Topic 6: Acids, Bases, and Buffers - Mastery
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
Salts and pH
When salts dissolve in water, their ions may react with water to produce solutions that are acidic, basic, or neutral, depending on the strength of the parent acid and base.
Neutral Salts
Formed when a strong acid (e.g., HCl, HBr, HClO4) and a strong base (e.g., NaOH, KOH) react. These salts produce neutral solutions because neither ion reacts with water as they are very weak (weak conjugate acids/bases).
Example: NaCl (s) → Na⁺ (aq) + Cl⁻ (aq)
Both Na⁺ and Cl⁻ are spectator ions that do not hydrolyze, so pH = 7.
Acidic Salts
Formed when a strong acid and a weak base react (e.g., NH₄Cl). The anion is the conjugate base of a strong acid, and thus a very weak base that does not affect the pH. The cation is the conjugate acid of a weak base and acts as a moderately strong acid
Example: NH₄⁺ (aq) + H₂O (l) ⇌ NH₃ (aq) + H₃O⁺ (aq)
The cation (NH₄⁺) hydrolyzes to produce H₃O⁺ ions, making the solution acidic.
pH of Acidic Salt Solutions
pH < 7 because the cation acts as a weak acid, generating additional hydronium ions.
Basic Salts
Formed when a weak acid and a strong base react (e.g., NaCH₃COO). The cation from the strong base does not affect the pH. However, the anion is the conjugate base of a weak acid and acts as a moderately strong base
Example: CH₃COO⁻ (aq) + H₂O (l) ⇌ CH₃COOH (aq) + OH⁻ (aq)
The anion (CH₃COO⁻) hydrolyzes to produce OH⁻ ions, making the solution basic.
pH of Basic Salt Solutions
pH > 7 because the anion acts as a weak base, generating hydroxide ions.
Salts of Weak Acid and Weak Base
Both the anion and the cation can undergo hydrolysis. The pH depends on the competition between the acid properties of the cation and the basic properties of the anion - depends on the relative Ka and Kb values of the ions.
Determining Salt Solution’s Nature
Compare Ka (of cation) and Kb (of anion):
If Ka > Kb → acidic
If Ka < Kb → basic
If Ka = Kb → neutral.
Hydrolysis Definition
Hydrolysis is the reaction of an ion in water (if it is a stronger acid or base than water) → causing it to donate or accept protons from water → to form H₃O⁺ or OH⁻ ions → affecting pH.
Buffer Solutions
Buffers resist changes in pH when small amounts of acid or base are added.
Composition of a Buffer
A buffer consists of a weak acid and its conjugate base, or a weak base and its conjugate acid, in roughly equal and substantial amounts.
Acid Buffer: CH₃COOH (aq) + CH₃COONa (aq)
Basic Buffer: NH₃ (aq) + NH₄Cl (aq)
Mechanism of Buffer Action
HA ⇌ H+ + A−
When H₃O⁺ is added, the conjugate base mops up the protons to neutralize it;
A− + H₃O+ → HA + H₂O
When OH⁻ is added, the weak acid donates protons to neutralize it;
OH− + HA → A− + H₂O
Buffer Capacity
The amount of strong acid or base that can be added before the buffer stops stabilizing the pH.
Buffers with higher concentrations of both the acid and base components have a larger buffer capacity
The ability of a buffer to resist pH change is greatest when [A⁻] ≈ [HA], i.e., when pH ≈ pKa.
Preparing a Buffer
Mixing a weak acid (e.g., acetic acid) with its conjugate base salt (e.g., sodium acetate).
Mixing a weak base (e.g., ammonia) with its conjugate acid salt (e.g., ammonium chloride).
Adding a strong base (e.g., NaOH) to a weak acid (e.g., acetic acid) to neutralize half of the weak acid.
Adding a strong acid (e.g., HCl) to a weak base (e.g., ammonia) to neutralize half of the weak base
Buffer Mechanism Example - Acetic Acid Buffer
Acetic Acid Buffer - CH₃COOH + CH₃COONa
CH₃COONa dissociates to give CH₃COO⁻, establishing equilibrium with CH₃COOH.
Adding a strong acid: Added H₃O⁺ is consumed by CH₃COO⁻ → CH₃COOH + H₂O, minimizing pH change.
Adding a strong base: Added OH⁻ reacts with CH₃COOH → CH₃COO⁻ + H₂O, again minimizing pH change.
Buffer Range
A buffer works best when the ratio [A−]/[HA] is close to 1
At Equal [A⁻] and [HA] → pH = pKa, because log(1) = 0.
Effective buffer range is typically pKa ± 1 pH unit.
To make a buffer at a desired pH, one must select an acid/base pair where the pKa is close to that target pH
![<ul><li><p><span><span>A buffer works best when the ratio [A−]/[HA] is close to 1</span></span></p></li></ul><ul><li><p>At Equal [A⁻] and [HA] → pH = pK<sub>a</sub>, because log(1) = 0.</p></li><li><p>Effective buffer range is typically pK<sub>a</sub> ± 1 pH unit.</p></li><li><p><span><span>To make a buffer at a desired pH, one must select an acid/base pair where the pK</span><sub><span>a</span></sub><span> is close to that target pH</span></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/000ae0d5-fc39-48b4-8ae8-25e83d3b3836.png)
Biological Buffers - Carbonic Acid-Bicarbonate Buffer in Blood
H₂CO₃ ⇌ H⁺ + HCO₃⁻
This system regulates blood pH around 7.4 by neutralizing excess acid or base.
Excess acid → HCO₃⁻ + H⁺ → H₂CO₃
Excess base → H₂CO₃ → H⁺ + HCO₃⁻.
Effect of Dilution on Buffers
Dilution changes both [HA] and [A⁻] equally, so pH remains nearly constant (slight shift due to water’s contribution).
Limitations of Buffers
If too much acid or base is added, buffer capacity is exceeded, and pH changes significantly.
Half-Equivalence Point
pH = pKa at this point because [A⁻] = [HA].
Titration
A quantitative technique in which a solution of known concentration (titrant) is added to a solution of unknown concentration (analyte) until the reaction is complete
Equivalence Point
The point during titration where the titrant has completely reacted with the analyte; stoichiometric amounts are present (moles of the acid = moles of the base), and no reactant is in excess.
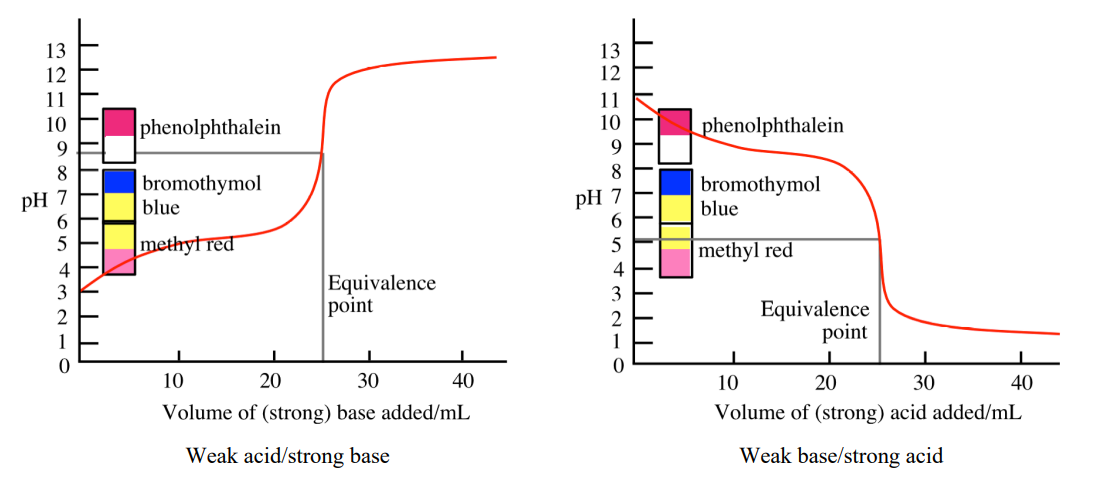
Endpoint
The point during titration at which the indicator changes color; ideally coincides with the equivalence point (specifically, near it).
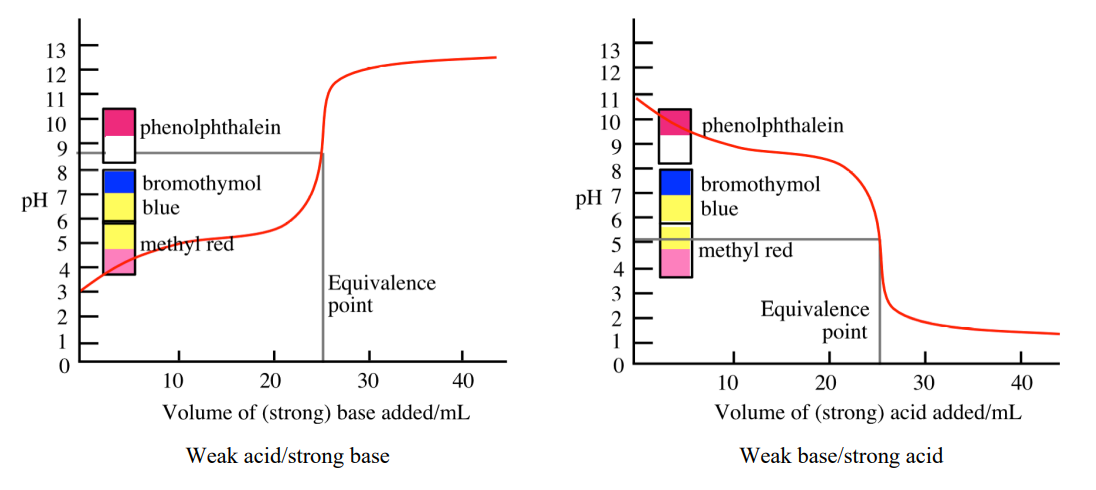
Indicator
A weak acid or base whose conjugate forms have different colors, used to signal the end of a titration.
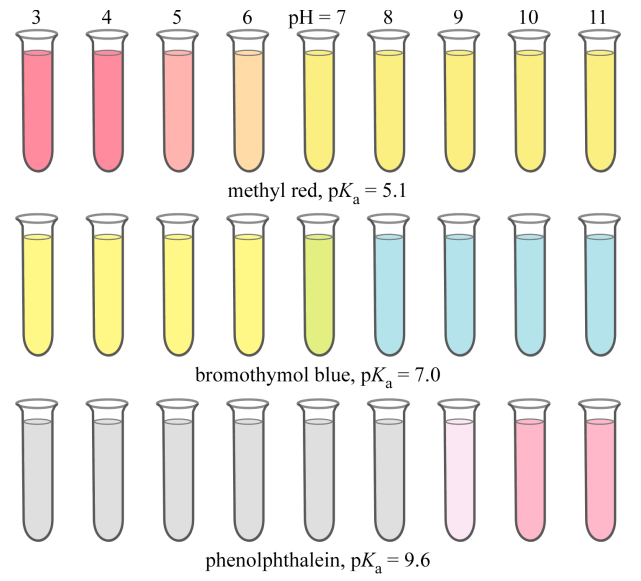
Indicator Equation
HIn ⇌ In⁻ + H⁺; the ratio of the two forms determines the observed color
The acid form (HIn) and conjugate base form (In−) display different colors.
This color difference arises because the loss of H+ changes the molecular structure, altering the energy levels and thus the wavelengths of light absorbed
pH at Equivalence Point
Determines the best indicator;
During titration, you want the end point (when the indicator changes color) to coincide as closely as possible with the equivalence point (where moles of acid = moles of base).
Therefore, the indicator’s pKa should be close to the pH at the equivalence point.
pH at Half-Equivalence Point
Determines pKa of the weak acid or the conjugate acid of the weak base
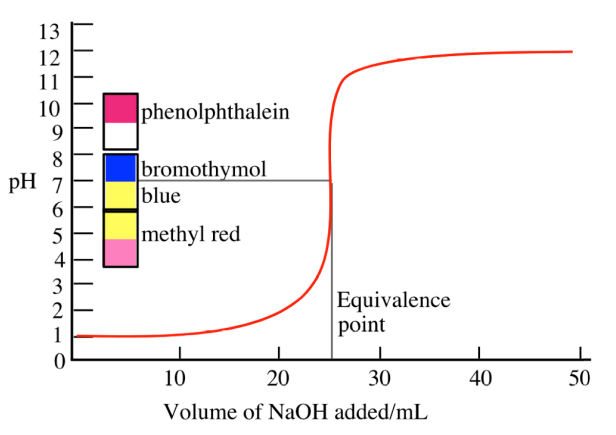
Titration Curve for Strong Acid + Strong Base
pH starts very low because of the strong acid, then at the equivalence point the pH = 7 because the salt formed (e.g., NaCl) does not hydrolyze
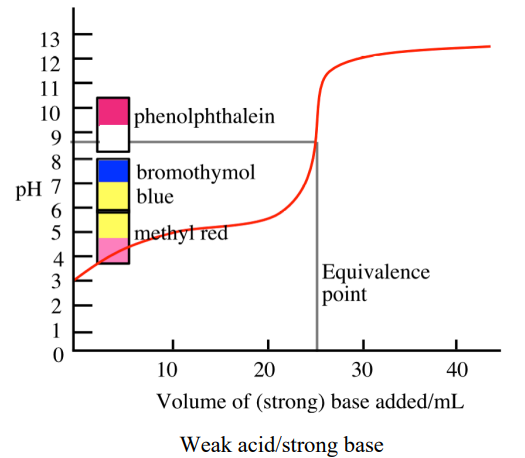
Titration Curve for Weak Acid + Strong Base
pH starts higher than a strong acid because the acid is only partially ionized, then at the equivalence point pH > 7 because the conjugate base (from the weak acid) hydrolyzes to produce OH⁻
Titration Curve for Weak Base + Strong Acid
pH starts high because the base solution is basic, then at the equivalence point pH < 7 because the conjugate acid hydrolyzes to produce H⁺
Buffer Region in the Titration Curve
A plateau in the titration curve where both the weak acid and its conjugate base are present, resisting pH change
Occurs before the equivalence point
Strong Acid + Weak Base Indicator Rule
Use indicator with color change in acidic region
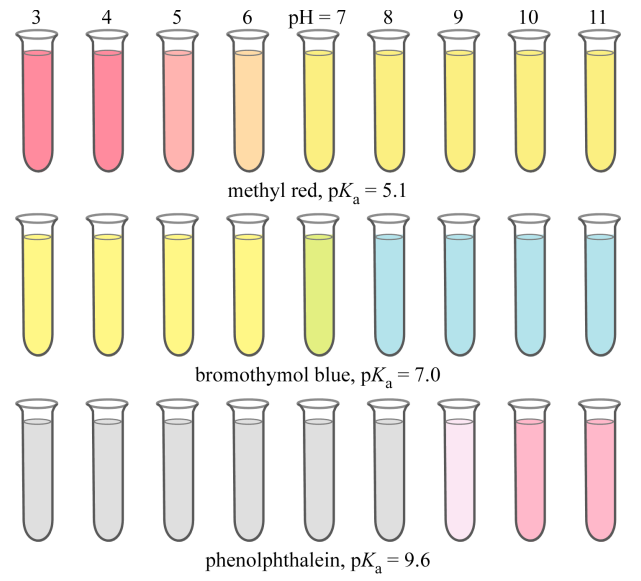
Strong Base + Weak Acid Indicator Rule
Use indicator with color change in basic region
Half Equivalence Point (For Weak/Strong Acids/Bases)
The point at which half of the acid (or base) has been neutralized → so [HA] = [A⁻] or [B] = [BH⁺] → so pH = pKa or pOH = pKb (because from the H-H equation, where the concentrations of acid and conjugate base/base and conjugate acid are equal, it becomes log(1), which is 0)
pKa Determination Practical Use
Allows experimental measurement of weak acid or weak base strength
Titration Curve Interpretation
Inflection point corresponds to equivalence point; flat region indicates buffer zone
Lewis Definition of Acids and Bases
A broader definition proposed by Gilbert Lewis that classifies acids and bases based on electron pair transfer rather than proton transfer.
Lewis Acid
A substance that can accept a pair of electrons to form a bond (electron pair acceptor).
Examples: H⁺ (prototype Lewis acid), electron-deficient molecules like BF₃, and metal cations such as Co³⁺
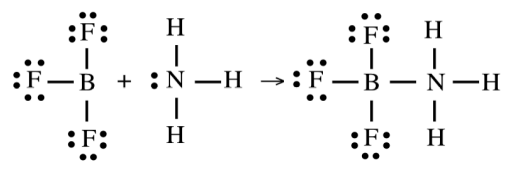
Lewis Base
A substance that can donate a pair of electrons to form a bond (electron pair donor)
Examples: Ammonia (NH₃), amines, and ligands containing lone pairs of electrons.
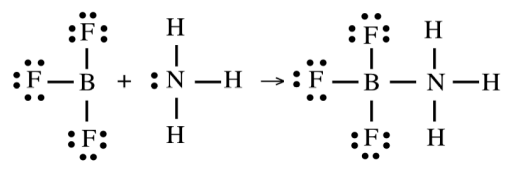
Alternative Names for Lewis Acid
Also called an electrophile (“electron-loving”).
Alternative Names for Lewis Base
Also called a nucleophile (“positive charge loving”).
Mechanism of Lewis Acid–Base Reaction
Involves the formation of a coordinate covalent bond when the base donates an electron pair to the acid
Example: BF₃ (Lewis acid) reacts with NH₃ (Lewis base) to form a coordinate covalent adduct
BF₃ acts as the electron pair acceptor because boron is electron deficient
NH₃ acts as the electron pair donor through its lone pair on nitrogen.
BF₃·NH₃ is the product, where nitrogen donates an electron pair to boron, completing its octet
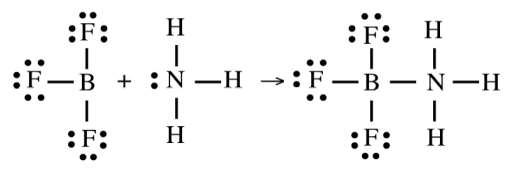
Product of a Lewis Acid–Base Reaction
An adduct — a compound formed by the combination of a Lewis acid and Lewis base via electron pair donation
Scope of Lewis Definition
Extends acid–base theory to reactions where no proton transfer occurs
Complex Ion Formation
A type of Lewis acid–base reaction where metal ions (Lewis acids) accept electron pairs from ligands (Lewis bases)
Example: Hydrated Cobalt Ion Reaction — [Co(H₂O)₆]³⁺ reacts with NH₃; Co³⁺ acts as Lewis acid by accepting electron pairs from ligands, and NH₃ or H₂O act as Lewis bases donating electron pairs to form coordinate bonds with the metal ion.
Significance of Lewis Theory
Provides a unified explanation for acid–base behavior in reactions without H⁺ or OH⁻ ions, including coordination and organic reactions.