oral suspensions 1
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
What is a disperse system?
A disperse system is a two-phase heterogeneous system in which an insoluble or immiscible dispersed phase is distributed through a continuous phase.
classification?
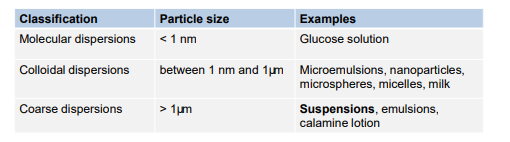
what is the definiton of Pharmaceutical suspensions?
-a pharmaceutical suspension is a liquid disperse system consisting of particles distributed within a liquid vehicle.
. • Classified as coarse or colloidal dispersion, depending on the size of particles (normally between 0.1 to 10μm)
• Suspensions are not optically clear and appear cloudy
Reasons for formulating of an oral pharmaceutical suspension? 4
• To deliver poorly water-soluble drugs which cannot be formulated as aqueous solutions
• To mask the bitter taste of the drug
• To increase drug stability
• To achieve controlled/sustained drug release
What forces affect particles in suspensions?
Particles in suspensions experience:
Van der Waals attractive forces: These draw particles together.
Electrostatic repulsion forces: These push particles apart, preventing aggregation.
How do forces at the surface of particles affect flocculation?
the balance between Van der Waals attraction and electrostatic repulsion at the surface of particles determines whether they flocculate (aggregate loosely) or remain deflocculated (dispersed individually).
What happens in a flocculated suspension?
: Suspended particles form floccules (loose aggregates) due to Van der Waals forces rather than remaining as separate particles.
What is the effect of flocculation on sedimentation and redispersion?
In flocculated suspensions, sediment is large, and redispersion is easy because the particles are loosely aggregated.
What is controlled flocculation used for?
Controlled flocculation is a mechanism used to prevent particle caking in suspension formulations, ensuring stability and easy redispersion.
How can flocculation be induced in a suspension?
Flocculation can be induced by reducing the surface charge (zeta potential) of particles, typically through the addition of surfactants or ionic salts.
What is a deflocculated suspension?
A deflocculated suspension is a system where particles are individually and uniformly dispersed throughout the liquid medium.
What keeps particles deflocculated in a suspension?
Particles remain deflocculated when the repulsive energy between the suspended particles is high, preventing aggregation.
What happens to deflocculated particles over time?
Deflocculated particles may settle slowly, forming a layer of particle sediment at the bottom, which is difficult to re-suspend.
Are deflocculated suspensions ideal for pharmaceuticals?
: No, deflocculated suspensions are not ideal for pharmaceuticals due to the slow settling and difficulty in re-suspending the particles.
To ensure a uniform dose, an oral suspension should have the following properties:4
Particles settle slowly.
Particles are readily and uniformly re-dispersed upon shaking.
Particle size remains consistent over time.
Viscosity is high enough to ensure a uniform dose, but not so viscous that the suspension cannot be easily poured from the bottle.
All particles show movement, either caused by:
• Brownian motion
• Gravity
• External agitation
How does particle motion affect flocculation in a suspension?
Particle motion affects the inter-particulate distance, influencing the flocculation status of the suspension.
How do particles behave in a deflocculated system?
In deflocculated systems, particles behave as individual small particles, remaining separate and dispersed.
How do particles behave in a flocculated system?
In flocculated systems, particles clump together and behave as individual large particles with a porous structure.
What type of motion do small particles (<2μm) experience?
Small particles (<2μm) are subject to Brownian motion, which is irregular movement within the medium and diffusion from high concentration to low concentration.
What is the effect of Brownian motion on particle distribution?
: Brownian motion helps provide a more homogeneous particle distribution by enabling random movement and diffusion.
: How does particle size affect diffusion?
Smaller particles (<2μm) will diffuse more rapidly, while particles larger than 2μm experience negligible diffusion.
How does the viscosity of the medium affect diffusion?
: Increasing the viscosity of the medium will reduce diffusion by hindering the movement of particles.
what is sedimentation?
It is the downward particle movement due to gravity.
• Particle movement is critical for successful suspension formulations
What are the two key factors for sedimentation in suspensions?
The two key factors are the speed of sedimentation and reversibility.
What type of system is ideal for a slow sedimentation rate?
A deflocculated system is optimal for slow sedimentation, as it keeps particles dispersed.
Why is reversibility important in suspension formulations? which is best achieved ?
Reversibility is required to ensure dosing is reproducible, which is best achieved with a flocculated system, where particles can easily be re-suspended.
How can a deflocculated system be developed for minimal sedimentation?
A deflocculated system with minimal sedimentation can be developed by increasing the viscosity to maintain dispersion.
How can a flocculated system be developed for controlled sedimentation?
A flocculated system can be developed with controlled slow sedimentation to ensure stability while maintaining ease of re-suspension.
what does particle size hace a direct effect on?
the ease of maintenance of a uniform suspended phase
what is Submicron suspension
Brownian motion helps to keep the particles in a dispersed state
Larger particles:
the effect of gravity becomes significant
stokes law?
According to Stokes’ law, the velocity of a suspended particle falling under gravity is directly proportional to the particle’s size
Ways to reduce sedimentation rate:
• Particle size reduction – increases diffusion.
• Reduce particle density – but at the same time increases particle size thus may have adverse effects.
• Increase medium density
. • Increase medium viscosity –l m
. • Increase temperature – increases diffusion constant, but effect is limited within normal range of temps.