Shapes of simple molecules and ions
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
39 Terms
what is the shape of a molecule determined by?
the number of pairs of electrons in the outer shell of the central atom
what are bonding pairs?
shared electrons taking part in the bond
what are lone pairs?
unbonded/ unshared electrons
what do electron pairs exist as?
charge clouds
what is a charge cloud?
an area where you have a big probability of finding an electron. electrons whizz around in their charge clouds
where are clouds formed?
in bonding pairs
describe electron pair repulsion:
charge clouds will repel one another as far apart as possible
What affects how far lone pair charge clouds can repel?
lone pair charge clouds differ in shape to bonding pair chair clouds
which pair repels more and why?
lone-pairs repel more as they are closer to the central atom- more squat and spread out
what is the order of size of bond angle created from the repulsion? (Valence shell electron pair repulsion theory (VSEPR) )
lone-lone pairs= biggest angle
lone-bonding= second biggest angle
bonding- bonding = smallest angle
what determines the shape of molecules?
the number of electron pairs around the central atom
what does molecular shape depend on?
electron pairs around the central atom
why do electron pairs repel each other?
they are negatively charged
what does VESPR explain?
the electron pairs around a central atom arrange themselves as far apart from each other as possible in order to minimise repulsion
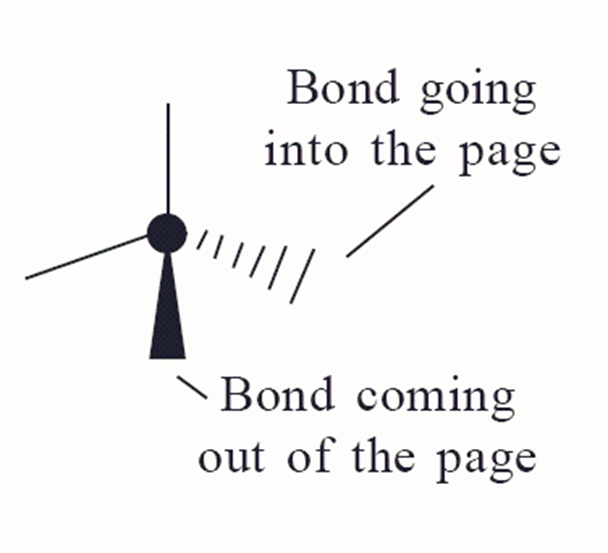
how do you draw a bond going into the page?
dashed lines
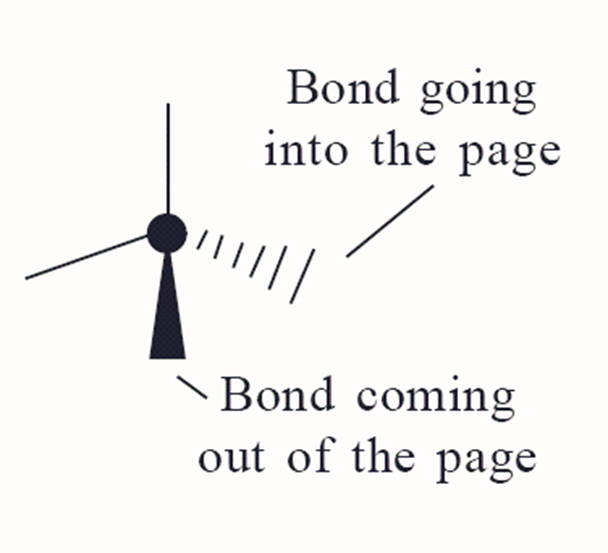
how do you draw a bond coming out of the page?
a shaded in wedge / triangle
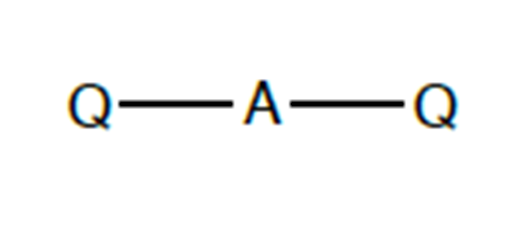
what shape would be found in a molecule with 2 bonding charge clouds? what is the angle?
linear. 180
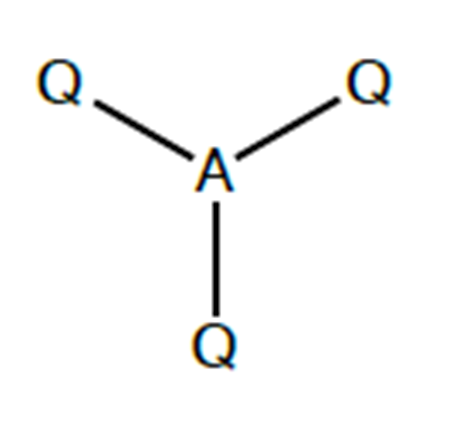
what shape would be found in a molecule with 3 bonding charge clouds? what is the angle?
trigonal planar. 120
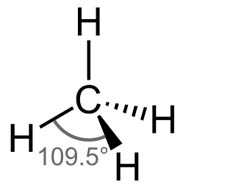
what shape would be found in a molecule with 4 bonding charge clouds? what is the angle?
tetrahedral. 109.5
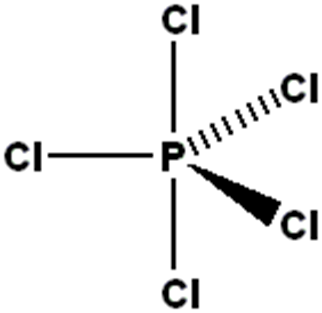
what shape would be found in a molecule with 5 bonding charge clouds? what is the angle?
trigonal bipyramidal. 120 and 90
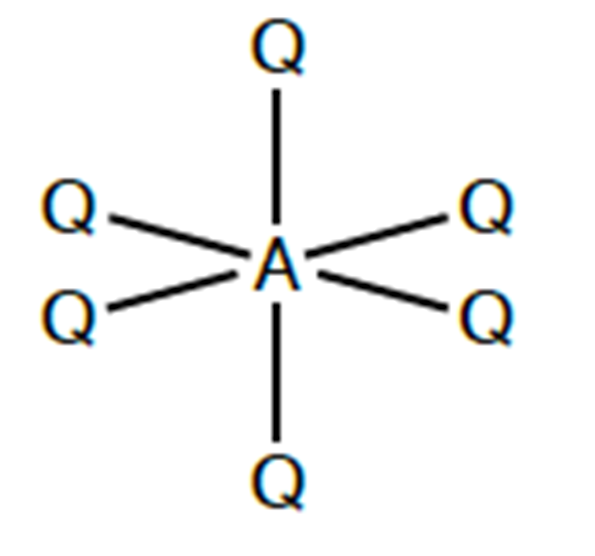
what shape would be found in a molecule with 6 bonding charge clouds? what is the angle?
octahedral. 90 and 180
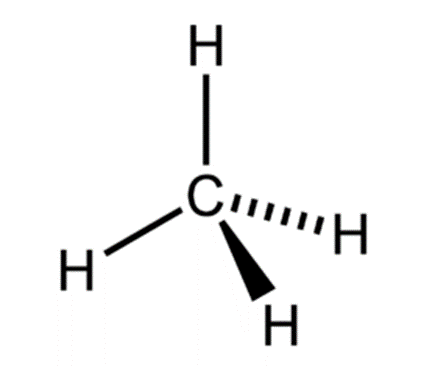
describe the charge clouds and bonding/lone pairs of methane? bond angle? molecular shape?
4 charge clouds
0 lone pairs and 4 bonding pairs
tetrahedral
109.5
describe the charge clouds and bonding/lone pairs of ammonia? bond angle? molecular shape?
4 charge clouds
3 bonding pairs and 1 lone pair
Trigonal pyramidal
107
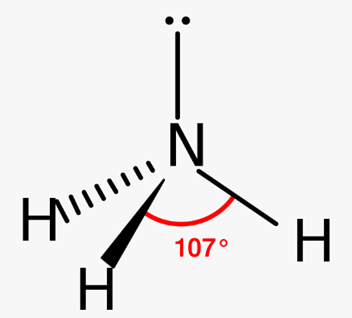
how do you calculate the bond angel when there are lone pairs?
decreases by 2.5 for each lone pair from 109.5
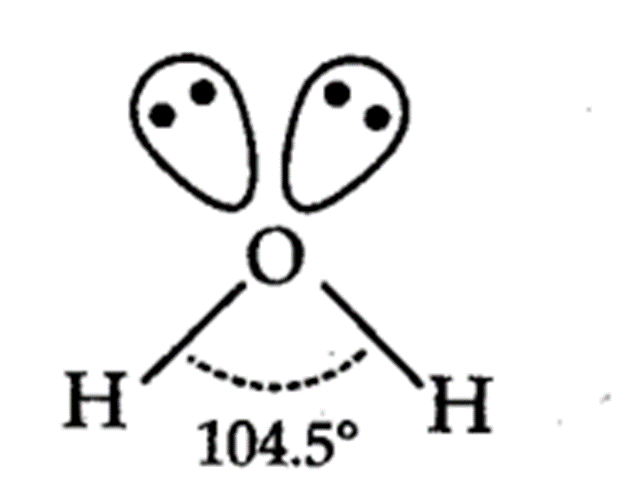
describe the charge clouds and pairs in water? what is the bond angle?
4 charge clouds
2 bonding pairs and 2 lone pairs
109.5
v-shaped / bent shape
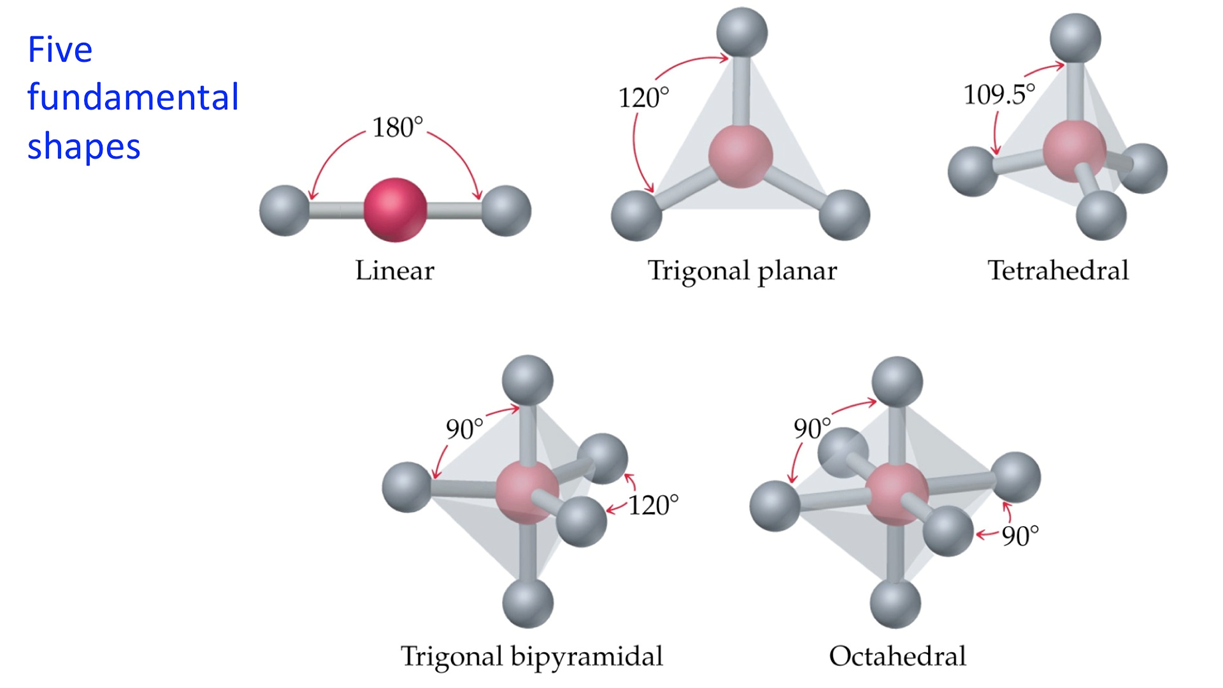
what are the 5 fundamental shapes of molecules?
linear (180)
trigonal planar (120)
tetrahedral (109.5)
trigonal bipyramidal (90 and 120)
octahedral (90)
which shape would be found in a molecule with 2bp 0lp? what angle?
linear 180
what shape and angle would be found in a molecule with 3bp 0lp?
trigonal planar 120
what shape and angle would be found in a molecule with 2bp 1lp?
bent. slightly less than 120
what shape and angle would be found in a molecule with 4bp 0lp?
tetrahedral 109.5
what shape and angle would be found in a molecule with 3bp 1lp
tetrahedral/trigonal pyramidal 107
what shape and angle would be found in a molecule with 2bp 2lp
bent 104.5
what shape and angle would be found in a molecule with 5bp 0lp?
trigonal bypramidal 120 and 90
what shape would be found in a molecule with 4bp 1lp?
seesaw
what shape and angle would be found in a molecule with 3bp 2lp?
trigonal bipyramidal 90
what shape and angle would be found in a molecule with 2bp 3lp?
trigonal bipyramid 180
what shape and angle would be found in a molecule with 6bp 0lp?
octahedral 90
what shape and angle would be found in a molecule with 5bp 1lp?
octahedral 90
what shape and angle would be found in a molecule with 4bp 2lp?
square planar 90