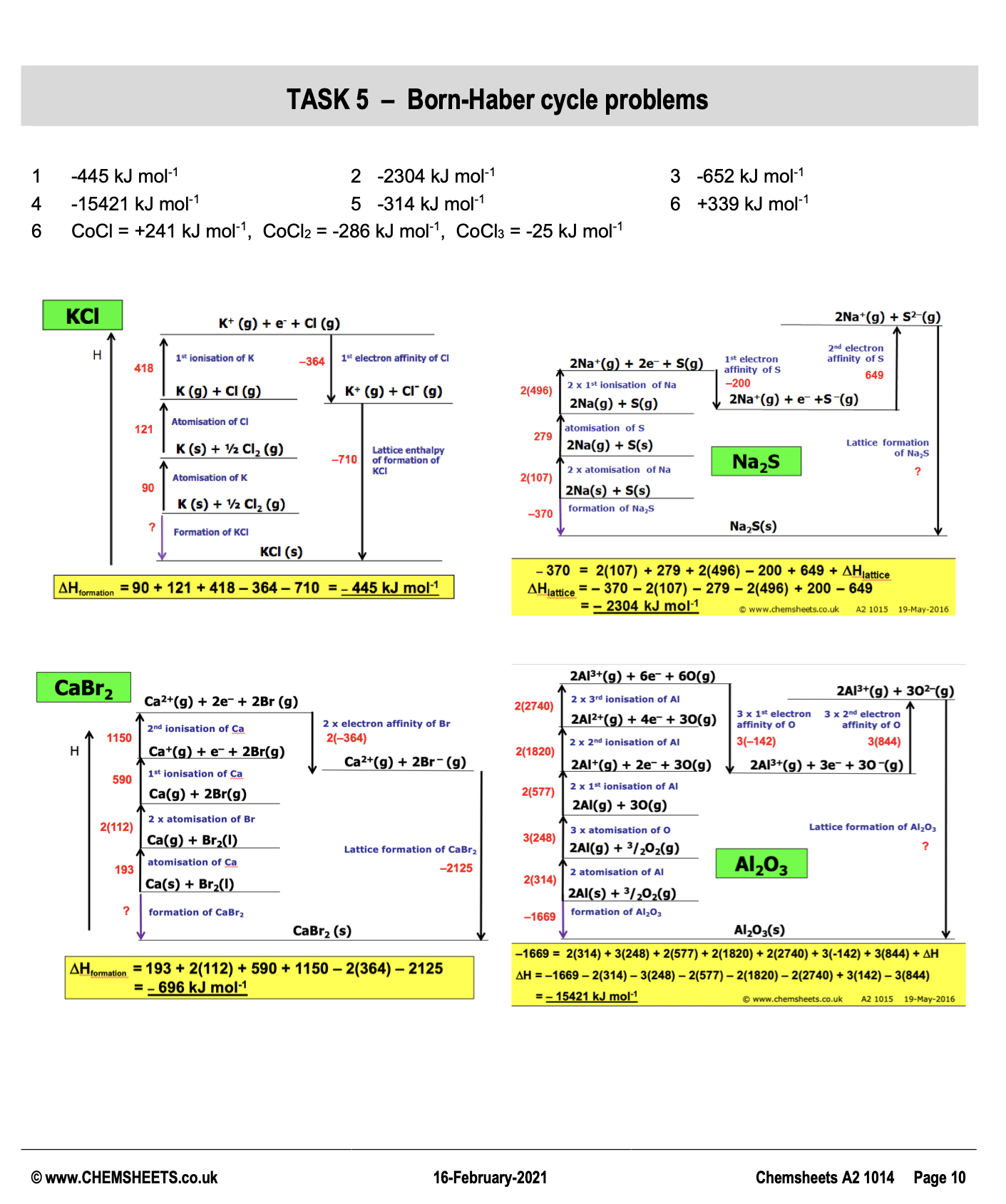
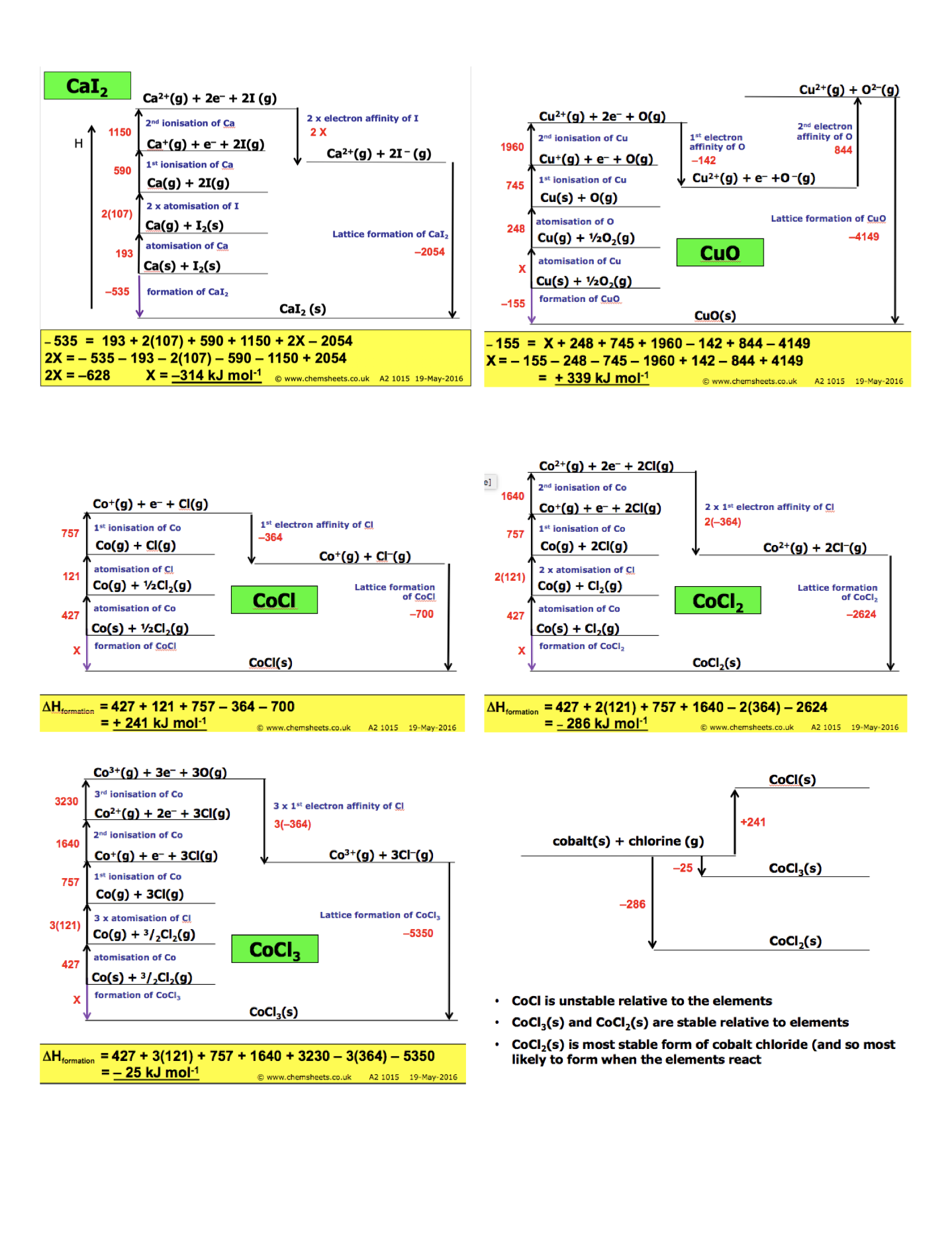
calculating enthalpy change
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
enthalpy change question (formation)
C6H6 + 7.5 O2 → 6 CO2 + 3 H2O
🔺H Overall : - 3267
🔺H CO2: - 394
🔺H O2: - 268
add pic
what is involved in forming an ionic bond
metal starts as giant lattice
non metal consist of molecules
metal starts as giant lattice
requires energy to separate ( enthalpy change of atomisation) and remove electrons (ionisation )
non metal consist of molecules
requires energy to separate ( enthalpy change of atomisation) and energy involved in adding electron to non metal ( electron affinity)
why is ionic crystal stable ?
Because of the large release of energy when oppositely charge ions come together
is lattice enthalpy exo or endo ?
Exothermic because energy is given out when an ionic bond formed from gaseous ions.
what does the lattice enthalpy shows
show strength of a ionic lattice, large negative values shows a strong bond strength.
can lattice enthalpy be measured ?
no it cannot because it’s impossible to form one mole of an ionic lattice from gases ion.
how to calculate the lattice enthalpy of formation ?
lattice enthalpy of formation = - (electron affinity) - (ionisation) - ( atomisation) - ( atomisation) + (enthalpy of formation)

questions
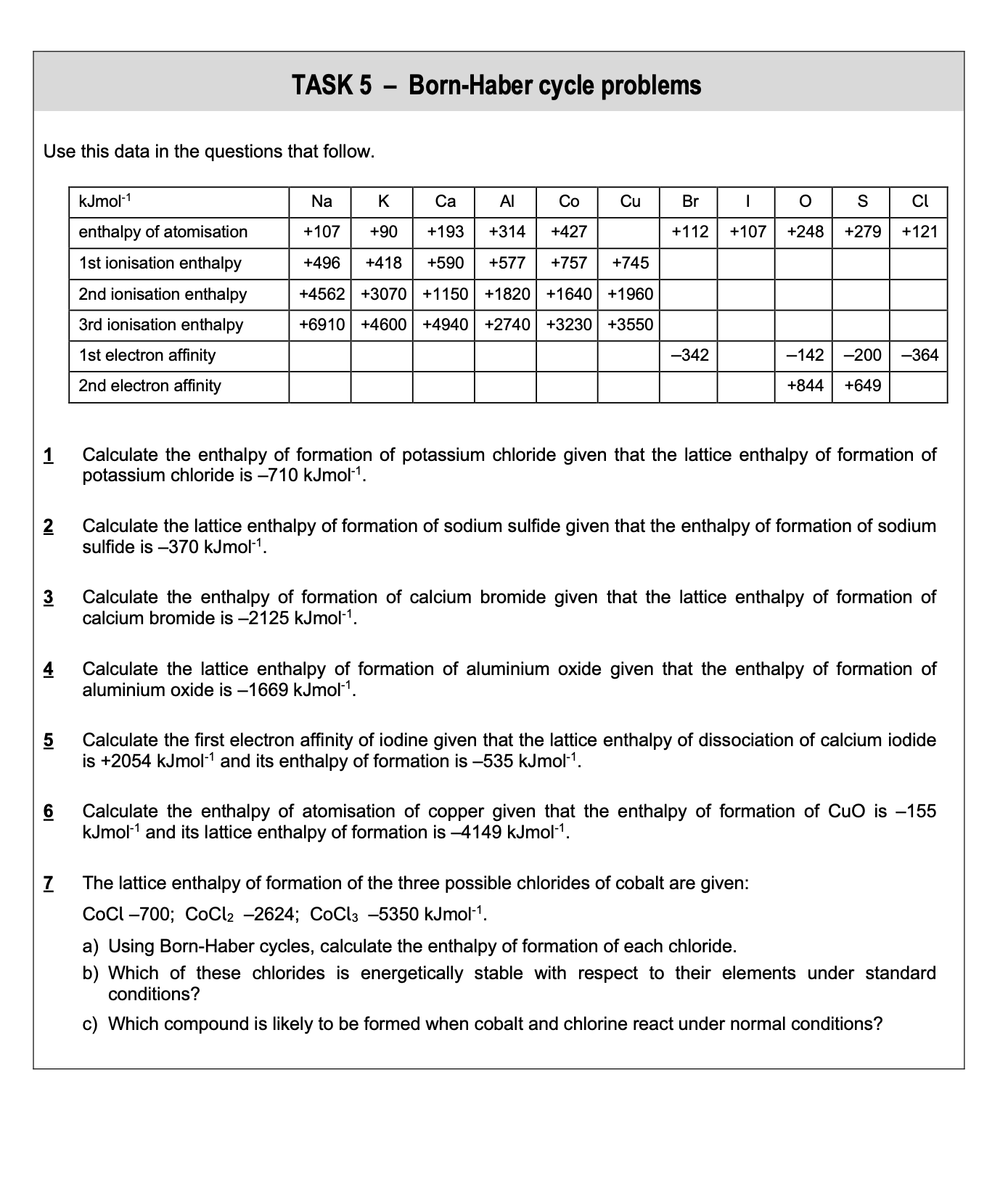
answer