CHEM 232 L Prelab Quiz 3 - Fractional & Simple Distillation of an Unknown Liquid
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
When does the boiling point range begin?
When the first drop is collected.
When boiling a pure compound what is the relationship of the pot temperature and the head temperature?
The pot temperature is the same as the head temperature.
Raoult’s Law states that the partial vapor pressure of a component in a mixture is directly proportional to:
the mole fraction of the component in the mixture.
Dalton’s Law explains that the mole fraction of a component in the vapor phase is equal to the ratio of its:
vapor pressure in the mixture to the total vapor pressure
What is the theoretical plate in fractional distillation?
A single step in a series of simple distillations.
In simple distillation, how many theoretical plates are typically involved?
1
Which of the following compounds has the lowest boiling point?
n-Hexane
When should simple distillation be used instead of fractional distillation?
When the boiling point difference between the compounds is 110°C or more
If a mixture contains equal amounts of carbon tetrachloride and toluene, the vapor phase will contain:
more carbon tetrachloride
Which statement is true about the heating mantle used in distillation?
It should never come in contact with liquid.
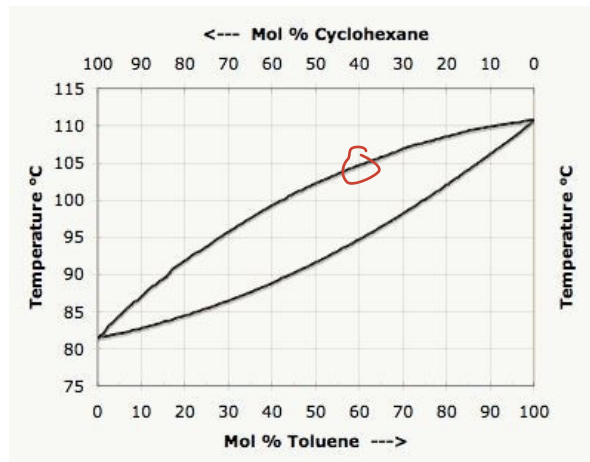
Consider this boiling point-composition curve for Cyclohexane and Toluene. At a temperature of 1050C, what is the approximate vapor mole ratio of cyclohexane to toluene?
40:60
Explain the main differences between simple distillation and fractional distillation.
Fractional distillation is used to separate liquids with similar boiling points
Explain the role of a theoretical plate in fractional distillation
It provides additional distillation events that help differentiate two boiling liquids and enhances the purity
Why is it important to ensure a steady flow of cooling water through the condenser during distillation? What could happen if there is not an adequate flow?
A steady flow allows the condenser to condense the vapes into a liquid. With too little flow, there will be less distillate collected