lec 3 (minko) - drug product design
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
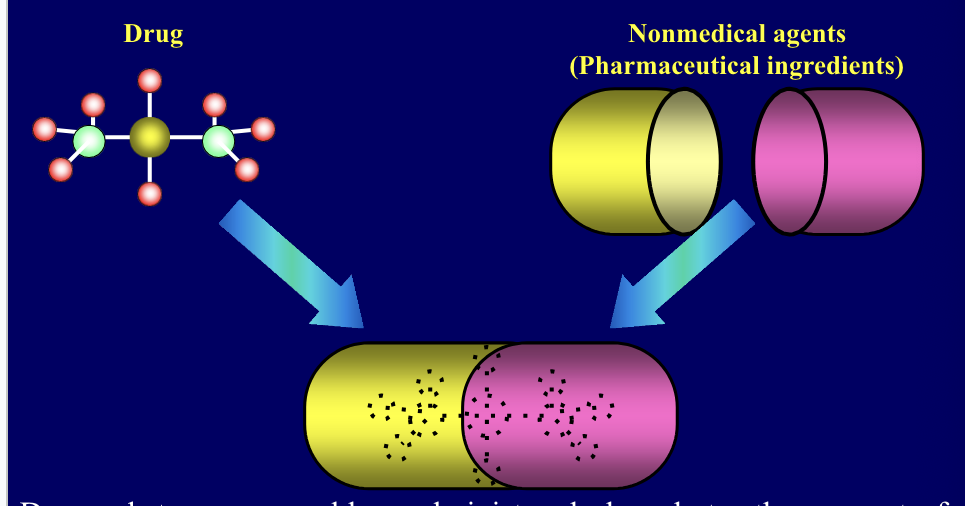
dosage form
drug + non-medical agents (pharmaceutical ingredients) come together to form drug
drug substances are rarely administered alone but rather as part of formulation in combo with one or more non-medical agents
dosage form = combination of a drug with there non-medical agents referred as pharmaceutical ingredients
why we need dosage forms?
for the safe and convenient delivery of an accurate dosage
for the protection of a drug from destructive influences of atmospheric oxygen or humidity
for the protection of a drug from the destructive influences of gastric acid after oral admin
to conceal bad taste or odor
to provide liquid preparations of substances that are either insoluble or unstable in the desired vehicle (e.g. suspensions)
to provide clear liquid dosage forms of substances (syrups, solutions)
to provide controlled release drug delivery
to provide optimal drug action through diff. admin sites
drug and drug product stability
chemical
each active ingredient retains chemical integrity and labeled potency within specified limits
physical
the original physical properties, including appearance, palatability, uniformity, dissolution and suspend-ability are retained
microbiologic
sterility or resistance to microbial growth = retained according to the specified requirements. antimicrobal agents taht are present retain effectiveness within specified limits.
therapeutic
therapeutic effect remains unchanged
toxicologic
NO significant increase in toxicity
stability = extent to which a product retains, within specific limits, and throughout its period of storage and use, the same properties and characteristics that it possessed at the time of its manufacture
drug stability: mechanisms of degradation
hydrolysis
process in which drug molecules interact with water molecules to yield breakdown products of different chemical constiutions
oxidation
process in which a substance combines with oxygen to form oxides
chemical instability of medicinal agents may take many forms since modern drugs are of such diverse chemical constiutions
chemically the most frequently encountered destructive processes = hydrolysis and oxidation
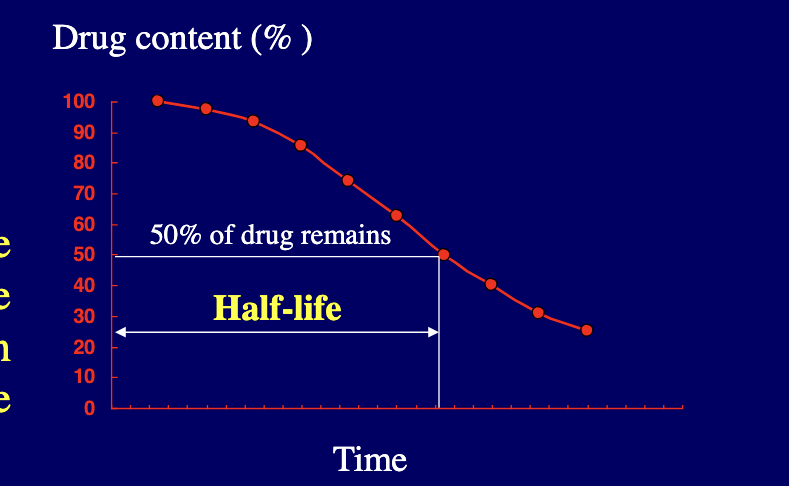
stability testing: kinetic study
half life = time required to decrease the conc. of an active component in the drug form by 50%
kinetic study begins by measuring the conc. of the drug being examined at given time intervals under a specific set of conditions:
temperature
pH
ionic strength
light intensity
drug concentrations
the measurement of the drug concentration at various time intervals reveals the stability or instability of the drug under the specified conditions with the passage of time
from this starting point, each of the OG conditions may be varied based on individual basis to determine the influence that such changes make on drug’s stability
from experimental data, half life can be determined
pharmaceutic ingredients
solvents = to dissolve drug substance
flavors and sweeteners = to make product more palatable
colorants = to enhance product appeal
preservatives = to prevent microbial growth
stabilizers = such as antioxidants and chelating agents to prevent drug decomposition
diluents and fillers = to increase the bulk of formulation
binders = to increase the adhesion of powdered drug and pharmaceutic substances
anti-adherents or lubricants = to assist the smooth tableting process
disintegrating agents = to promote tablet break-up after admin
to prep. a drug substance into final dosage form, pharmaceutic ingredients are required
sterilization and preservation
physical methods
autoclaving for 20 minutes at 15 PSI and 120 degrees C
dry heat at 180 degrees C for 1 hour
bacterial filtration
chemical method
use of preservatives
although some types of pharmaceutical products like ophthalmic and injectable preparations are sterilized by physical methods during manufacturing, many of them additionally require an antimicrobial preservative to maintain their aseptic conditions throughout the period of their storage and use
dissolution and drug absorption
dissolution = process by which a drug particle assimilates into the fluid at the absorption site
for instance, drug administered orally in tablet or capsule CANNOT be absorbed until the drug particles are dissolved by the fluids at some point within the GI tract → drug will be dissolved in the stomach (acidic conditions) or intestines (low acidic, neutral or basic conditions) depending on the pH-dependent solubility of the drug
pH of GI tract
duodenum pH = 5-7
stomach pH = 1-3
ascending colon pH = 7-8
jejunum pH = 6.5
bioavailability and bioequivalence
bioavailability = rate and extent to which an active drug ingredient or therapeutic moiety is absorbed from a drug product and becomes available at the site of drug action
bioequivalence = comparison of bioavailability of different formulations, drug products, or batches of the same drug product
blood concentration-time curve
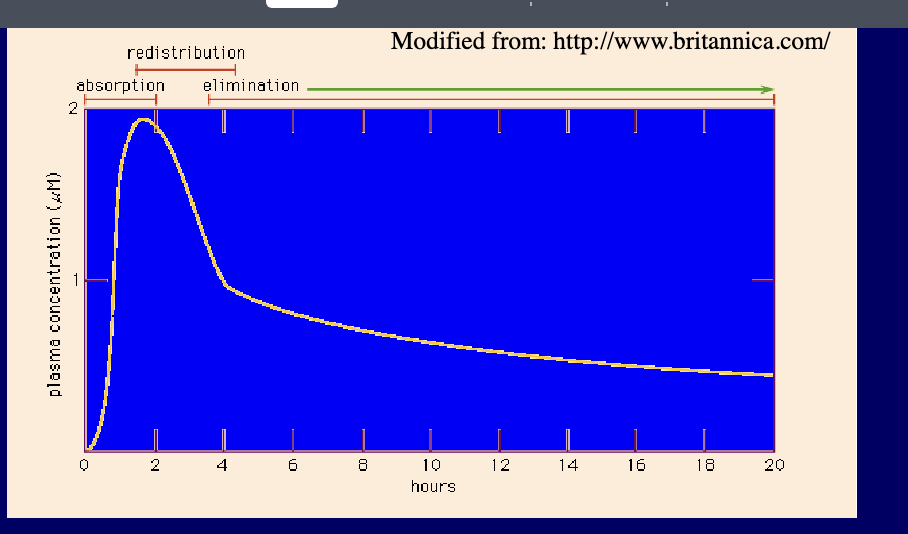
bioavailability of drug is presented by concentration-time curve of the administered drug in an appropriate tissue system e.g. plasma
rise and fall of the concentration of the drug in the blood plasma over time determines the course of action of most drugs
if drug is given orally, 3 phases can be distinguished
absorption phase → leads to peak in plasma concentration
redistribution phase → plasma concentration falls rapidly as the drug is taken up by various tissues
elimination phase → slower phase of decline as drug is metabolized or excreted
parameters for assessment and comparison of bioavailability
when the drug is first administered (time zero), the blood concentration of the drug should also be zero
as drug passes into stomach and/or intestine, it is released from dosage form, dissolved, and absorbed
blood sample reveal increasing concentrations of drug until max (peak) concentration is reached → blood level of the drug progressively decreases and if no additional dose is given → eventually falls to zero
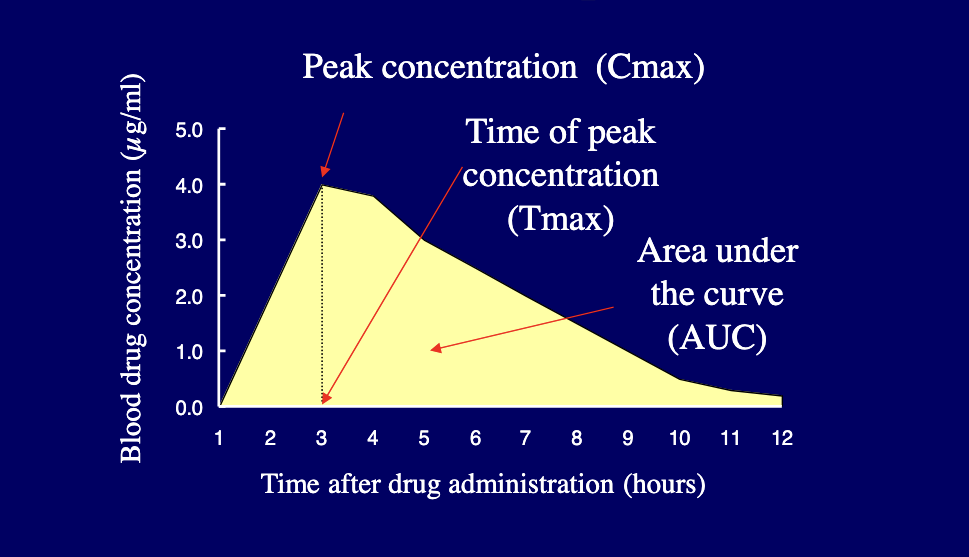
following parameters are usually used for describing and comparison of bioavaliability
Cmax = peak concentration
Tmax = time of peak concentration
AUC = area under the curve
excretion of drugs
urine (kidney)
feces
bile
lungs
sweat, saliva, milk
excretion of drugs and their metabolites terminates their activity/presence in body
drugs may be eliminated by various routes with kidney playing dominant role by eliminating drugs via urine
drug excretion with feces = also important especially for drugs that are poorly absorbed and remain in GI tract after oral admin
exit through bile is significant only when the drugs reabsorption from GI tract is minimal
lungs provide the route of elimination for many volatile drugs through expired breath
sweat glands, saliva, and milk play only minor roles in drug elim; should be noted that if a drug gains access to the milk of a mother during lactation it could easily exert its effects in the nursing infant
novel dosage forms and drug delivery technologies
new drug delivery system development is largely based on:
promoting the therapeutic effects of a drug
minimizing its toxic effects by:
increasing the amount and persistence of a drug in the vicinity of a “target” cell
reducing the drug exposure of “nontarget” cells
new drug delivery systems: mechanisms
novel drug delivery systems can include those based on physical mechanisms and biochemical mechanisms
physical mechanisms (controlled drug delivery systems) include:
osmosis
diffusion
erosion
dissolution
electrotransport
biochemical mechanisms include:
monoclonal antibodies
gene therapy and vector systems
polymer drug abducts
etc.
novel drug compositions
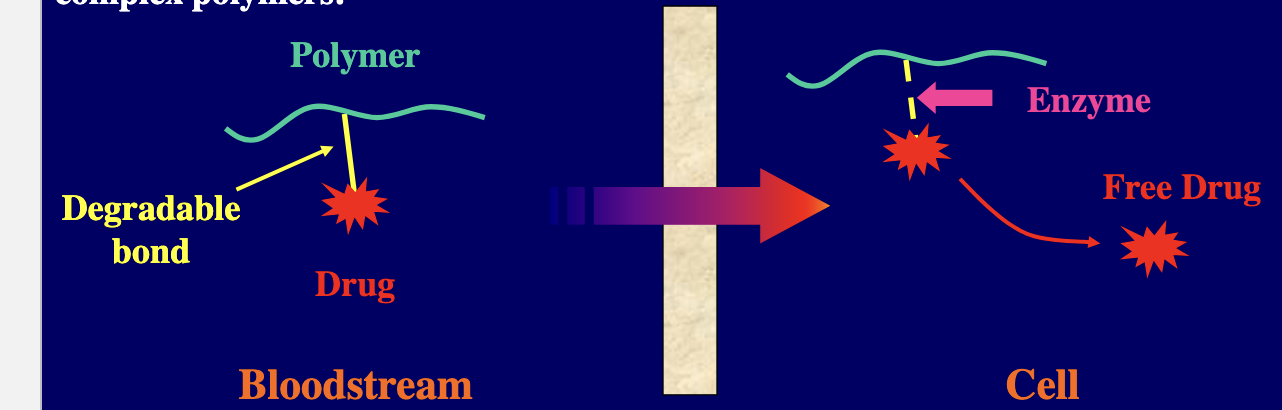
composition of newer drug delivery systems can be quite variable ranging from naturally derived substances such as gelatin and sugars → more complex polymers
therapeutic efficacy of selected products can be enhanced and toxicity can be decreased by incorporating novel polymer technology
for example, degradable bonds can be used to attach an active drug to a synthetic or naturally occuring polymer → upon delivery to the target site and in the presence of certain enzymes or through hydrolysis the product can be cleaved releasing the active drug at a specific site of action
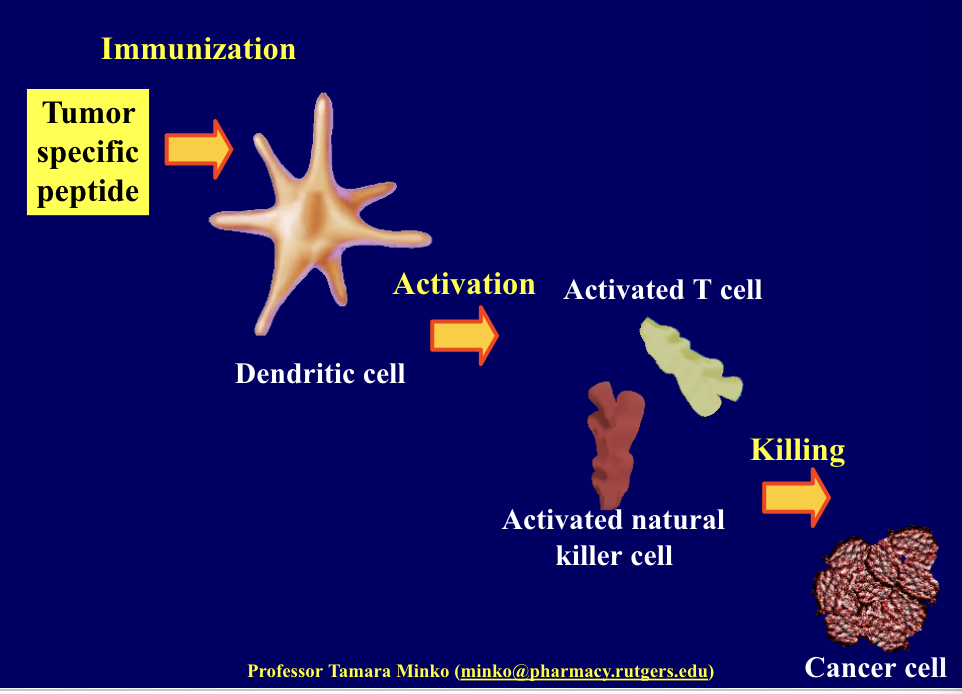
cancer vaccines
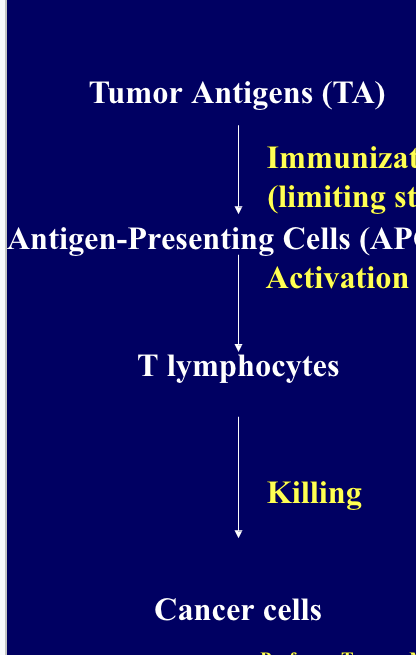
cancer treatment by vaccines include 3 main steps
immunization (limiting step)
activation of immune system (dendritic cells, T-lymphocytes and natural killer cells)
killing of cancer cells
treated cancer cells, cancer cell-dendritic cell conjugates or cancer specific peptides might be used as tumor antigens
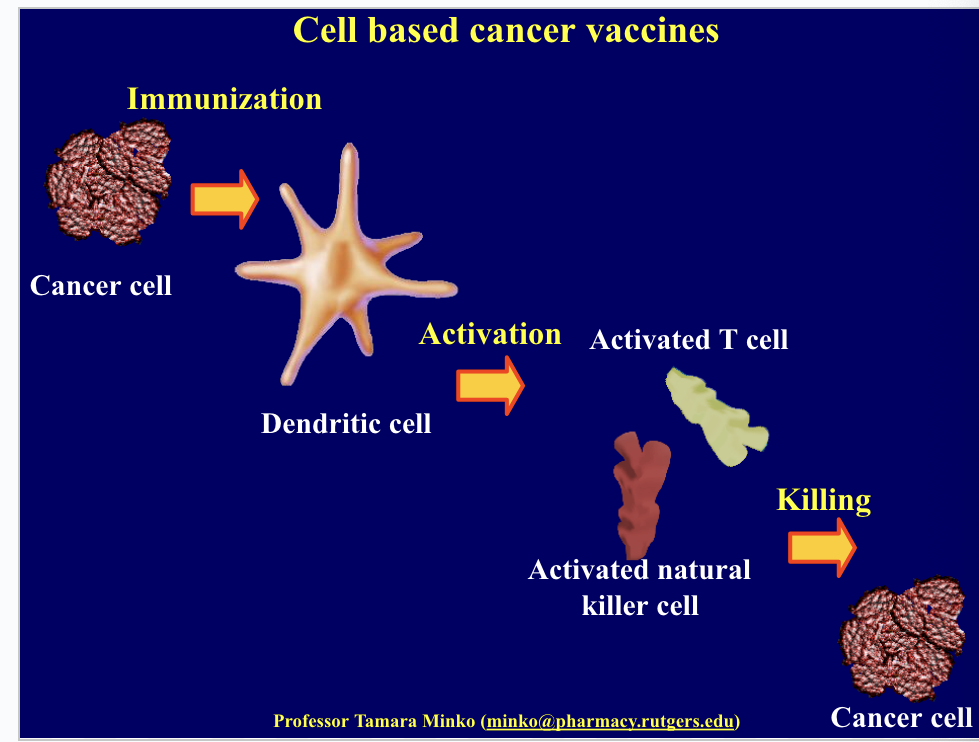
cell based cancer vaccines
form of immunotherapy used to treat cancer by stimulating the immune system to recognize and attack cancer cells
immunization using pt’s own cancer cells or tumor antigens
involves using the pt’s own dendritic cells (a type of immune cell that helps initiate the immune response)
dendritic cells are extracted from the pt’s blood → dendritic cells exposed to tumor-specific antigens → re-injected into pt
the dendritic cells present the tumor antigens to T cells which activate T cells and natural killer cell → immune response against cancer which kills the cancer cells
cell free cancer vaccines
do NOT involve the use of whole cells but instead focus on using tumor derive components such as proteins, peptides, or nucleic acids to stimulate the immune system
use tumor specific peptides to formulate vaccine → peptides taken up by dendritic cells → activates T cells and natural killing cell → kills cancer cells