix and in so power the regeneration of ATP.
*then also need to get ATP out of mito!
• In this aerobic cellular respiration, the last electron acceptor of the chain is molecular O2. This final oxidation is irreversible, and the addition of protons results in H2O.
-The electron transport chain yields 30-something ATP and several molecules of H2O.
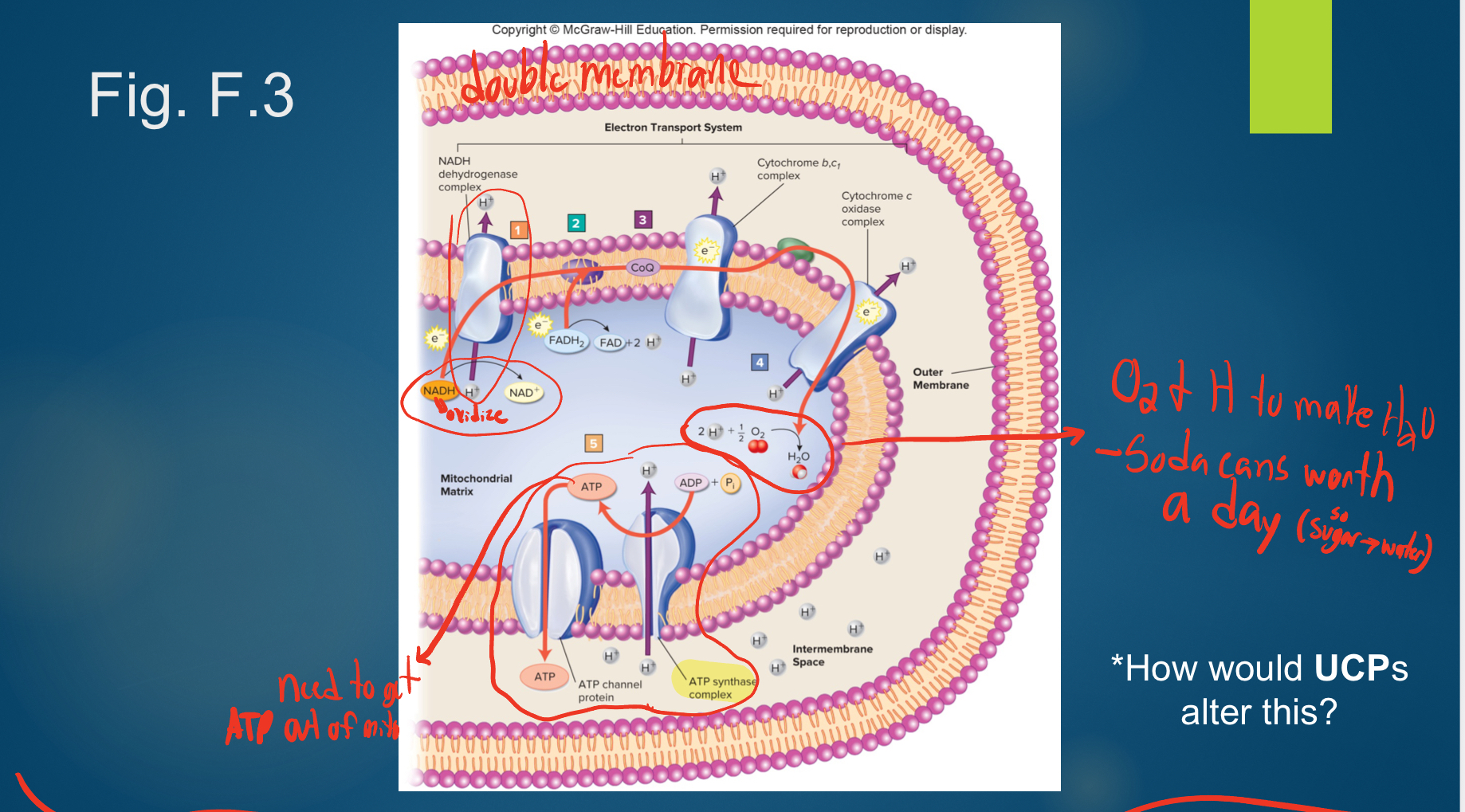
how would UCP alter ETC?
-uncouple oxidation and phosphorylation so less efficent
*2nd doesn’t happen even though first did
-make proton slides so metabolism has to increase
ex- if same # slides and ATPase: make ½ ATP, thus metabolism doubles in rate to make the same amount of ATP
-we all have UCP; brown fat burned to make heat
-pill as weight loss tool, but not complete (=cynainde!)
how many kcal=1lb fat?
3500kcal=1lb fat
if 3500 kcal = 1lb fat, and burn 5000kcal/day, how much would you eat to burn 1lb?
1500 kcal
Summary of complete glucose oxidation
-The complete oxidation of glucose can be represented as follows:
C6H12O6 + 6O2 + #ADP + #Pi → 6CO2 + 6H2O + 30-something ATP
-About 40% of the energy in glucose is captured by ATP; the remainder is given off as heat.
-Glycolysis, the Krebs cycle, and especially the electron transport chain provide all of the ATP for cellular activities.
-Because the Krebs cycle and electron transport chain depend on aerobic processes, cells cannot carry on their activities for long without sufficient oxygen.
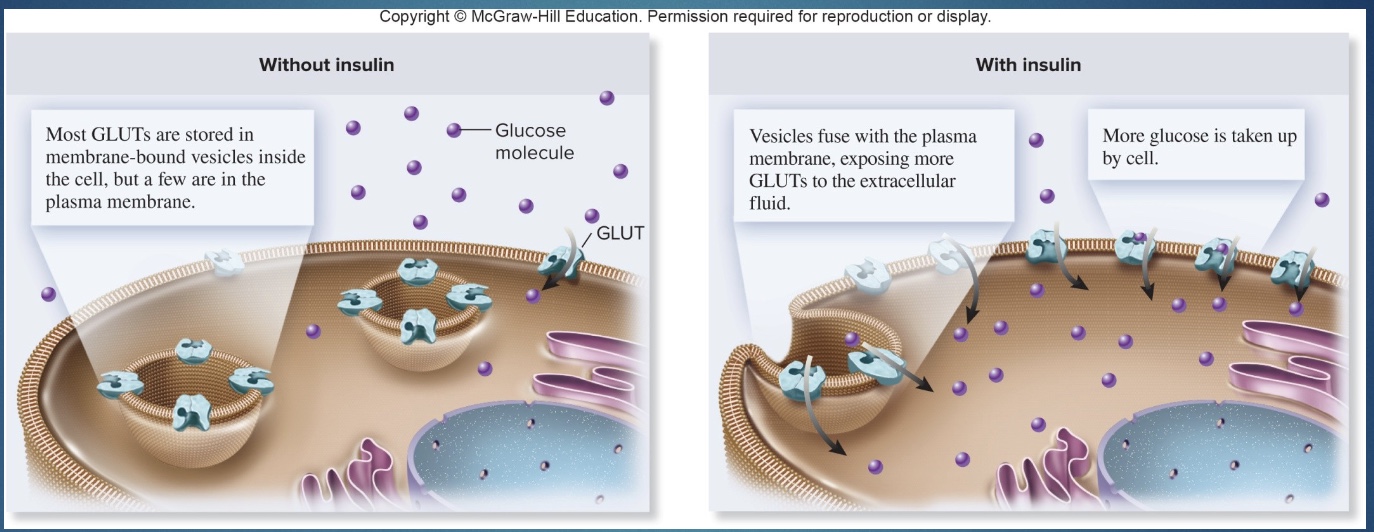
Glucose entry into muscle cells requires
GLUT-4 translocation and is stimulated by both insulin and exercise-related pathways.
*liver doesn’t use Glut-4
Glycogenesis (+ liver vs muscle)
-Glycogenesis is the conversion of glucose to glycogen by glycogen synthase for storage in the liver and skeletal muscle.
-The process is stimulated by insulin and occurs following meals
*liver stores for time not eating so maintain constant blood sugar (kcal not full in morning for liver), 100g=400kcal glucose
*muscle for itself, never leaves cell, 400g=1600kcal glucose (so 2000 kcal total carb between liver and muscle)
starch and fiber are both forms of
glycogen (→ in animals)
-in plants….
*starch=digestible
*fiber=non-digestible
carbohydrate loading
-is the practice of consuming large amounts of carbohydrates prior to an endurance event (such as marathon running), in an effort to maximize glycogenesis.
-By providing the largest possible glucose storage, one might avoid the fatigue associated with glycogen depletion
glycogenolysis (+ stim)
-Glycogenolysis is conversion of glycogen back to glucose by glycogen phosphorylase (break glucose off glycogen; trap in cell by sticking P on glucose).
-This process occurs between meals and during exercise and is stimulated by glucagon and epinephrine (fight or flight).
-In the liver it is done to maintain blood glucose levels. In skeletal muscle it is done to fuel activity.
gluconeogenesis (+when happen?)
-is the conversion of amino acids or glycerol molecules into glucose.
-It occurs in the liver and is done to provide the blood with a continual glucose supply.
-This is important when a person is starving, eating a very low carbohydrate diet, or is exercising for a long period of time.
-Gluconeogenesis is stimulated by glucagon, along with other hormones.
*make glucose from scratch
Glut-4 (with and without insulin)
-Glut-4 stimulated to translocate by:
1) insulin (can’t go in cell so receptor on surface)
2) exercise (but dif pathways than insulin; imp for type II diabetes)
-With out insulin AND no exercise/at rest, glut-4 stays in the cell
*endurance exercise up regulates genes that make glut-4 transporters, so more glut-4 in cells and can more easily regulated blood sugar
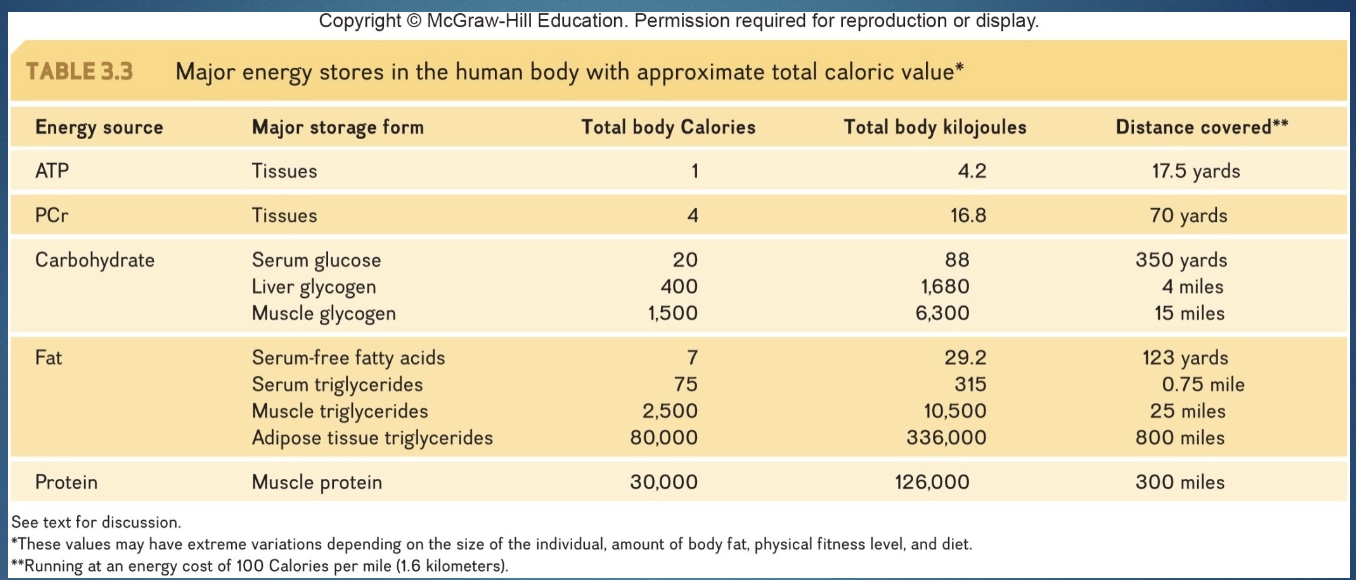
scale of how many kcal stored where in body
*don’t store much fat in muscle; if do in dark meat/twitch fibers
-This stored fat represents the largest amount (~80%) of the stored energy in the body (way more kcal than stored glycogen).
-And at rest, roughly half the body’s energy is derived from its breakdown
lipid metabolism (overview)
-During digestion, triglycerides are broken down into fatty acids and monoglycerides.
-Longchain fatty acids and monoglycerides are carried in micelles for entrance into villi, digested to glycerol and fatty acids in epithelial cells, recombined to form triglycerides, and transported by chylomicrons through the lacteals of intestinal villi into venous circulation
Fate of Lipids
1. Oxidized to reform ATP (*burn)
2. Stored in adipose tissue (*in between meals)
3. Used as structural molecules or to synthesize essential molecules such as: (*build)
• Phospholipids and cholesterol of plasma membranes; Myelin sheaths of neurons; Thromboplastin for blood clotting; Cholesterol used to synthesize bile salts and steroid hormones; Lipoproteins (e.g. LDL) that transport lipids such as cholesterol
Fat storage
-Triglycerides are gathered from chylomicrons or lipoprotein molecules and stored primarily in adipose tissue, mostly in the subcutaneous layer.
-This stored fat represents the largest amount (~80%) of the stored energy in the body (way more kcal than stored glycogen).
-And at rest, roughly half the body’s energy is derived from its breakdown
*if 150 lb and 20% fat: 30lb fat and 100,000 kcal (prob store 2000 kcal carb)
*if 400lb and 50% fat: 200lb fat and ~1,000,000 kcal
Lipid catabolism: lipolysis
-Triglycerides are hydrolyzed by lipases into free fatty acids and glycerol (a process called lipolysis) and mobilized throughout the body.
-This process is under the influence of hormones such as epinephrine, norepinephrine, and cortisol.
-Glycerol can be converted by the liver into glucose via gluconeogenesis.
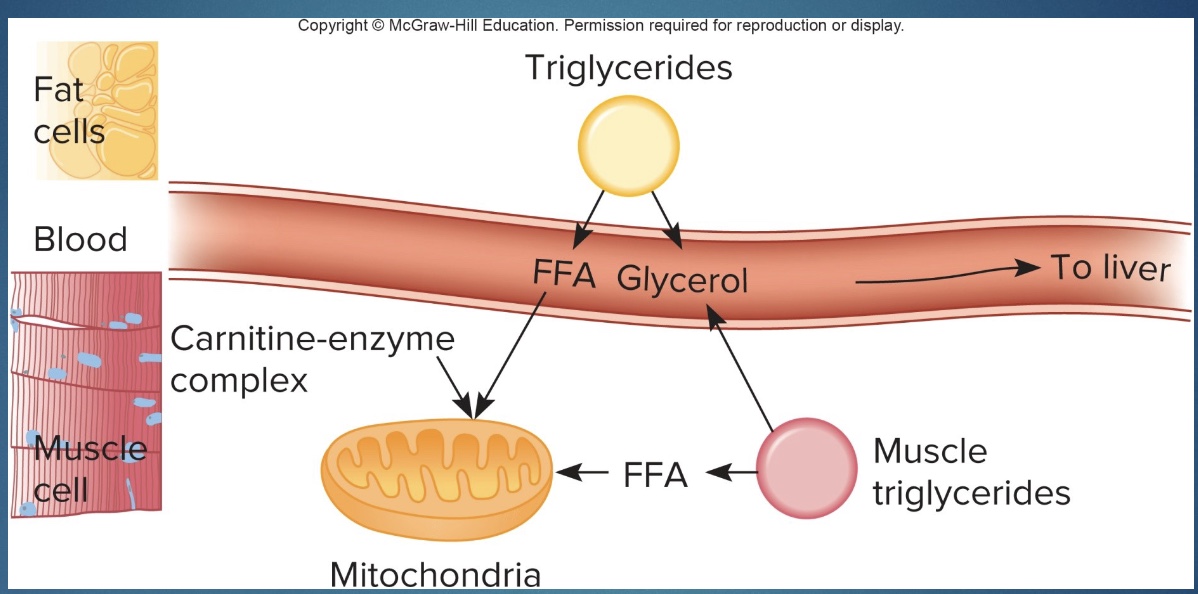
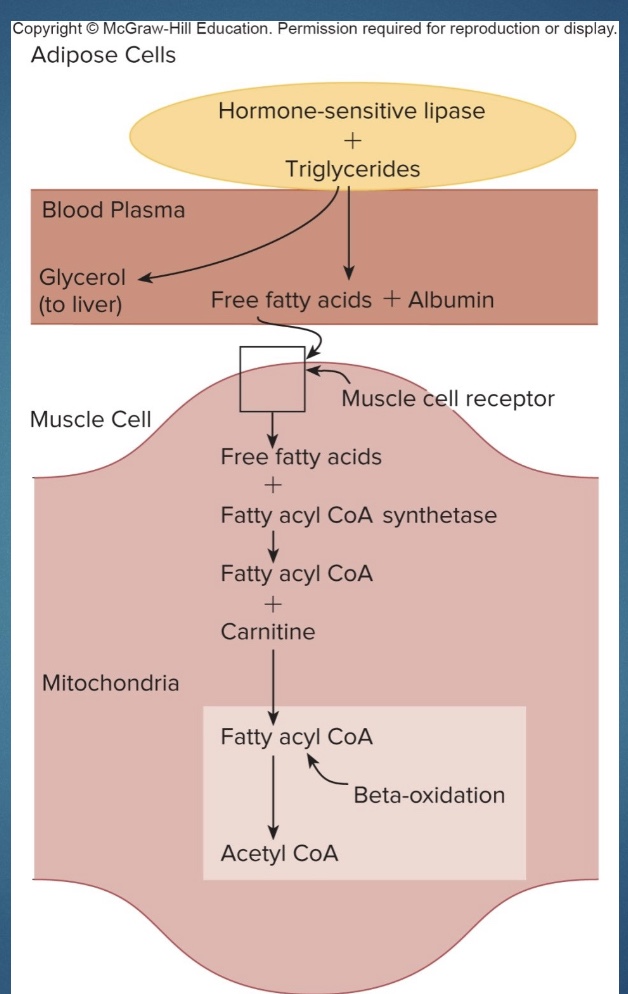
Lipid catabolism: Carnitine and Beta-Oxidation
• In muscle cells, fatty acids are combined with CoA, and with the help of carnitine are transferred into the mitochondria.
*sold to burn fat but not the rate limiting step; to make start as AA and add vit and minerals until get “mini protein”
• In the mitochondria, fatty acids undergo beta oxidation, in which their carbon atoms are removed in pairs. Those carbon pairs result in the formation of acetyl CoA and enter the Krebs cycle. ATP resynthesis then occurs through cellular respiration.
*Albumin= fat protein transporter
*Beta-Oxidation: chop up 2C at a time; oxidation bc H pops off; speeds up w/ metabolism; higher in morning when burning more fat
ketones
-ketone= 2 acetyl groups slapped together (so 4C like Butaric Acid/”mini sugar)
-The formation of ketone bodies (ketogenesis) by the liver is a normal phase of fatty acid catabolism, but this process is elevated if someone limits carbohydrates—putting them into nutritional ketosis.
-Ketones fuel nervous tissue alongside blood glucose.
Finish the Statement
-most fat is in ___
-beta oxidations does not equal ____
-to burn fat, you should ___
-adipose tissue
-lypolysis
-eat fat (if weight stable)
Lipogenesis
-The conversion of glucose or amino acids into lipids is called lipogenesis, occurs in the liver, and is stimulated by insulin.
-This will occur when excess daily calories are consumed.
*insulin goal: store stuff in body
*”beta-oxiidation backwards”: link acetly groups together
do we “burn” kcal when making or breaking ATP?
Both!
-happen simultaneously
-can measure them being broken w/ VO2
true or false: FFA have an even number of carbons?
true!
-even #’s and long
Carnitine
*don’t have to know steps!
-Helps get fatty acid and CoA into mito
-add vitamins and minerals: minerals: skeletor, enzymes; vitamins: enzymes
-end w/ “mini protein”
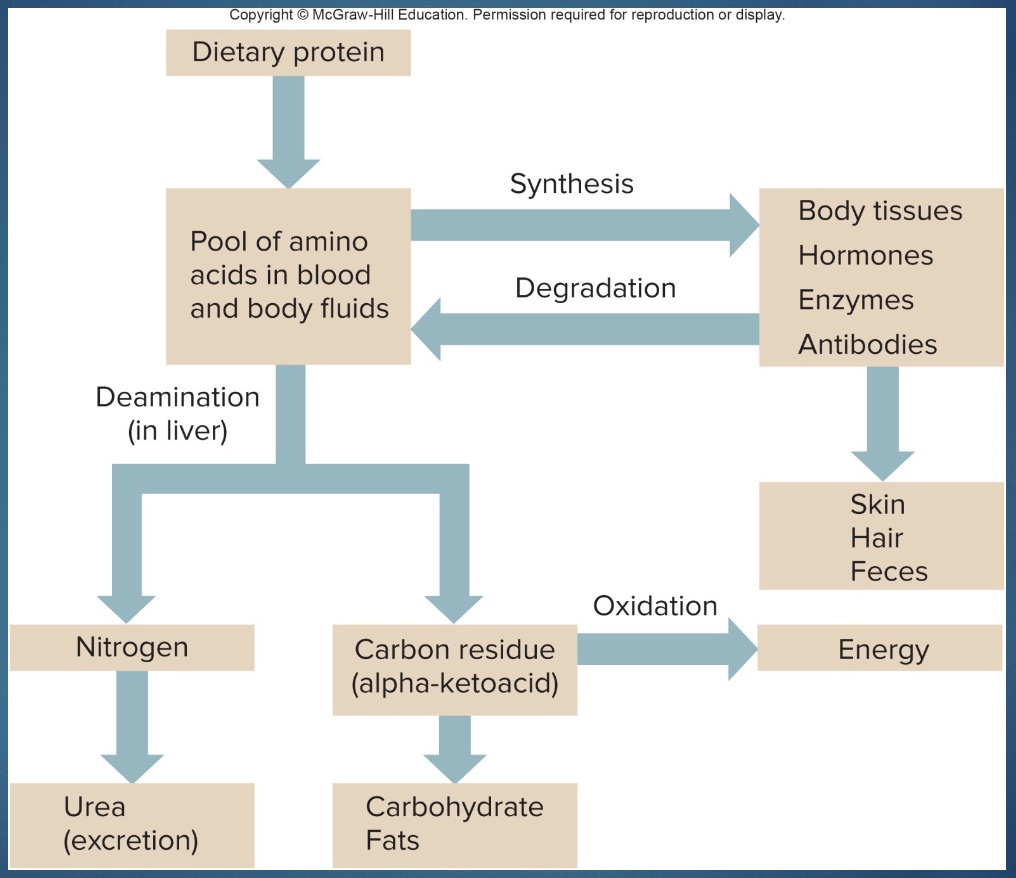
Protein metabolism (overview)
-During digestion, proteins are hydrolyzed into amino acids.
-Amino acids are absorbed by the capillaries of villi and enter the liver via the hepatic portal vein
Fate of Proteins
-Amino acids, under the influence of human growth hormone (hGH) and insulin, enter body cells by active transport.
-Inside cells, amino acids are synthesized into proteins that function as: Enzymes, Contractile elements in muscle fibers, Antibodies, Collagen, Hormones, Clotting chemicals, Transport molecules, Structural elements, DNA
-Some protein is lost from the body as hair and nails or when shed as cells from the GI tract or skin
Protein Catabolism (all different pathways)
-Before amino acids can be catabolized, they must be converted to substances that can enter the Krebs cycle or become glucose.
-These conversions involve deamination and occur in the liver.
The amine group (NH2) is removed, converted into urea, and excreted in urine.
What remains is converted into such things as pyruvate or acetyl CoA. This can then be oxidized.
*some protein in blood, build other; constant turnover protein so degrade
“carbon residual”=carbon backbone; “oxidation” could be lipogenesis; nitrogen=amine group
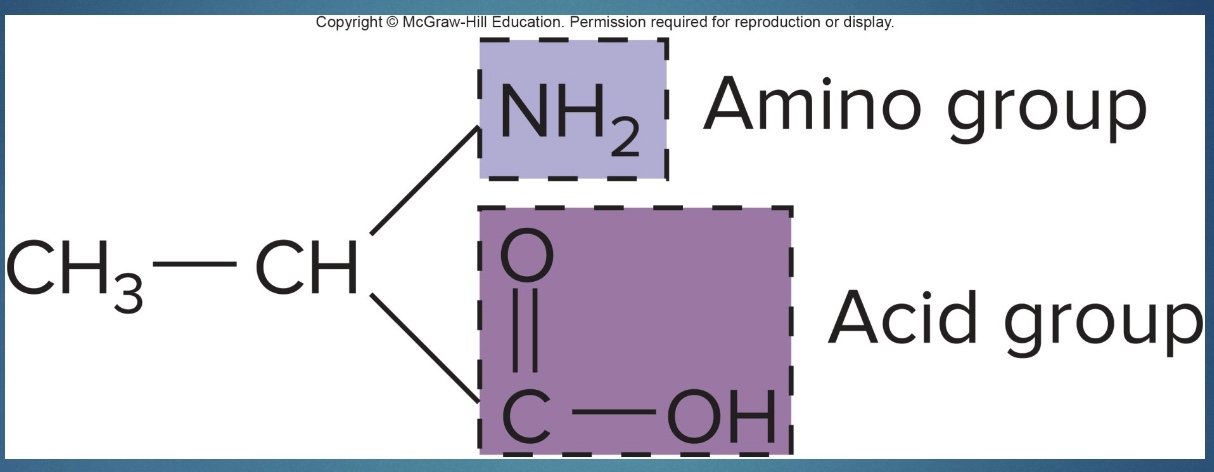
Deamination
-take amine group of AA; amine group becomes urea that is excreted in urine
-left with a glucose molecule that can enter metabolism/the Krebs cycle (can become pyruvate or acetyl CoA)
-occurs in liver
*N unique to AA
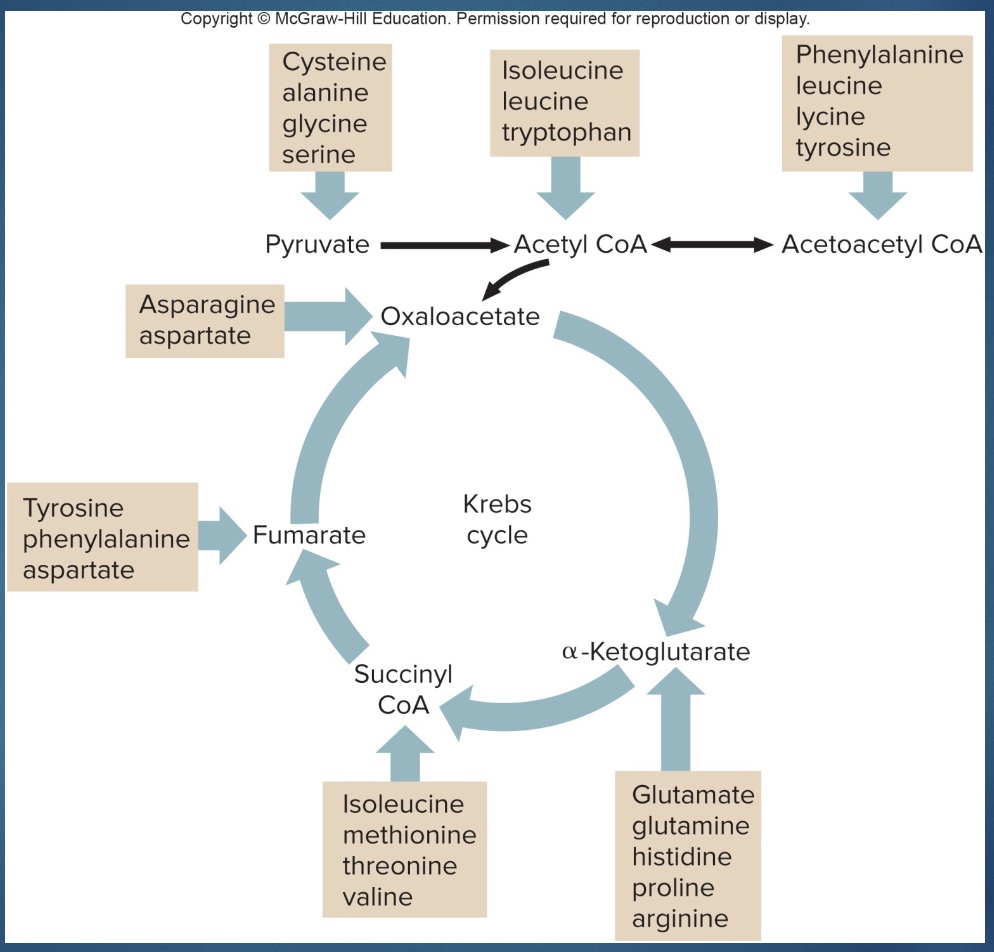
How AA feed into metabolism
*deaminate AA in the liver, what left can become other molecules (pyruvate or acetyl CoA) that can go into metabolism/Krebs
*can come into beginning or middle
Protein Anabolism
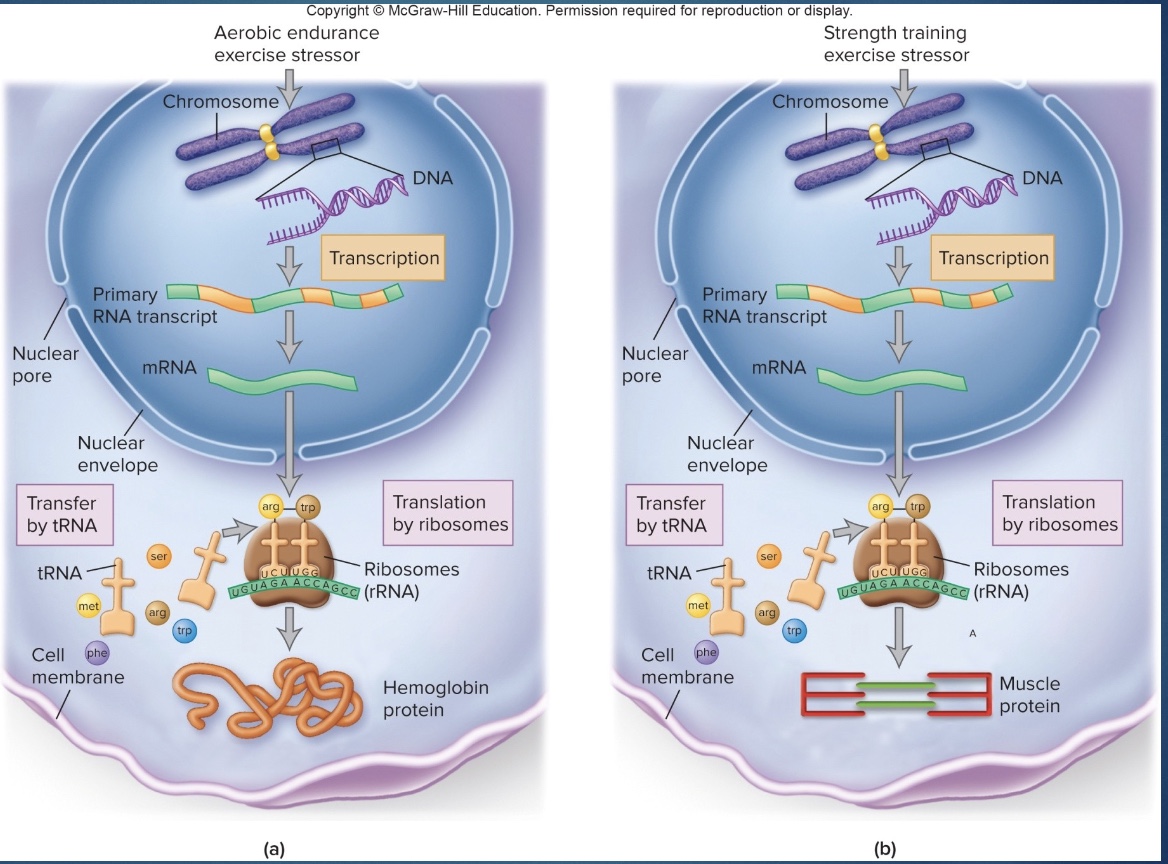
-Protein anabolism involves the formation of peptide bonds between amino acids to produce new proteins
-This protein synthesis is carried out on the ribosomes of almost every cell in the body, directed by the cells' DNA and RNA.
DNA
-blueprint for building proteins
-transcribe, translocate to make protein
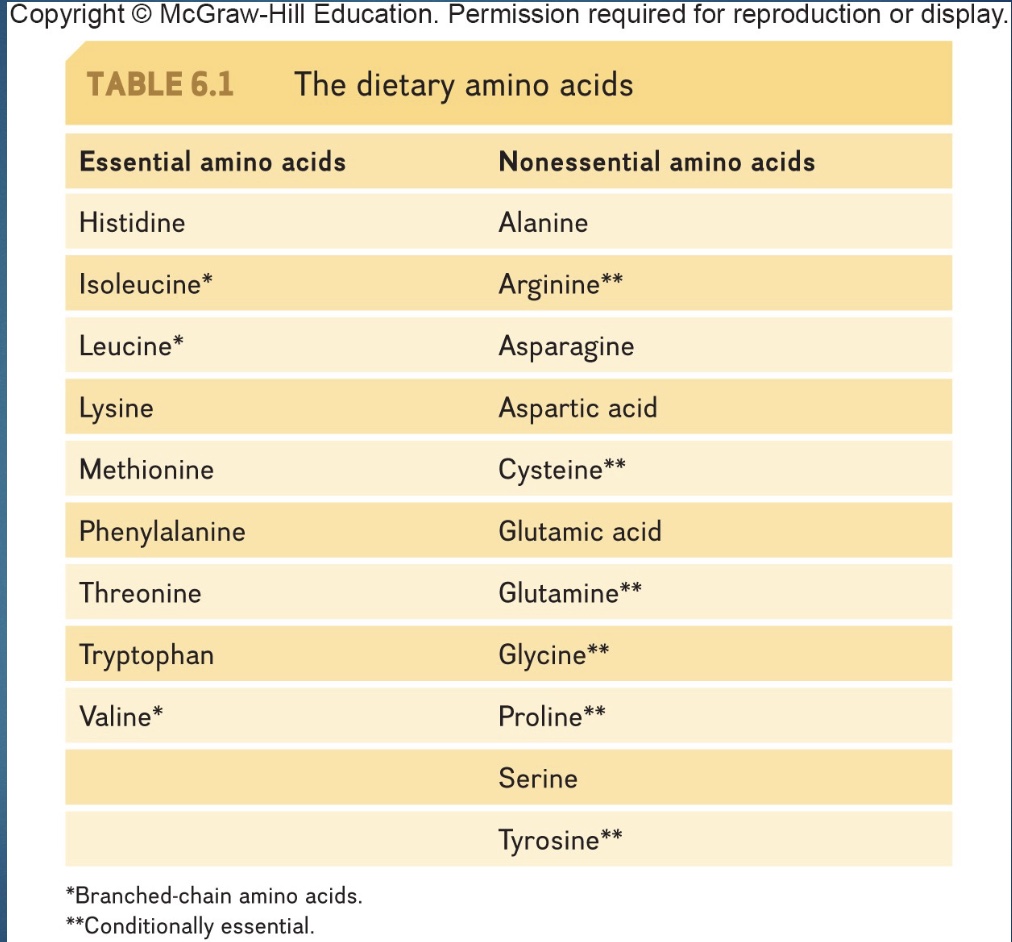
Essentail & Non-Essential Amino Acids
- ½ AA are essential (of the 20 amino acids in your body, 9 are referred to as essential amino acids)
-can make non-essential out of essential
*branched-chain= in muscle
Summary of Key Molecules in Metabolism
Although there are thousands of different chemicals in your cells, three molecules play key roles in metabolism: glucose 6-phosphate, pyruvate, and acetyl CoA
-When glucose enters a (muscle or fat) cell, it is immediately phosphorylated to become glucose 6-phosphate. This traps glucose inside the cell. Glucose 6-phosphate may be converted into: Pyruvate, Glycogen, Glucose (only in the liver, helps maintain blood sugar), or Ribose (the pentose sugar needed for synthesis of RNA, DNA, ATP)
*not in liver where pop P off so glucose can go to blood
-When ATP is not needed quickly (at rest or moderate exercise) and oxygen is sufficient, pyruvate is converted to acetyl CoA. When ATP demands are intense (high-intensity exercise) or oxygen supply insufficient, pyruvate is converted to lactate. Pyruvate also can be used to generate nonessential amino acids
*gluconeogenesis= glycolysis backwards
-Acetyl CoA is the gateway into the Krebs cycle and is also used to make fatty acids and cholesterol
*joining point all macro nutrients
Absorptitive and Postabsorptive states (overview + time frames)
-your metabolic reactions depend on how recently you have eaten
-During the absorptive (fed) state, which alternates with the postabsorptive (fasting) state, ingested nutrients enter the blood and lymph from the GI tract, and glucose is readily available for ATP production.
-An average meal requires about 4 hours for complete absorption, and given three meals a day, the body spends about 12 hours of each day in the absorptive state.
-The other 12 hours, during late morning, late afternoon, and most of the night, are spent in the postabsorpative state/”fasting phase”
*rest=1kcal; fast=0kcal; dinner=5kcal/min
Absorptive state
-Most body cells produce ATP by oxidizing glucose to carbon dioxide and water.
• Glucose transported to the liver and skeletal muscle is converted to glycogen
• Most dietary lipids are stored in adipose tissue (prioritize fat into storage)
• Amino acids in liver cells are converted to carbohydrates, fats, and proteins.
-Insulin stimulates absorptive state metabolism. Soon after eating, the rise in blood glucose and AA concentration stimulates insulin release from pancreatic beta cells
*higher RER
postabsorptive state
-absorption is complete, and the energy needs of the body must be satisfied by nutrients already present in the body
-The major concern of the body during the postabsorptive state is to maintain normal blood glucose level (70 to 100 mg/100 ml of blood). This involves conversion of liver glycogen into glucose
• Glycerol, produced by hydrolysis of triglycerides primarily in adipose tissue, is converted to glucose. During prolonged fasting, large amounts of amino acids from tissue protein breakdown (primarily from skeletal muscle) are also released to be converted to glucose in the liver by gluconeogenesis.
• Although the nervous system continues to utilize blood glucose normally, all other body tissues reduce their oxidation of glucose and switch over to fatty acids as their main ATP source in order to help minimize the use of blood glucose. As a result of this glucose sparing and fatty acid utilization, a person can fast for several weeks, provided water is consumed, and the glucose level will not drop more than 25% from its normal range.
• Glucagon is a hormone that stimulates metabolism in the postabsorptive state. It belongs to a group of hormones (such as epinephrine, cortisol, and GH) sometimes called antiinsulin hormones because they counter the insulin effects that dominate the fed state.
*lower RER
*liver put sugar in blood
Total Body Energy Balance: thought
"Instead of asking why people persist in eating too much and exercising too little, the key questions of obesity research should address those factors (environmental, behavioral or otherwise) that lead to dysregulation of the homeostatic mechanism of energy regulation."
“Kcals in” vs “Kcals out”
-Energy stored = Intake of food energy – (External work + Internal heat produced)
-Energy stored = Intake of food energy – (BMR + thermic effect of food + physical activity)
^all mean same thing!; true statement
• Metabolism of 2500 kcal/d and diet intake of 2000 kcal/d ≈ loss of 1 lb/wk (eat 500kcal less each day for a week)
• Seems simple, but more complex…
Energy Balance: Thought questions + what regulates in and out?
-In: hunger, satiety, social factors
-Out: figet, BMR, PA, night hormones (Ex: thyroid)

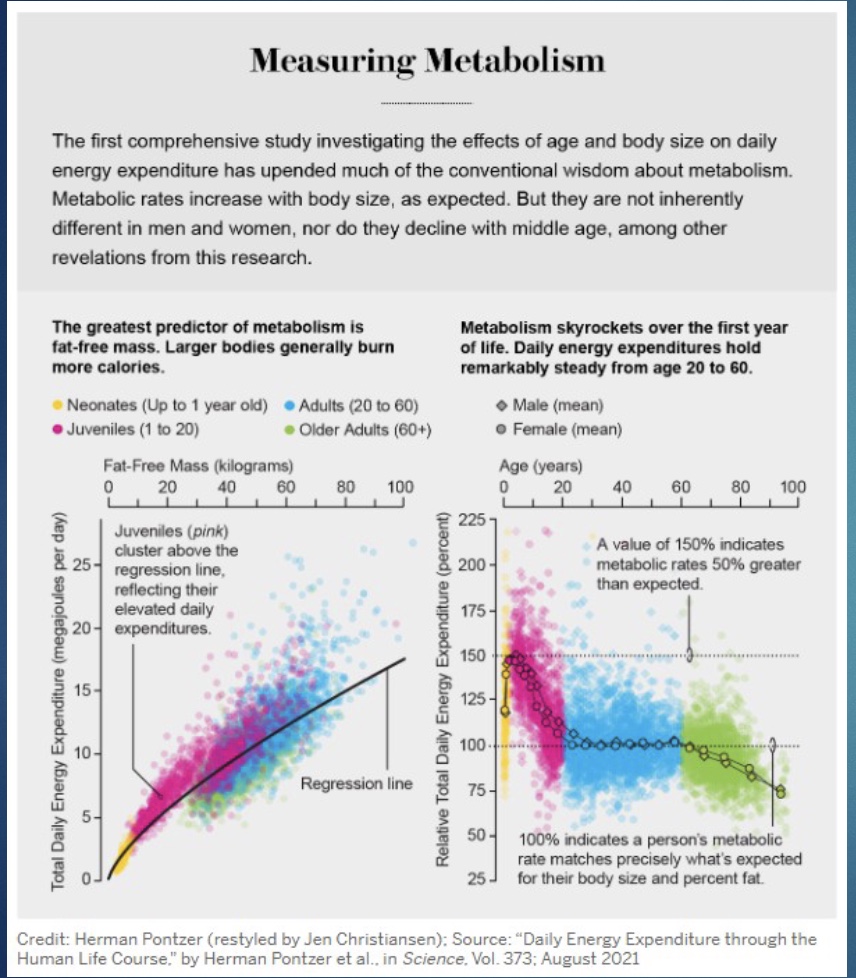
Measuring Metabolism Study: LBM and Age
-LBM: increase LBM=increase TDEE
-Age: younger=higher metabolism; level out age 20; decline once hit 60 years and older (where metabolism actually starts to slow down; can lead to decreased immune)
*study had people drink non normal H and O, see how fast went though them
Is counting calories the answer to weight loss?
-depends on person
-but gets you to stop thinking about eating pattern and quality of food (which is not good!)
-self-control a factor? Physiological awareness?
How is hunger and satiety regulated?
-Hypothalamic “food intake center” feeding signals vs. satiety signals (limbic: emotions w/ food)
-hunger and satiety=experiences in brain based on signals
-Special in that have more conscious physiological awareness of our hunger
-Regulated by hormonal and neural signals that give sense if full or not
hormonal signals: leptin and insulin (tells you full)
senses like taste (ex: eat more if taste good= “hyperpalitability”)
gastric distension: more full if stuff in stomach
*2nd info graph: shows pathway monosaccharides, artificial sweeteners and receptor, “bitter toxins”=phytochemicals that bind to receptor
*Ghrelin when stomach empty; GLP-1=ozempic; CCK
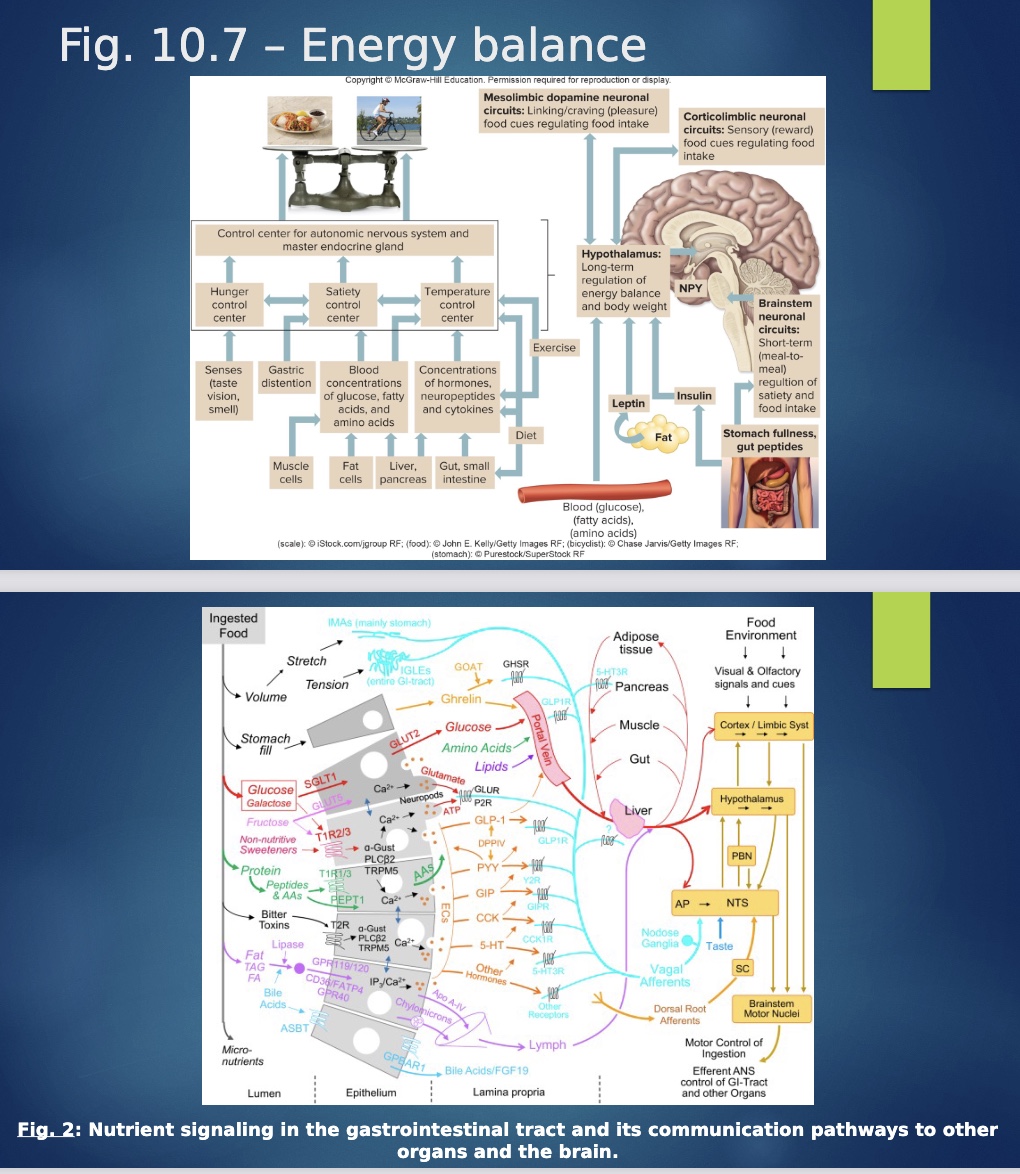
“anti-insulin” hormones
-Glucagon, epinephrine, cortisol, and GH
-called antiinsulin hormones because they counter the insulin effects that dominate the fed state
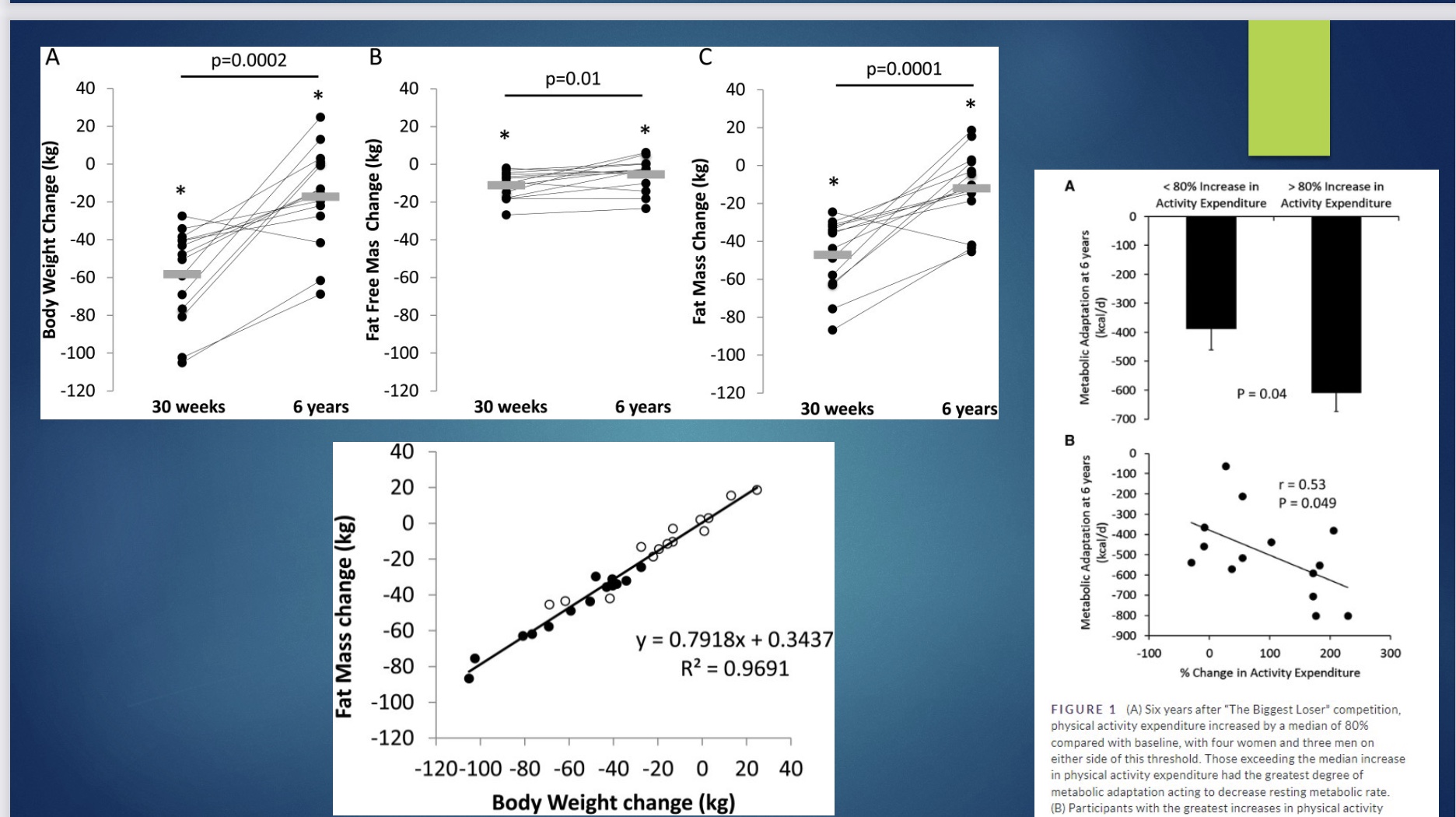
Biggest Loser Study
-14/16 contestants participated; took data before show, end of show, and 6-year follow up; measured BMR and body composition; once study published, show never aired again
-During show participants did LOTS of exercise and had very low kcal diet
definition of growing
eat more kcal than body burns; where anabolism dominates over catabolism
Definition of Overweight and Obesity
-BMI: normal=18.5-25; OW=25-30; Obese=>30 (different classes)
*in kg/m²
-difficult to define; drawbacks like LBM, so do body comp assessment to make more sense
-obese: excess weight/fat
U.S. Trends for Obesity
-average BMI went up from 70’s to 2000’s teens, but slowing down now (bc of GLP1 drugs???)
-measure every year, but research still hard
-now, 70% population overweight OR obese
Consequences of Obesity
-Risk factor for chronic disease (driver of poor health)
-orthopedic problems
-expense
-quality of life
-lose productivity
Trend TDEE (+ possible reasons why)
-has declined over the past 3 decades due to declining BMR, and NOT reduced PA
-PA up, but BMR down at a faster rate
Why?
-too much temperature controlled envionrments? (less energy expended to control body temp)
-envionrment too clean? (spend less energy to fight pathogens)
-animal studies have found that decreased sat fat consumed (what guidelines recommend for humans!) leads to decreased metabolism
Metabolic adaptation
evolutionary process to preserve body tissue by slowing down metabolism/BMR
*so don’t “vanish”
Big Takeaways from Biggest Loser Study
-Metabolic rate down for contestants
-”Metabolic Adaptation”: evolutionary process to preserve body tissue
-”set point theory” vs “setting point” (if spend enough time at new weight)
-Hormone regulated
-Envionrmental factors (ex: what food have in home)
*Ken: cognitive behavioral theory is quite successful
Bariatric Surgery + Study
-take apart stomach and staple stomach so smaller; pretty successful
-Study: BMR went back to normal and mainted weight loss
why dif than biggest loser?: bc did intense exercise in biggest loser
Biggest Loser Study Results: weight loss and slowing metabolism
*BMR relative to size
-not surpising the BMR decreased as lost weight during show
-but after show and as gained back weight, would expect BMR to increase (BMR increases even if fat)
-BUT, their metabolism slowed to an even greater extent than expected after the show
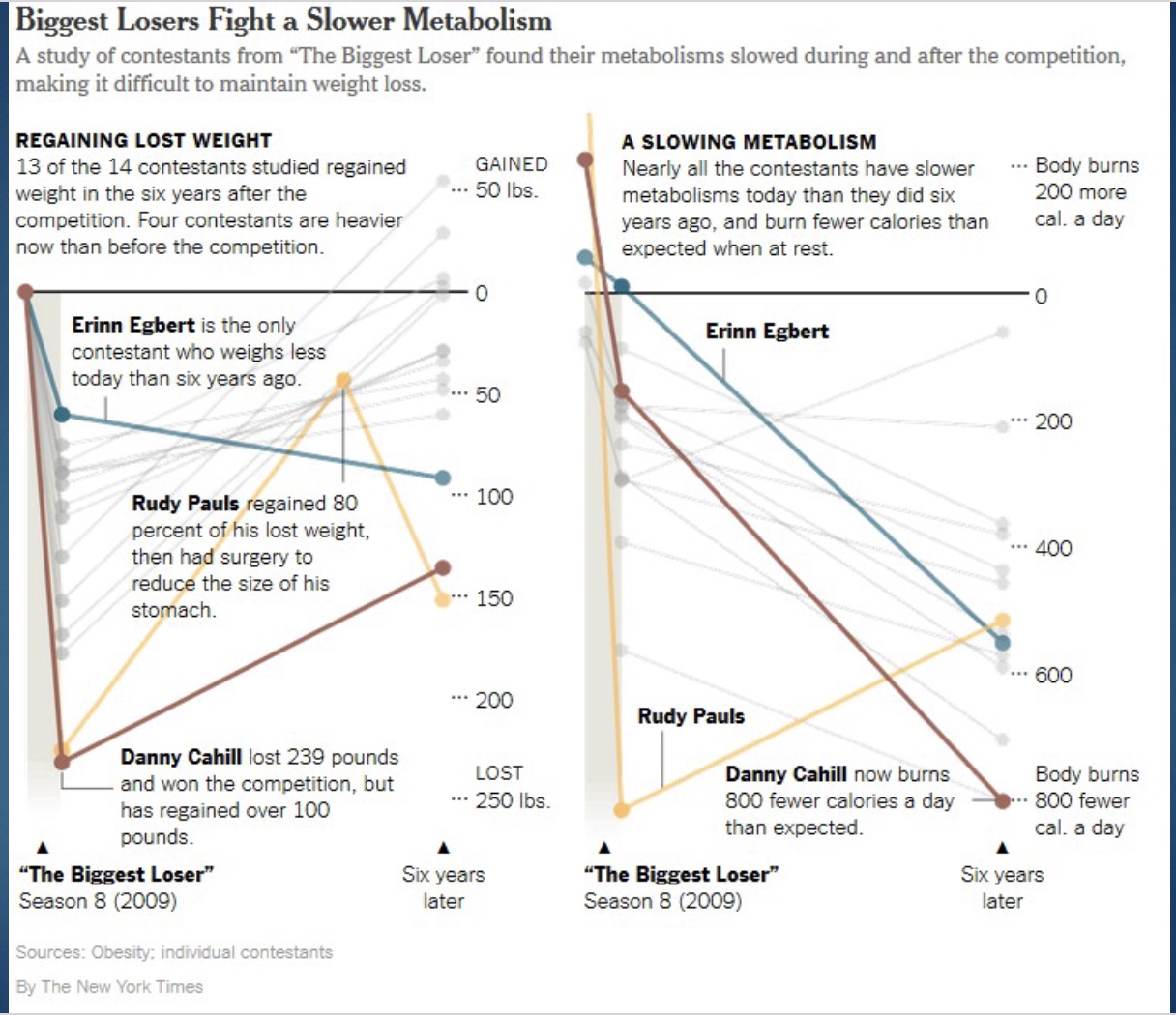
Biggest Loser Study Results: How much Metabolism Changed
*metabolic adaptation
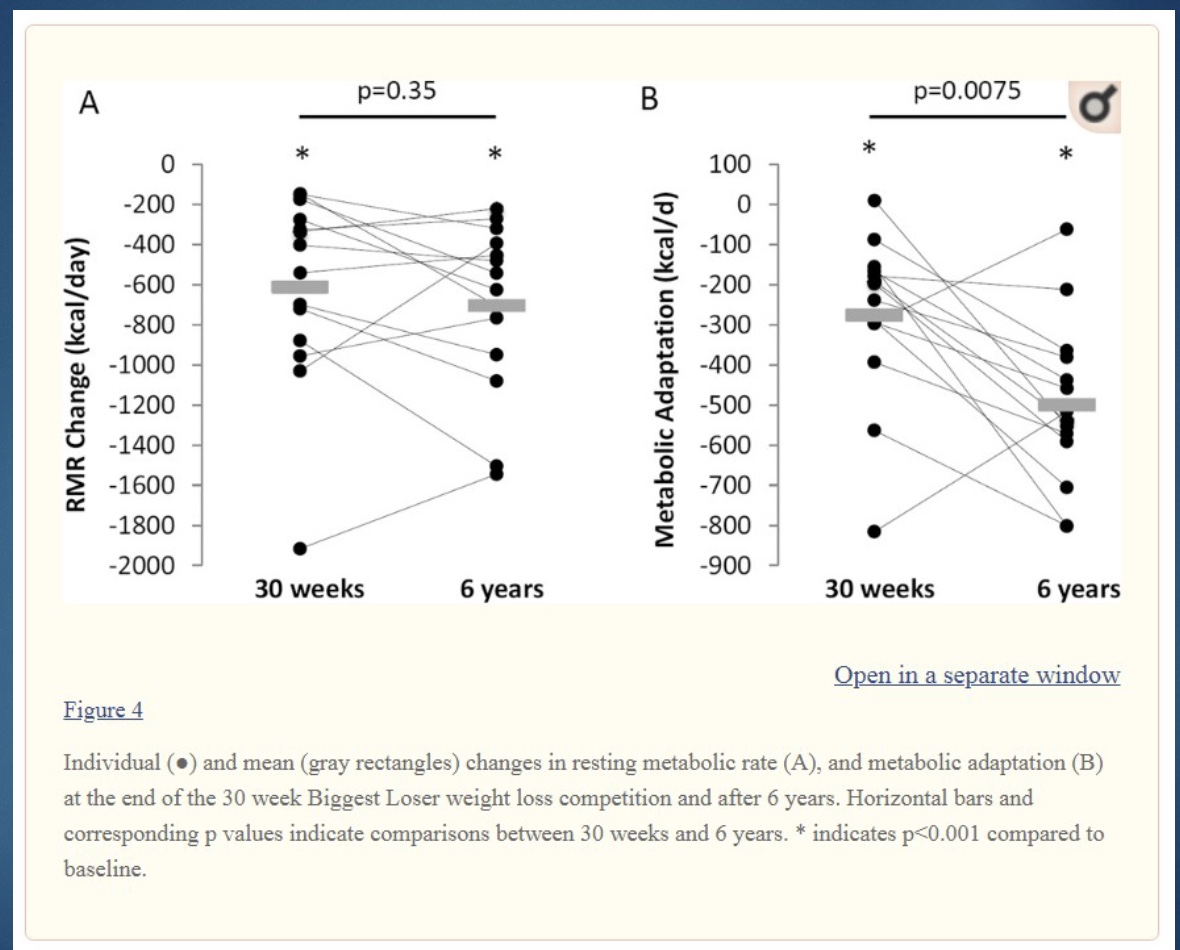
Biggest Loser Study Results: Body Composition
-BW went up over the 6 years
-lost 10kg/20lb of lean tissues (like organs and bone density) start of show; after show (when gained weight) gained lean body mass back
-gained fat mass back
-change in fat mass correlates well w/ BW (ex: lost 90% fat and 10% LBM, and gained back 90% fat and 10% LBM)
-How much exercised: increase exercise= increase metabolic adaptation (thus the exercise played some role!)
*Ken: like food, don’t label exercise as “good” or “bad”