Chemistry 7.3 - Nanoparticles
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
How many atoms are in a nanoparticle and what size are they?
Nanoparticles contain a few hundred atoms and are 1-100 nm in size.
What is 1 nanometre (nm) in metres?
0.000000001 m = 1×10-9 m
What is the radius of an atom?
About 0.1 nm
How many atoms are in 1 nm?
About 5 atoms
A gold nanoparticle has a diameter of 21 nm. Calculate the diameter of the nanoparticle in meters.
Number of nm x size of 1 nm = 21 x (1×10-9 m) = 2.1 × 10-8
How big is the nucleus of an atom?
About 10,000 times smaller than an atom.
Estimate the radius of a nucleus. (a) nm (b) m.
(a) Radius of nucleus = 1/10,000 × 0.1nm = 1×10-5 nm
(b) Number of nm x size of 1 nm = (1x10-5) x (1×10-9 m) = 1× 10-14 m
Why are gold nanoparticles more reactive than ‘bulk’ gold (larger pieces such as granules or powder)?
The nanoparticles have a much greater surface-to-volume ratio.
What is the surface to volume ratio formula?
Surface-to-volume ratio = Surface area / volume
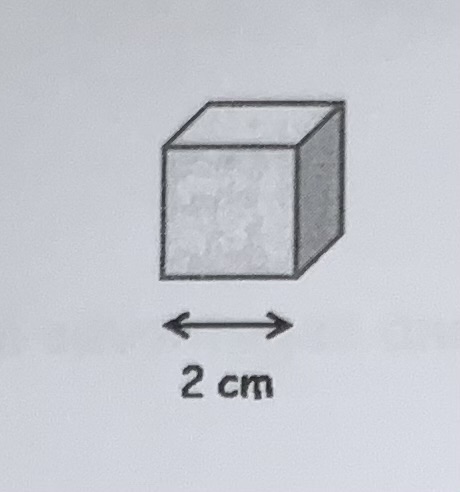
Calculate the surface-to-volume ratio for the image of the cube provided.
Surface area: 2×2×6 = 24 cm2
Volume: 2×2×2 = 8cm3
Surface to volume ratio: 24 / 8 = 3
Why are zinc oxide nanoparticle used instead of ‘bulk’ zinc oxide in suncream?
They are transparent and easier to spread over skin, giving better coverage and better protection against UV light
Why could zinc oxide nanoparticles be a health risk?
They may penetrate cell membranes, or could be inhaled, and may be more reactive or more toxic than ‘bulk’ zinc oxide.
What problem is caused by nanoparticles being so new?
The potential health risks posed by nanoparticles are not understood.
What could nanoparticles lead to?
Damage to whole ecosystems by accumulating in food chains.