Pathophys - CREDIT 2 (25)
1/48
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
49 Terms
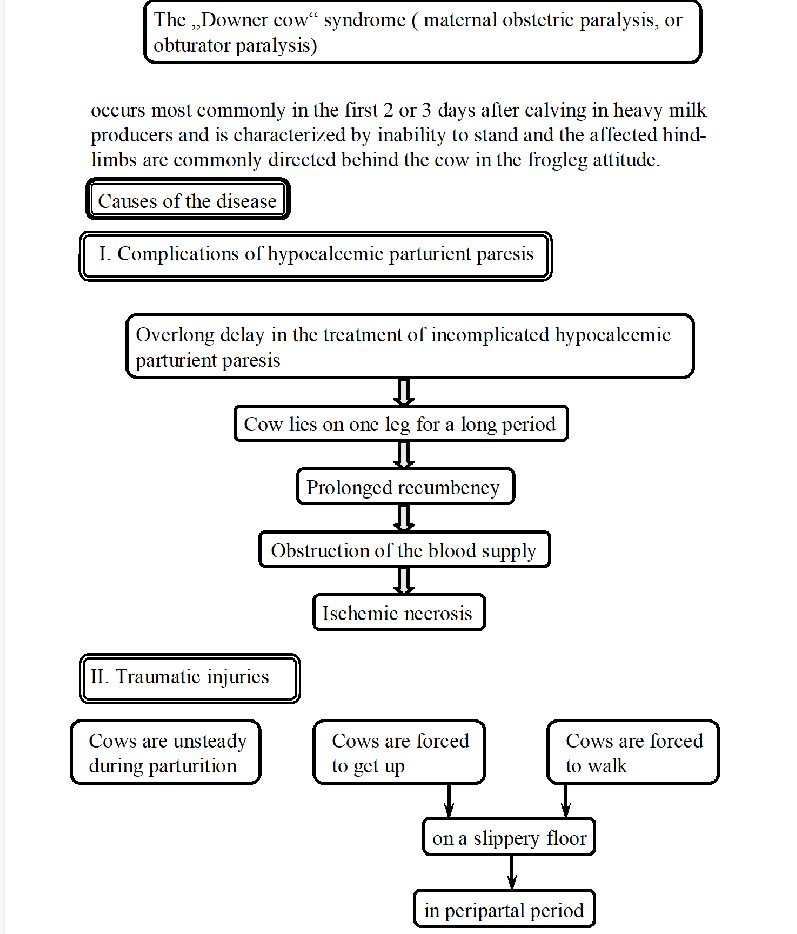
Downer cow syndrome in cow
Condition, ischemic myopathy, where a cow is unable to stand, often seen 1-3 days after calving in heavy milk producers. Frog-leg position of hind limbs.
causes:
complications of hypocalcemic parturient paresis - milk fever: delay in treatment, cow lies on one leg, prolonged recumbency → obstructed blood supply → necrosis (lack of blood)
traumatic injuries: cows are unsteady after giving birth, injuries are more likely on slippery floor, oversized calf can give peripelvic traumatic injury → prolonged recumbency.
consequences: leading to compression of muscle cells, inflammation and anoxia - cell damage, myoglobinuria, necrosis, acute mastitis, limb injuries, ulceration, death/euthanasia need.
1st degree AV block, definition, ECG changes
simple delay in the electrical signal as it passes through AV node.
P-R interval is longer with normal P-wave and QRS complexes at a 1:1 ratio.
Causes: hyperkalemia, meds, digitalis intoxication
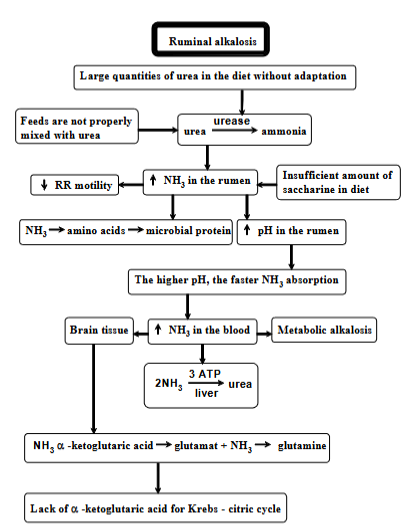
Acute ruminal alkalosis
Condition of high pH (alkaline) in rumen, due to large quantities of urea in the diet, where the animal has no time to adjust. Process:
too much urea in diet
urea conversion: urea → ammonia (by enzyme urease in rumen)
feeds are not mixed with urea = worsen
consequences:
increased NH3 in rumen
insufficient amount of saccharine in diet (not enough carbs) = worsen
ammonia → AA → microbial protein (normally used, but if ammonia is produced faster than it is used = problem)
decreased RR motility (slowing digestion)
increased pH in rumen => faster ammonia absorption into blood → higher levels → metabolic alkalosis (increased in blood pH) → can affect brain tissue.
liver - tries to convert ammonia back into urea, tries to detoxify it by combining it to form glutamate → glutamine.
krebs cycle disruption, as liver takes the enzyme to detoxify ammonia.
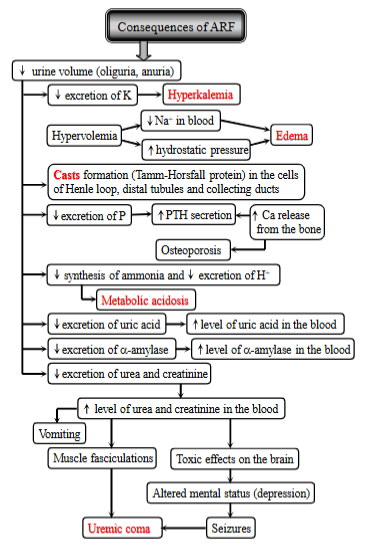
acute renal failure, definition, 3 causes, consequences
ARF is the rapid deterioration of kidney function → imbalance of the internal environment and azotemia (buildup of nitrogenous waste products in the blood). It is also usually linked with oliguria (red. urine).
3 general categories of causes:
prerenal (decr. renal blood flow, due to hypovolemia and cardiac failure)
renal (direct or indirect damage to the nephron from inflammatory diseases or toxins)
postrenal (urinary tract obstruction, bladder rupture → increased tubular backleak → oliguria).
These causes → decrease renal blood flow → not enough medullary blood flow → activates mediators of vasodilation + vasoconstrction of vascular beds → redistribution of blood, from cortex to medulla → ischemia of cortex → cortical necrosis → decreased glomerular filtration.
Consequences:
decreased urine volume (oliguria, anuria)
electrolyte imbalance: hyperkalemia, fluid overload - hypervolemia, decreased sodium, increased hydrostatic pressure → edema
cast formation - in cells of nephron
mineral imbalance: decr. excretion of P, increased PTH, increased Ca release from bone, osteoporosis.
acid-base imbalance: decr. ammonia, decr. excretion of H+, metabolic acidosis.
waste buildup - decreased excretion of uric acid, urea + creatinine
more urea + creatinine in blood, vomit, muscle fasciculations, toxic effects on the brain, seizures, uremic coma
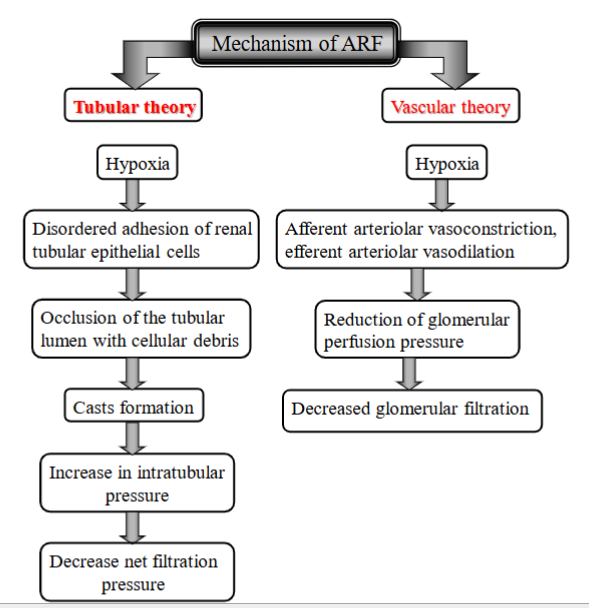
2 Mechanisms of ARF (acute renal failure)
tubular theory (clogged tubules inside kidney)
Hypoxia (damage to tubules can be by toxins, lack of O2, inflammation)
tubular cells will act abnormal → some die, detach from the tubule walls, other may change/become sticky
detached cells, proteins + debris → clump together → blockage within tubules. (casts formation).
these “sticky” cells can also contribute to the clogging by trapping debris → stops blood flow in the tubules
Kidney tubes gets too thick, clogged → cannot filter blood properly (net filtration pressure reduced).
vascular theory (traffic jam in BV leading to the kidney)
Hypoxia → afferent arteriolar vasoconstriction + efferent arteriolar vasodilation. (BW gets too narrow, blood cannot flow properly to kidneys)
Glomerular perfusion pressure reduced
glomerular filtration reduced
Acute pancreatitis - pathogenesis
Rapid inflammation of pancreas, arising from three main routes → damaging pancreatic cells (acinar cells) and activating digestive enzymes within the pancreas itself. Enzymes will start eating the pancreas itself, leading to inflammation, necrosis, hemorrhage.
Duct obstruction - gall stone, blocking → edema, ischemia (reduced blood flow)
acinar cell injury - alcohol, drugs, trauma, releasing digestive enzymes
defective intracellular transport - stopping normal movement of enzymes inside the pancreatic cells
Explain primary hyperthyroidism, causes, 4 consequences
Thyroid disorder, from excessive circulating concentrations of T4 or T3. common in cats, but rare in dogs. Includes primary, secondary, tertiary.
Primary: Problem is within the thyroid gland itself, causing it to make too much thyroid hormone (T4/T3), leading to decreased TSH secretion.
Causes: thyroid hyperplasia (general enlargement), thyroid adenoma (benign tumor) and toxic multinodular goiter (enlargement of gland with many overactive nodules)
General Consequences of hyperthyroidism:
Calorigenesis and thermoregulation: increased basal metabolic rate and O2 consumption → hyperthermia, heat intolerance
Growth and Maturation: Bone resorption exceeds bone formation, potentially leading to hypercalcemia, hypercalcinuria and fractures.
Metabolism: increases hepatic gluconeogenesis, carb absorption, and insulin degradation, decreases cholesterol, incr. need for vitamins → weight loss, deficiencies.
cardiovascular system: increased output, heart rate, pulse pressure, potentially leading to supraventricular tachycardia, incr. R-wave, atrial/ventricular arrhythmia
NS: hyperactivitiy, restlessness, pacing and irritability.
GIT: vomit, diarrhea, malabsorption
warm and sweaty skin, may lead to relative polycythemia, tachypnea, decreased fertility + change steroid metabolism in male, increased sodium + water excretion by kidney → hypovolemia.

Explain secondary hypothyroidism, what, causes, consequences
Comes from pituitary dysfunction. (not from thyroid gland itself). The pituitary does not produce enough TSH, which is needed to tell thyroid to make hormones.
Causes: Pituitary destruction, tumors, isolated TSH deficiency, Congenital pituitary malformation (panhypopituitarism) - birth defect.
Physiological consequences:
Calorigenesis + thermoreg.: decreased basal metabolic rate + O2 consumption → cold intolerance, hypothermia, weak.
Growth + maturation: abnormal synapse development, mental retardation in young, dullness in adults
carbohydrate metabolism: decreased glycogenolysis + glycolysis
neuromuscular: polyneuropathy + muscle atrophy
GIT: constipation
dry, cool and yellow-orange colored skin, bradypnea
decreased GH, cortisol, gonadotropins, secondary GH deficiency. decreased reproductive function.

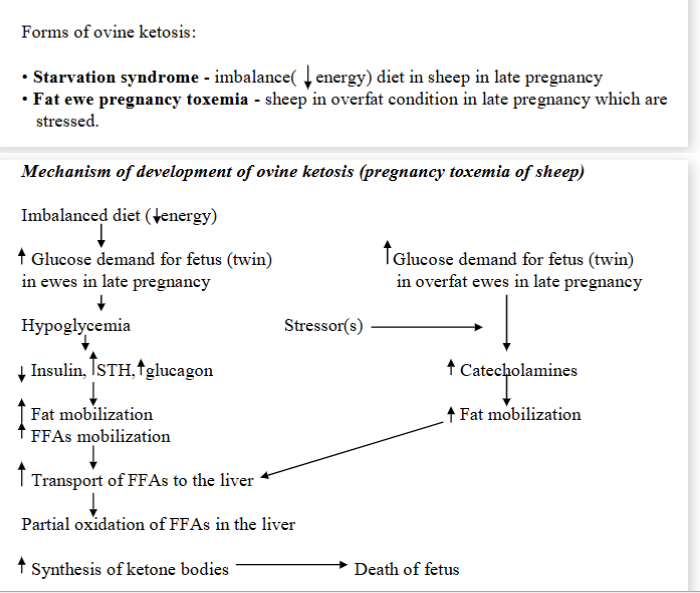
Pregnant toxemia in sheep (Ovine ketosis)
Metabolic disturbance, characterized by hypoglycemia, low level of hepatic glycogen, ketonemia + abnormally high level of cortisol in plasma (in stress).
Occurrence: in ewes in late pregnancy carrying more than one fetus. Occurs when the sheep cannot meet the energy demands of the late pregnancy, leading to the low blood sugar, fat breakdown + ketone production.
causes: Excessive glycogen demand for the fetus + decreased ability of the liver to make glucose by gluconeogenesis.
Mechanism:
increased glucose demand (growing fetuses need energy)
hypoglycemia (blood sugar of ewe drop as the fetuses are using it up too quickly)
hormonal changes: low glucose → insulin decreases, STH and glucagon increases.
Fat mobilization: to compensate for lack of glucose → body breaks down fat → FFAs
FFAs → liver → partial oxidation → ketone body synthesis
Special case: Fat ewes and stress:
stress in overweight ewes trigger release of catecholamines like adrenaline
fat mobilization → increased FFAs → liver cannot process → ketone body synthesis
Consequences: ketone bodies build up → toxic, death of fetus.
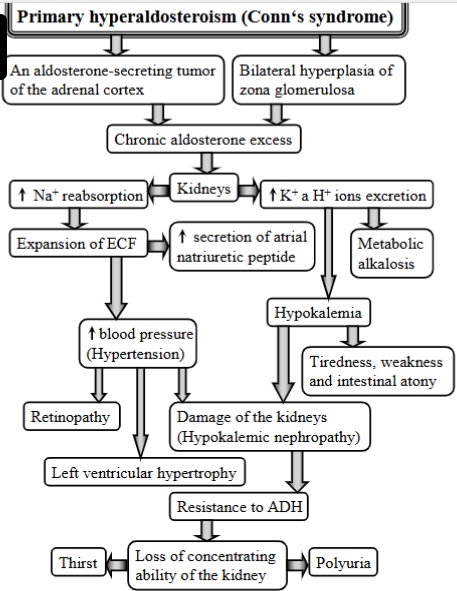
Explain conn`s syndrome
(primary hyperaldosteronism)
Condition that causes high BP, K+ loss + other electrolyte imbalances due to excessive aldosterone production by the adrenal gland.
Causes:
aldosterone-secreting tumor of the adrenal cortex
chronic aldosterone excess
bilateral hyperplasia of the zona glomerulosa
Mechanism:
causes → affects kidneys
excretion of K+ and H+ → hypokalemia + metabolic alkalosis
reabsorption of Na+
expansion of ECF + secretion of atrial natriuretic peptide
high BP (hypertension) → retinopathy, left ventricular hypertrophy, damage to kidneys
tiredness, weakness, intestinal atony
resistance to ADH, loss of concetrating ability of kidney, thirst and polyuria.
SIADH, definition, causes, consequences
Syndrome of inappropriate Antidiuretic Hormone Secretion.
Occurs when the body makes too much vasopressin (Antidiuretic hormone/ADH).
Occurs by:
Excess ADH
water retention - body will hold on to too much water
decreased sodium levels in the blood
Causes: different conditions and drugs, certain tumors like lung cancer or by chronic lung diseases.
Medicines linked with SIADH - common meds such as antidepressants, antianxiety agents, antipsychotic agents, seizure meds, and desmopressin (DDAVP)
Consequences:
Low urinary output
high levels of ADH
hyponatremia (retaining too much fluid dilutes the sodium in the blood)
over hydrated
retaining too much fluid

Malabsorption diarrhea
Occurs when the ability to digest or absorb nutrients is impaired, leading to increased fecal volume and fluidity.
Pathogenesis: disorders of mixing, disorders of digestive enzymes, damage to enterocytes or their surface transporters
Diarrhea due to changed motility
increased tone of PNS (stress, fear)
excessive motility of intestine → decreased transit time + mucosal surface contact → less chance of absorption → large-volume diarrhea
Altered gastric Secretion
hypersecretion of gastric acid → reduced activity of pancreatic enzymes in the duodenum
changes pH → gastric diarrhea
changes of activity of pancreatic enzymes
chronic pancreatitis or pancreatic cancer can change the activity of pancreatic enzymes with lipase activity being lost most rapidly
changed bile salts function
liver damage, obstruction, paralytic ileus
treatment with ATB, bacterial overgrowth in SI
bile salt deficiency results in steatorrhea, insufficient fat digestion
damage to enterocytes/their surface transporters
infectious agents such as pig corona virus, rota virus in calf and parvovuis → mucosal damage
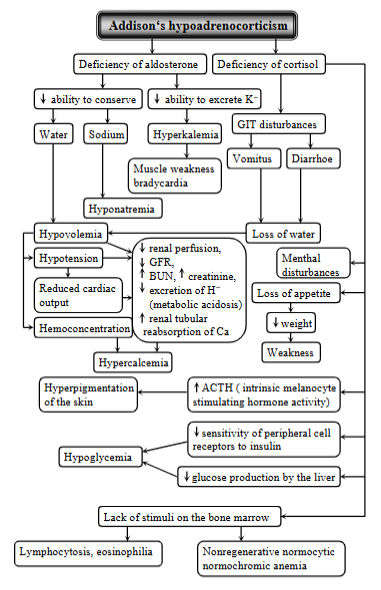
Addison disease
(Primary hypoadrenocorticism)
Syndrome resulting from deficiency of both glucocorticoid and mineralcorticoid secretion from the adrenal cortex. Occurs more frequently in dog, rare in cat.
Causes: idiopathic (autoimmune) adrenocortical insufficiency (body`s immune system attacks the adrenal cortex), surgical removal of both glands, hemorrhage or blockage of blood supply, fungal infections or tumors, drug - mitotane damaging the adrenal cortex, or tuberculosis infection.
Mechanism:
deficiency of aldosterone (mineralcorticoid def.)
inability to conserve sodium, ability to secrete potassium + loss of water → hyponatremia, hyperkalemia, hypovolemia (low blood volume)
Muscle weakness + bradycardia - from electrolyte imbalances
hypotension + reduced cardiac output, hypercalcemia
Deficiency of cortisol (glucocorticoid deficiency)
GIT disturbances (vomiting, diarrhea) → loss of water
mental disturbances, loss of appetite, hyperpigmentation of skin (Incr. ACTH), hypoglycemia
Escape complex (beat), AV junctional rhythm (Definition, ECG)
Occurs when the SA node stops or slows to a rate less than that of a subsidiary automatic pacemaker.
ECG: QRS is normal, P-wave is negative and may precede, be superimposed on, or follow the QRS complex. P-wave location depends.
Subsidiary pacemakers (AV nodal, junctional tissue and Purkinje fibers) function as a rescue mechanism during severe bradycardia.
Escape complex (Beat): single spontaneous impulse from a lower pacemaker. (lower pacemaker takes over when higher pacemakers fail (SA node fails).
Escape rhythm: run of three or more such complexes.
Acute (ruminal/lactate) acidosis
Caused by sudden intake of excessive quantities of highly fermentable feeds. Process:
increased production of VFAs → shifting proportion
pH drops, harming protozoa
shift in microbial population, increased lactic acid production and further decreasing rumen pH
if there are no fibers i the diet: decreased salivation, rumination and regurgitation, decreased alkali in rumen, lactic acidosis
Consequences: metabolic acidosis, inhibition of breathing, lysis of gram-negative bacteria (release of endotoxins and histamine). Dehydration, hemoconcentration, diarrhea.
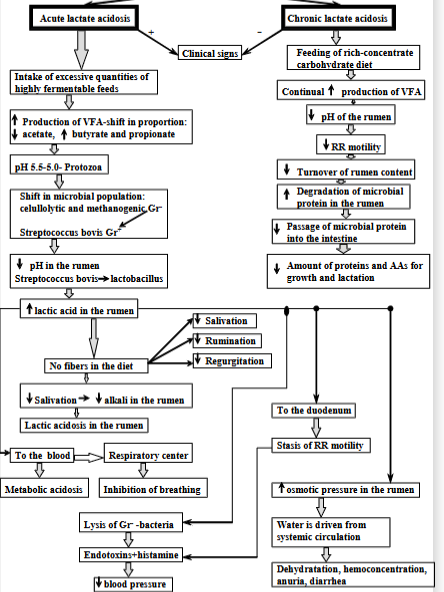
Chronic renal failure - 4 consequences
Gradual decline in kidney function. Prolonged azotemia leads to combination of adaptive metabolic changes + clinical signs known as the uremic syndrome.
Causes: toxic effect of retained products normally excreted by kidneys (urea), normal products - hormones but not present in increased amounts, loss of normal products (erythropoietin).
Loss of nephron mass → decreased renal biosynthetic capacity and renal excretory function.
Consequences:
Decreased renal excretory function:
Buildup of waste products → toxic metabolites → reduced appetite, weight loss, poor hair coat, protein-calorie malnutrition.
Bone problems: not being able to excrete acid + generate buffers → metabolic acidosis → bone decalcification, osteoporosis.
P builds up → low Ca + increased PTH
Fluid imbalance: reduced excretion of sodium + water → edema, ascites, hypertension
increased urea in blood, changes in WBC, oral unceration, melena.
Decreased renal biosynthetic capacity:
hormone regulation issues: impaired degradation of insulin in kidneys
Ca + bone: reduced production of vitamin D3 → decreased absorption of calcium → hypocalcemia
secondary hyperparathyroidism - as the body tries to fix the low levels of ca by PTH
anemia - reduced erythropoietin, uremic toxins + RBC damage, platelets defects, phosphate imbalance.
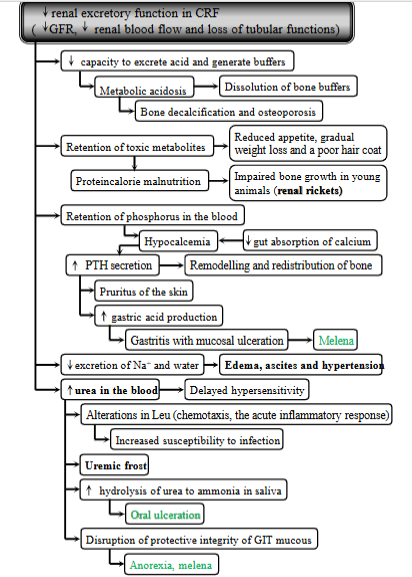
diabetes insipidus
Syndrome characterized by polyuria (excessive urination) + polydipsia (excessive thirst). Resulting from inability to concentrate urine due to lack of vasopressin (antidiuretic hormone, ADH). Mostly affects dog, horse, cat.
two main types:
central Diabetes Insipidus: diseases of CNS, total, partial
head trauma, intracranial tumor, post-intracranial surgery or idiopathic, inflammatory or parasitic lesions of CNS
Nephrogenic diabetes insipidus: diseases of kidney
congenital or secondary to a variety renal + metabolic disorders including CRF, tubular necrosis or drug-induced.
Mechanism:
Large damage of CNS → decreased synthesis + secretion of ADH → polyuria + nocturia → pressure receptors stimulated → polydipsia → inability to maintain water intake → dehydration → hypernatremia → cell shinkage → neurological symptoms + coma.
Pathological: decreased ADH → decreased water reabsorption in renal tubules → decreased fluid volume → increased serum osmolality + excessive urine output.
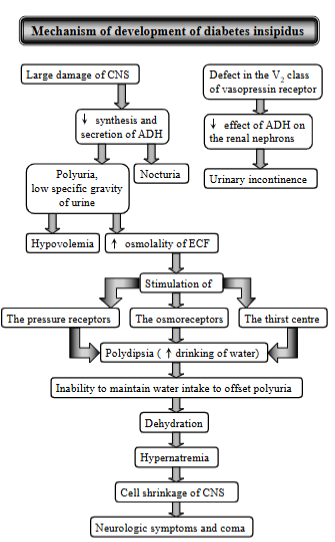
enzootic calcinosis - pathogenesis
Chronic disease in cattle, sheep by eating certain plants containing calcinogenic glycosides leading to calcium deposits in soft tissues, esp. blood vessels and heart.
Process:
grazing on plants
glycosides are broken down in rumen
vitamin D mimicking substance is made (vitamin D3 is made)
calcium overload as the imposter vitamin D will:
increase Ca absorption from intestine
increase Ca mobilization from bones
hypercalcemia
calcification
signs: stiff gait, reluctance to move.
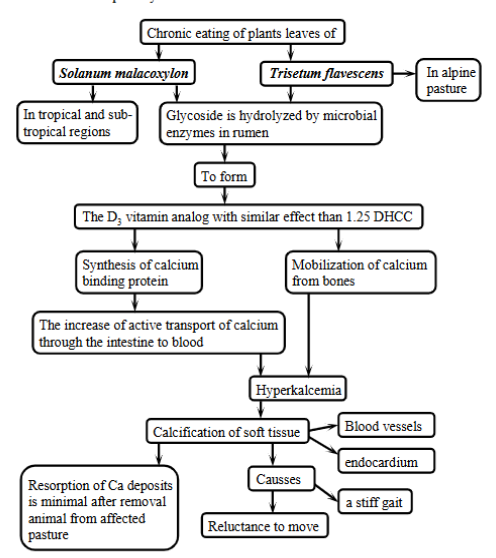
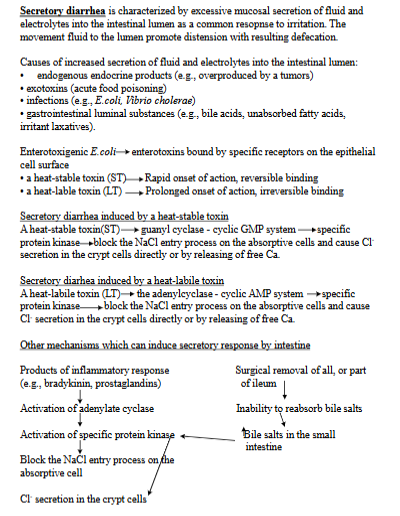
secretory diarrhea - pathogenesis
Characterized by excessive mucosal secretion of fluid and electrolytes into the intestinal lumen. Resulting in distension, increased frequency of defecation.
causes: endogenous endocrine products (overproduced by tumors), exotoxins (acute food poisoning), infections (E. coli), GI luminal substances - bile acids, unabsorbed FA.
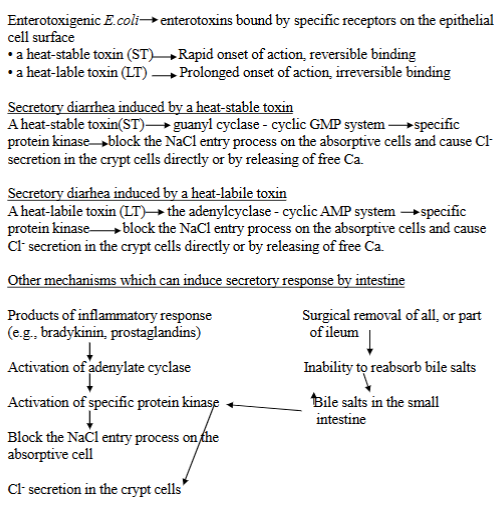
Enterotoxins - bacteria like E.Coli produce these
heat-stable toxin (ST): activates the guanyl cyclase-cyclic GMP system, blocking NaCl entry + causing Cl-secretion.
heat-labile toxin (LT): activates adenylcyclase-cyclic AMP system, also blcoking NaCl entry + causing Cl-secretion.
Other mechanisms: products of inflammatory response (bradykins), surgical removal of all/part of ileum → inability to reabsorb bile salts
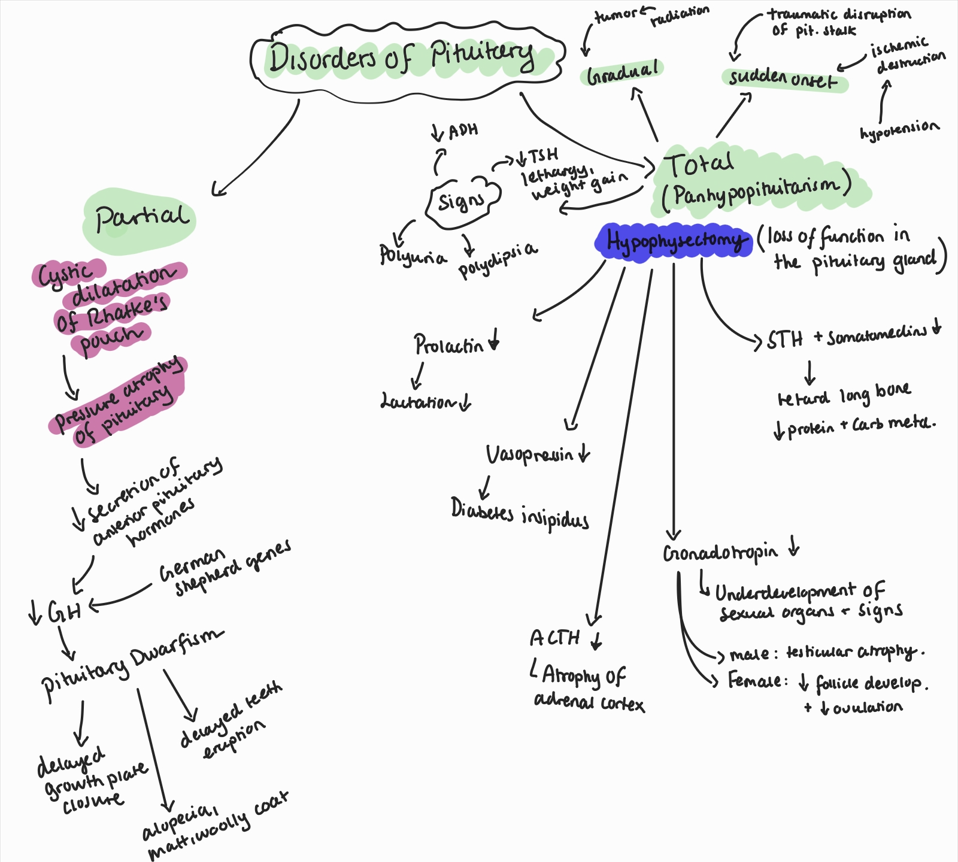
Hypopituitarism, definition, types, causes, consequences
Condition characterized by decreased secretion of one or more of the hormones produced by the anterior pituitary gland.
Types:
partial: cystic dilatation of Rathke`s pouch → pressure atrophy of the pituitary → red. secretion of anterior pituitary hormones → decr. in GH
can lead to pituitary dwarfism - loss of hair coat, alopecia, matted + woolly appearance, delayed growth plate closure
common in german shepherds, genetic.
total (panhypopituitarism): complete loss of pituitary function.
caused by hypohysectomy
consequences:
prolactin deficiency: ionterruption of lactation
gonadotropin deficiency: underdevelopment of sexual organs, secondary sexual signs
vasopressin deficiency: diabetes insipidus
ACTH deficiency: atrophy of adrenal cortex
STH deficiency: retard growth of long bone + diminishing of protein + carb metabolism.
there are 2 patterns of total hypopituitarism:
sudden onset: by traumatic disruption of the pituitary stalk or ischemic destruction
gradual: tumors, pressure on osmoreceptors, reduction in throphic hormone. → decr. ADH, polyuria, polydipsia, decr. TSH, lethargy, weight gain.
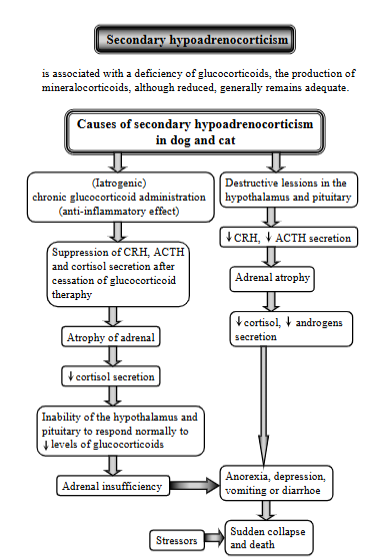
secondary hypoadrenocorticism, definition, causes
Condition from a deficiency of ACTH (adrenocorticotropic hormone) secretion from the anterior pituitary. While it can cause deficiency in glucocorticoids, the production of mineralcorticoids, although reduced, generally remains adequate.
Causes:
(latrogenic) chronic glucocorticoid administration (anti-inflammatory effect) → suppression of CRH, ACTH + cortisol secretion → atrophy of adrenal → decreased cortisol secretion → inability of hypothalamus + pituitary to respond normally to reduced levels of glucocorticoids → adrenal insufficiency
Destructive lesions in the hypothalamus, pituitary → decreased CRH + ACTH → adrenal atrophy → decreased cortisol + androgens
Results in anorexia, depression, vomiting, diarrhea → sudden collapse + death
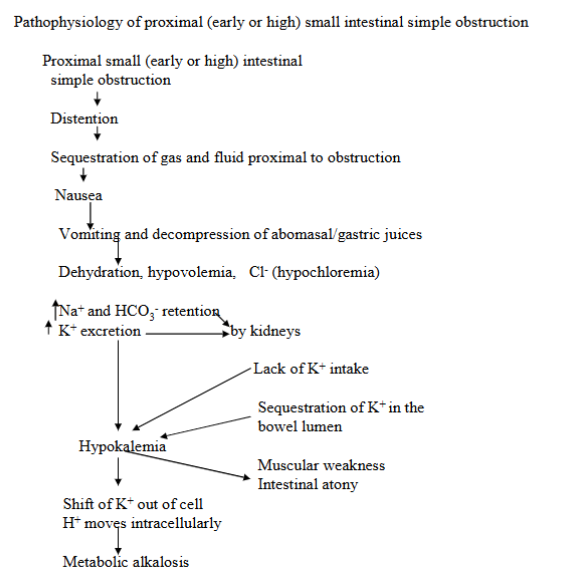
small intestine obstruction (proximal SI simple obstruction)
Distension + sequestration of gas and fluid proximal to the obstruction → nausea, vomiting and dehydration.
Metabolic abnormalities include hypochloremia, sodium and bicarbonate retention, hypokalemia, and metabolic alkalosis.
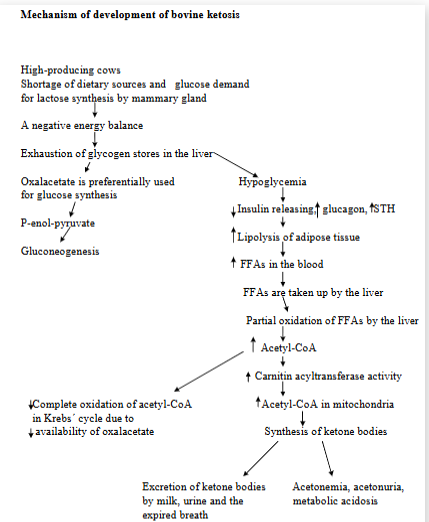
ketosis in dairy cattle (Bovine ketosis)
Metabolic disorder characterized by an increased concentration of ketone bodies in the blood, urine, milk. The animal`s body is not getting enough energy from carbs in the diet.
Hypoglycemia, low levels of hepatic glycogen, increase in plasma FFAs, ketonemia, ketonuria, metabolic acidosis.
Primary due to increased energy demand (lactation), secondary due to poor appetite as a result of pathological conditions (mastitis, infections).
Predisposing factors: importance of saccharide (carb) metabolism in liver of ru → dietary carbs are fermented by rumen microbes → VFAs
acetate used by peripheral tissues + mammary gland for long-chain FA, propionate used for glucose, and butyrate is converted and enters blood.
ruminants absorb very little dietary carbs directly as glucose → due to most being fermented
poor glycogen reserves → limited meaning that they cannot release glucose quickly when needed.
increased glucose demand is for mammary gland (lactose prod.) and fetus (growth and glycogen deposition)
Mechanism:
High-producing cows have not enough dietary sources + glucose demand for lactose synthesis by mammary gland → negative energy balance
exhaustion of glycogen stores in liver
hypoglycemia
reduced insulin → FFAs are taken up by liver → partial oxidation of FFAs by liver → increased Acetyl-CoA → ketone bodies formation
Oxalacetate is preferentially used for glucose synthesis → thus promoting buildup of acetyl-CoA.
For 3rd degree AV block - definition, changes on ECG tracing
3rd degree: Complete heart block, no electrical signals between sinus node and ventricles, the ventricles create their own, resulting in slower rhythm.
ECG: no relationship between P-waves and QRS complexes, resulting in varying P-R interval from beat to beat.
AV block: type of conduction disturbance, where the electrical signals in the heart gets delayed/blocked when going through the conduction system → prevents ventricles from contracting properly after atria. Caused by increased vagal tone, drugs, structural diseases of AV node.
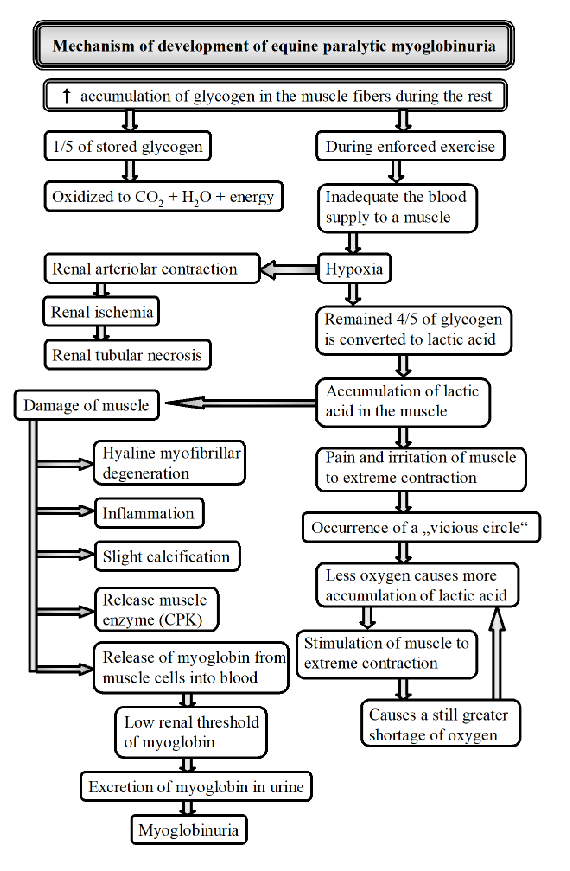
Explain pathogenesis of equine paralytic myoglobinuria/monday morning disease
Occurs after light exercise in horses undergoing training who are rested for 1 or more days being maintained on full carb diet.
Clinical signs: stiff, tilted gait, sweating, rapid breathing, muscles of gluteal/femoral are swollen and hard.
causes: Sudden increase in duration/intensity of training, environment (hot), factors changing blood supply, diet, decreased thyroid function, herpes, genetics.
Mechanism:
increased glycogen accumulation in muscles
sudden exercise - impaired blood supply to muscles
hypoxia - lack of O2 → lactic acid buildup → pain and muscle contraction → more O2 reduction
muscle damage → inflammation and release of CPK
Myoglobinuria - damaged muscle releases myoglobin into blood → urine
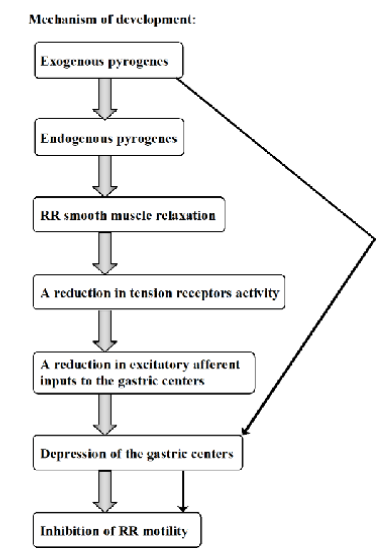
Central and peripheral inhibition of Ruminoreticular motility induced by pyrogens in febrile stage of disease.
Exogenous pyrogens stimulates release of endogenous pyrogens, which will act centrally and peripherally.
Effects:
directly affect RR smooth muscle, relaxes → reduces activity of tension receptors in rumen and reticulum. Resulting in reduction of excitatory signals.
Central effects: depressing activity of gastric centers
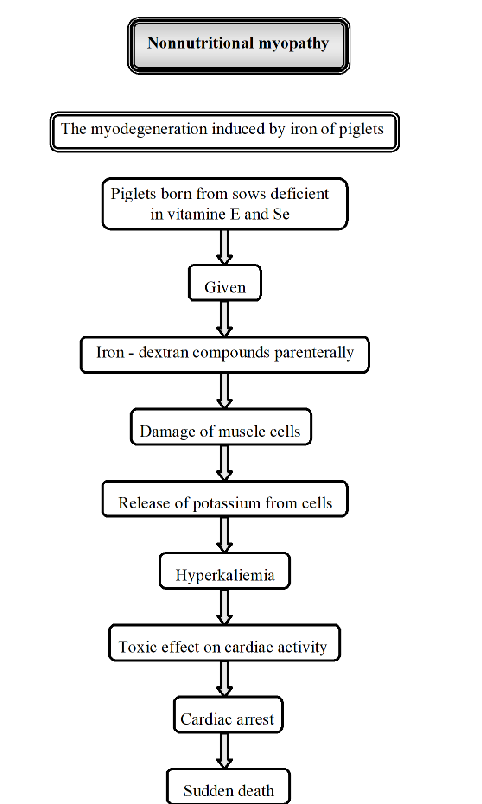
Pathogenesis of myodegeneration induced by iron of piglets.
“Non-nutritional myopathy in Piglets”. Muscle diseases not primarily caused by dietary deficiencies. It is caused by other factors like excessive iron injections.
Iron-induced myodegeneration:
Piglets are born from sows deficient in Se and vitamin E
Trigger: piglets are given iron-dextran compounds injection
The injection cause muscle damage, releasing potassium → hyperkalemia.
consequence: toxic effect on cardiac activity, cardiac arrest
sudden death
explain nephrotic syndrome
Condition characterized by severe proteinuria, albuminuria, hypoalbuminemia, peripheral edema, hyperlipidemia and fat bodies in the urine.
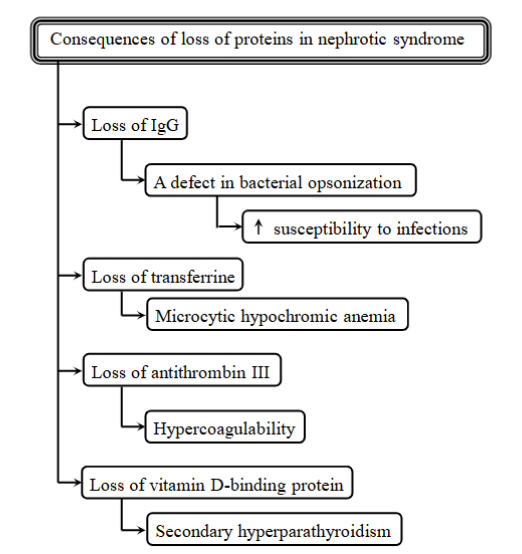
Consequences of loss of proteins in nephrotic syndrome:
immunodeficiency - loss of lgG
Anemia - loss of transferrin
hypercoagulability - increased risk of blood clots
secondary hyperparathyroidism - loss of vitamin-D binding protein, affecting calcium metabolism
Pathogenesis:
increased glomerular permeability → filters in kidneys are letting through products they should not, like proteins
leads to albuminuria (albumin in urine), urinary losses of proteins carrying hormones, metals and vitamins, hypoalbuminemia (not enough in blood), edema (low albumin → fluid leaks out of blood vessels), malnutrition, increased infections, thromboembolism (changed coagulation), tubular dysfunction
explain uremia
Condition that arises from chronic renal failure (CRF). Prolonged azotemia leads to a combination of adaptive metabolic changes and clinical signs known as the uremic syndrome.
caused by loss of nephron mass, reducing renal excretory and biosynthetic functions.
leading to buildup of waste products → level of urea and creatinine increase in the blood
explain renal metabolic acidosis
Occurs as a consequence to disorders of the renal tubules.
Characterized by impaired bicarbonate reabsorption and H+ secretion by the renal tubules → acid-base imbalance in the body.
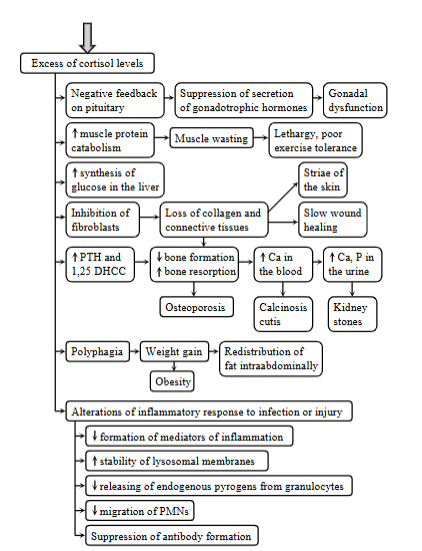
4 metabolic consequences of cushing`s syndrome
Cushing syndrome - hyperadrenocorticism.
Excess of cortisol levels, due to a failure of negative feedback mechanism of cortisol on ACTH.
Excess of glucocorticoids:
spontaneous cushing: excessive secretion of ACTH from anterior pituitary adenomas
latrogenic cushing: chronic glucocorticoid administration
Both lead to a failure of negative feedback mechanism of cortisol on ACTH, due to the chronic exogenous corticoids administration, increased secretion of ACTH by pituitary dependent hypercortisolism.
Metabolic consequences:
increased muscle protein catabolism - muscle wasting, leathargy, poor exercise tolerance
inhibition of fibroblasts → loss of collagen and CT → decreased bone formation, increased bone resorption → increased Ca → calcinosis cutis, kidney stones
changes of inflammatory response to infection or injury: suppression of Ab formation, decreased formation of inflammatory mediators, decrease releasing of endogenous pyrogens.
increased synthesis of glucose in the liver
explain
tertiary hypothyroidism
neophrogenic diabetes insipidus
Tertiary hypothyroidism: state where the body does not maintain normal levels of target tissue stimulation due to inadequate thyroid hormone secretion. In this case, the issue lies in the hypothalamus (hypothalamic dysfunction).
hypothalamus does not properly release thyrotropin-releasing hormone (TRH), which is repsonsible for triggering the release of TSH.
Nephrogenic diabetes insipidus: body is unable to concentrate urine. Results in polyuria and polydipsia.
caused by diseases of kidney reducing sensitivity to vasopressin (ADH).
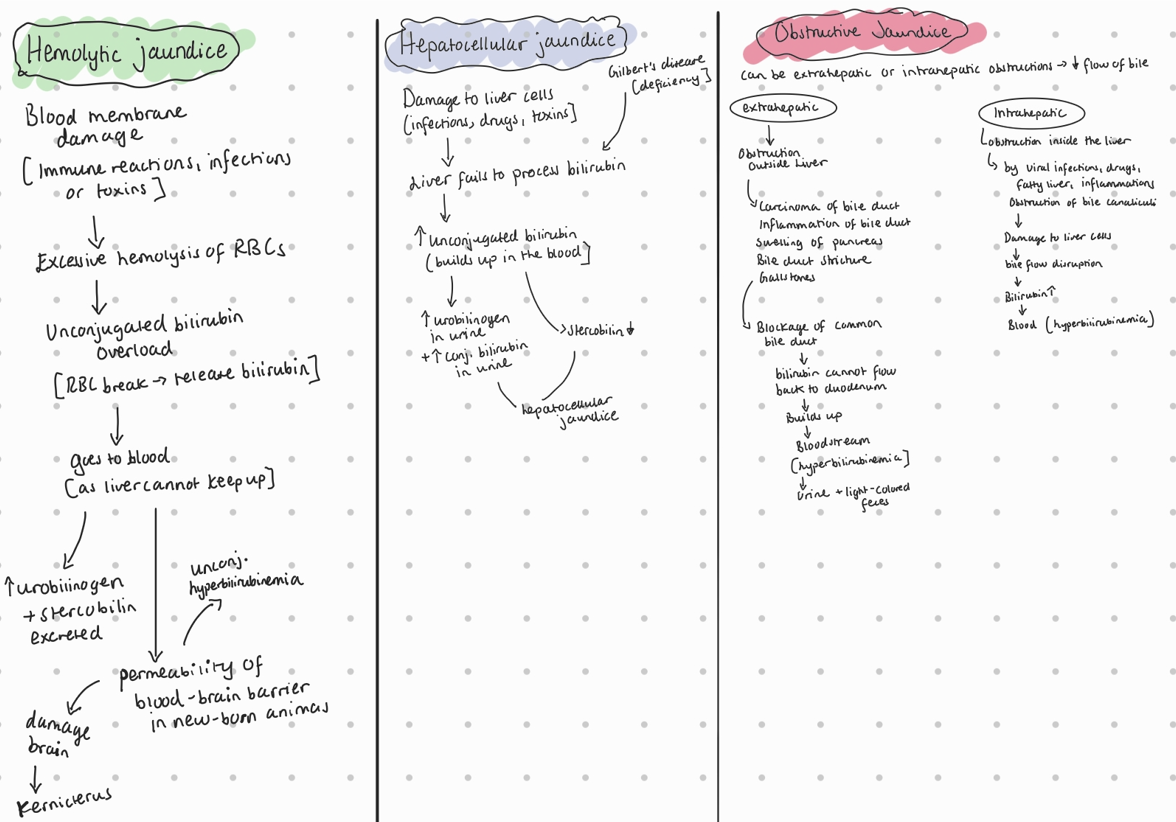
Name 3 types of hyperbilirubinemia and to each write one cause
3 types of hyperbilirubinemia which results in jaundice:
hemolytic jaundice: caused by excessive hemolysis (breakdown) or RBC due to blood membrane damage (immune mechanisms, severe infections, toxic substances like snake venom)
hepatocellular jaundice: by damage to the liver cells, by infections, tumors, therapeutic drugs like steroids or toxic chemicals.
obstructive jaundice: obstruction of the common bile duct (mechanical blockade), which can be caused by carcinoma of the bile duct, cholangitis, swelling of the pancreas, bile duct stricture, or obstruction by gall stones.
Hyperbilirubinemia → results in jaundice/icterus (yellow discoloration of scleras and skin). Too much bilirubin by excessive lysis of RBC, liver not being able to conjugate or excrete bilirubin and obstruction of bile duct system.
Atrial flutter, definition, changes on ECG
Type of ectopic arrhythmia, occurring from a site other than the sinus node.
Characterized by extremely rapid P-waves, which are sometimes called “flutter” waves. The atrial frequency is more than 300 beats per minute in dogs.
The AV node cannot conduct all these rapid impulses, so it blocks some of them, meaning the ventricular rate is slower than atrial rate.
The conduction of impulses through AV node to ventricle may follow a pattern, like 2:1 or 3:1 AV conduction pattern. QRS configuration is normal.
Causes: Mitral stenosis, myocarditis, hyperthyroidism.
Explain 3 cases of hyperammonemia
liver dysfunction: changes the urea cycle, which is needed for converting ammonia into urea. When the liver is damaged (due to cirrhosis or hepatitis), it cannot efficiently process ammonia. So, the ammonia accumulates in the blood. hepatic encephalopathy.
Hepatic shunting: occurs when blood bypasses the liver. This can be due to congenital or acquired conditions that create abnormal connections between the portal and systemic circulation. Ammonia-rich blood enters systemic circulation without being detoxified.
Ruminal alkalosis: excessive urea in the diet → converted to ammonia in rumen → produced faster than it is processed → blood → hyperammonemia.
Name 3 types of diarrhea and for each type write one cause
Secretory Diarrhea: Caused by the excessive mucosal secretion of fluid and electrolytes into the intestinal lumen.
cause: Infections (e.g., E. coli, Vibrio cholerae) that produce exotoxins. Heat-stable toxin (ST) → blocks NaCl entry and cause Cl secretion.
Osmotic Diarrhea: Caused by high luminal osmotic pressures due to faulty digestion, impaired particle absorption, or increased intake of indigestible molecules.
cause: Lactase deficiency, where lactose isn't digested or absorbed, drawing water into the intestine.
Malabsorptive Diarrhea: Develops when the ability to digest or absorb a particular nutrient is defective.
cause Damage to enterocytes from infectious agents like Coronavirus in pigs, Rotavirus in calves, or Parvovirus in carnivores.
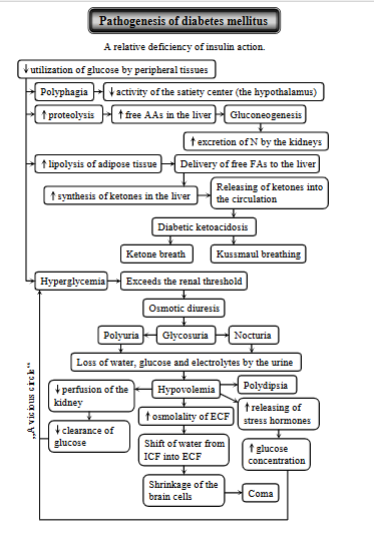
Diabetes mellitus - pathogenesis
Disorder in the dog and cat, characterized by a relative or absolute functional deficiency of insulin action → hyperglycemia + metabolic disturbances.
Causes: decrease in insulin secretion by B-cells of pancreas, decreased response to insulin, increase in counter regulatory hormones that oppose the effect of insulin.
Key process: decreased utilization of glucose leading to:
due to lack of insulin → breakdown of proteins → releases free AA → liver, undergoing gluconeogenesis (making glucose) → hyperglycemia.
kidneys will excrete excess N
Lipolysis of adipose tissue - due to insulin deficiency, causing the breakdown of fats → releases FFAs → sent to liver → ketogenesis (used to make ketones).
ketones are released into the circulation → diabetic ketoacidosis (DKA)
ketone breath (fruity odor on breath due to acetone)
kussmaul breathing (attempt to expel excess CO2 + counteract acidosis).
Hyperglycemia - increased blood glucose levels due to impaired glucose uptake by cells + incr. glucose production in liver.
exceeding the renal threshold → osmotic diuresis → glucose in urine, polyuria, nocturia (frequent urination at night)
the effects lead to loss of water, glucose and electrolytes in the urine → hypovolemia, triggering release of stress hormones.
Shift of water from ICF to ECF: hyperglycemia increases osmotic pressure in ECF, drawing out water of cells → shrinkage of brain cells → coma
explain anemia
Anemia: condition by a deficiency of RBCs or Hb in the blood → reduced O2 transport to tissues. This can be in several conditions:
malabsorption: lack of vitamin B12 → anemia
hypoproteinemia: lack of transferrin synthesis due to liver damage can limit iron availability for Hb synthesis → microcytic, hypochromic anemia.
nephrotic syndrome: loss of transferrin
Chronic renal failure: anemia due to reduced erythropoiesis + GI blood loss
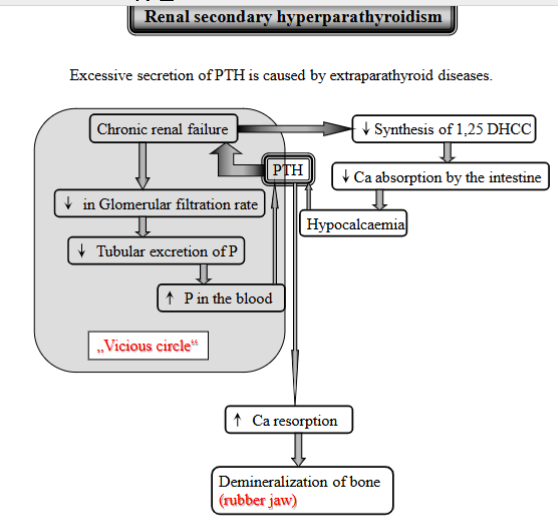
explain Renal secondary hyperparathyroidism (not sure it was this)
Excessive PTH secretion caused by kidney disease. (extraparathyroid diseases).
Process:
CRF - kidneys fails
decreased glomerular filtration rate
decreased tubular excretion of phosphorus - kidneys cannot get rid of enough
increased P in the blood + kidneys cannot make vitamin D → decreased Ca absorption from intestine → hypocalcemia
increased PTH secretion - due to low Ca
PTH will get Ca from bones, demineralization of bone → rubber jaw
Vicious cycle: increased PTH → further contributes to bone problems → kidney failure gets worse and so on. (constant stimulation → glands enlarge + secrete PTH)
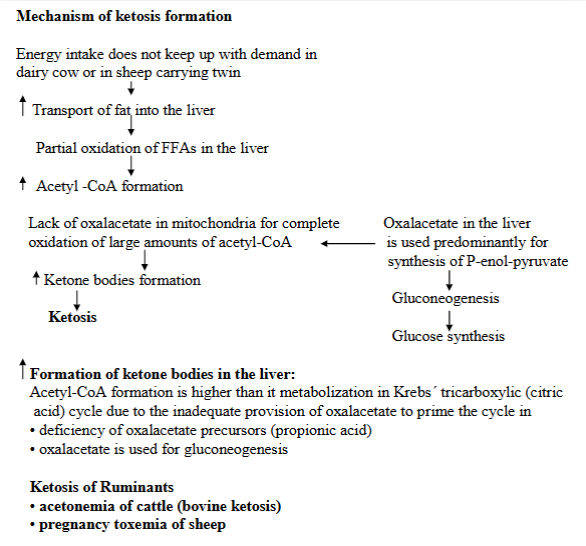
explain 3 metabolic states associated with ketosis
Ketosis is defined as the increased concentration of ketone bodies in the blood, urine, milk + expired breath.
Carbohydrate or their precursors deficiency or loss, hypoglycemia:
Deficiency or loss of carbs or their precursors, where the body cannot produce enough glucose for energy → low blood sugar (hypoglycemia) → triggers breakdown of fats → alternative way of energy.
liver then produce ketone bodies from these fats → ketosis.
case: high-fat, low carb diet, starvation, insulin hypoglycemia, glycogen storage disease, cobalt deficiency → vit. B12 deficiency.
Increased metabolic requirements:
During periods of increased metabolic demands, where body needs more energy → body starts to break down fats → ketosis.
case: pregnancy, lactation, rapid growth (fetus), wound healing, fever.
Metabolic blocks:
refers to conditions that disrupt the normal metabolism of carbohydrates. If there is an issue with glucose usage or a block in the glycolytic pathway → glucose cannot be used properly. Body will thus compensate by increasing fat metabolism → ketone body production, ketosis.
case: diabetes mellitus, various drugs
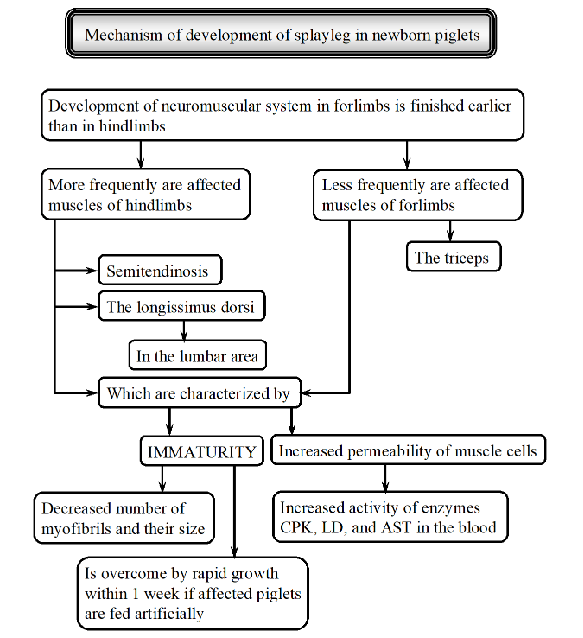
explain splayleg syndrome in piglets/Inherited Myopathies
Condition where newborn piglets cannot stand properly, mostly affecting hind legs, splayed to the sides/forwards. They mostly lie on their sternum, struggling to nurse.
Causes:
genetics, some breeds more susceptible (landrace, large white)
maternal stress (stress in mother → excess glucocorticoids → myopathy)
maternal nutrition (choline deficiency)
toxins (ingestion of fusarium by sow)
slippery flows can worsen the condition
Mechanism: neuromuscular system in the forelimbs develops earlier than in hindlimbs → because of this, hindlimbs are more affected.
muscles affected:
immaturity - decreased number/size of myofibrils
increased permeability of muscle cells - increased activity of muscle enzymes in blood → indicating muscle damage
explain pathogenesis of 2 systemic consequences of chronic ruminal acidosis.
Consequences: Laminitis, ruminal parakeratosis, rumenitis, liver abscesses, demineralization of bone, cerebrocortical necrosis and low-milk fat syndrome.
Ruminal acidosis: increased lactic acid, VFAs, decreased pH and increased osmotic pressure → damaging rumen epithelium.
Ruminal parakeratosis: affecting rumen lining.
Ruminal papillae becomes dark, enlarged and clump together.
leads to thickening of the cornified (outer) layer of rumen lining.
results in decreased absorption of rumen
leads to rumenitis (inflammation)
ulceration by fusobacterium necrophorum - damage leads to susceptibility
the bacteria can then travel to the liver, causing liver abscesses.
name 3 types of ileus and for each one type, write one cause
Ileus (intestinal obstruction): prolonged transit time or cessation of passage of digesta from the pylorus to the anus.
Type of ileus:
mechanical ileus (mechanical obstruction, a simple obstruction)
internally obstructing mass (foreign bodies, colon tumors), compression or distortion of the bowel by an extrinsic lesion (abscess, tumor), stricture of bowel wall (neoplasia).
malposition of bowel: hernia (protrusion of intestine), intussusception (telescoping of segment of SI), volvulus (twisting of intestine)
Adynamic ileus
caused by electrolyte imbalance (hyperkalemia)
involves loss of motility in intestine, paralytic and vascular ileus
spastic ileus
caused by strangulation obstruction, involving obstruction of the bowel lumen and simultaneous vascular compromise
parasympathicomy, characterized by increased tonus of intestinal walls + tonic spasm
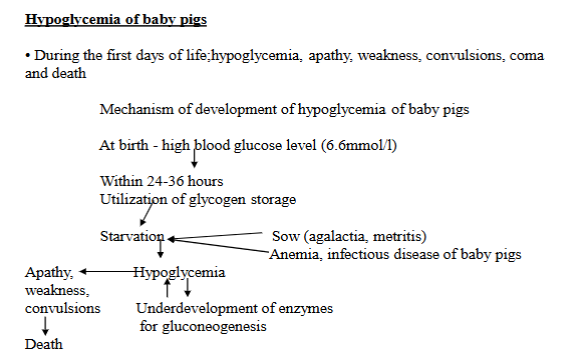
explain pathogenesis of hypoglycemia in piglets
Condition where newborn piglets experience abnormally low blood glucose levels. Issue during the first few days of life. Leads to weakness, apathy, convulsions, coma and death.
Pathogenesis:
High blood sugar at birth
glycogen use - within 24-36h, piglets use up their stored glycogen
starvation - if the piglets do not get enough milk, they become starved.
sow problems - mother pig might have agalactia (not producing milk) or metritis (uterine infection)
piglet problems - anemia, infectious diseases
Limited gluconeogenesis - as the piglets do not have fully developed enzymes for gluconeogenesis
Lack of food + immature glucose production → low blood sugar (hypoglycemia)
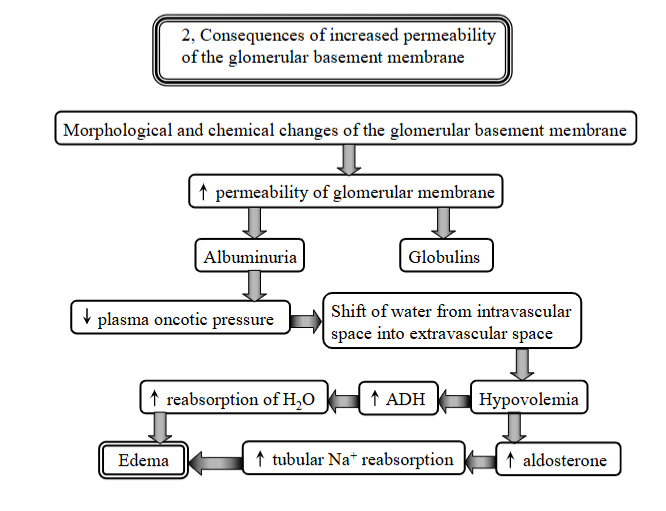
explain 3 consequences of increased permeability of glomerular membrane?
Disorder of glomeruli - increased permeability of glomerular membrane.
morphological + chemical changes of the membrane → increased permeability:
albuminuria + globulins - allows albumin to leak into urine
decreased plasma oncotic pressure - albumin is lost in urine, thus the blood looses its ability to hold onto water → decrease in pressure.
Edema - due to shift of water from intravascular to extravascular space → hypovolemia, body responds with aldosterone secretion for sodium reabsoprtion + ADH for water → Edema.
pathogenesis of secretory diarrhea associated with enterotoxicogenic E. coli.
Bacterial infection - process starts with an infection by enterotoxicogenic E. Coli in the intestinal tract → where it produces enterotoxins that disrupt the normal function.
Specific enterotoxins:
Heat-stable Toxin (ST): activates the guanylate cyclase-cyclic GMP system. Blocks NaCl entry into intestinal cells → increased Cl secretion into lumen.
Heat-labile toxin (LT): activates the adenylate cyclase-cyclic AMP system. Blocks NaCl, Increases Cl secretion into lumen.
Leads to increased luminal distension (swelling) due to the fluid. Distension promotes increased frequency of defecation + fecal fluidity → secretory diarrhea.
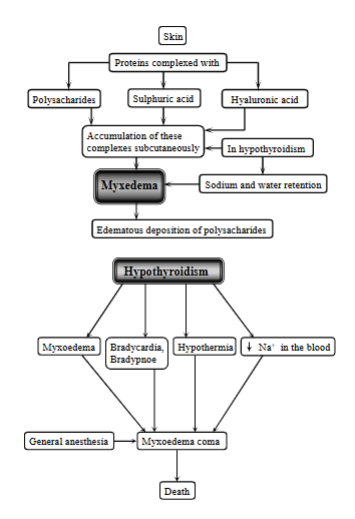
explain myxedema
A severe form of hypothyroidism resulting in swelling and changes in skin and muscle, which can lead to myopathy.
Condition of accumulation of complexes (proteins complexes with polysaccharides, sulfuric acid and hyaluronic acid) under the skin.
In hypothyroidism, there is an accumulation of these subcutaneously → sodium + water retention → edematous deposition of polysaccharides.
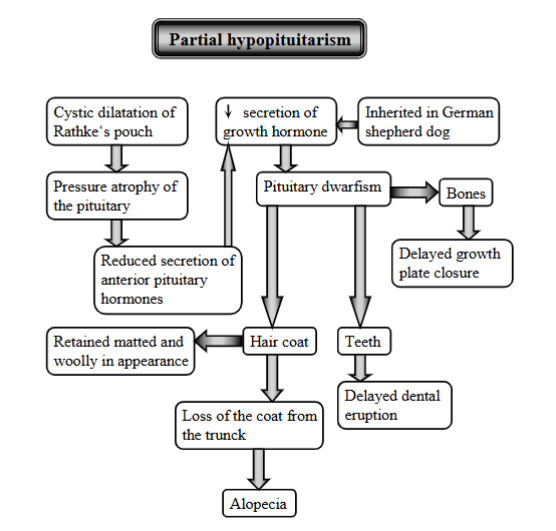
explain partial hypopituitarism
Condition of reduced secretion of anterior pituitary hormones, it is when the gland does not produce enough of some hormones, but not all (partial loss).
Mechanism:
cystic dilatation of rathke`s pouch (fluid filled sac forming that puts pressure on the gland)
pressure atrophy of the pituitary
reduced secretion ofr the hormones → reduction of GH
pituitary dwarfism
affecting hair coat (matted + woolly, alopecia)
affecting bone (delayed growth plate closure)
affecting teeth (delayed dental eruption)
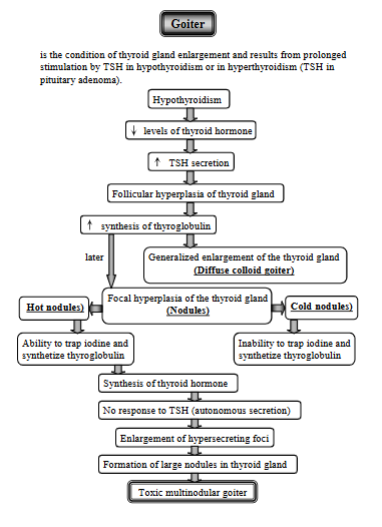
explain gouu`s disease (Goiter)
Enlargement of thyroid gland, due to prolonged stimulation by TSH in hypothyroidism or in hyperthyroidism.
Low Thyroid Hormone Levels (Hypothyroidism)
decreased levels of thyroid hormone → TSH increases in response
Follicular hyperplasia - constant TSH stimulation → thyroid grows, increased synthesis of thyroglobulin, leading to:
diffuse colloid Goiter (generalized enlargement of the gland)
Focal Hyperplasia (Nodules) - Sometimes, specific areas of the thyroid enlarge, forming nodules. These can be:
Hot Nodules: Nodules that can trap iodine and make thyroid hormone.
These nodules stop responding to TSH.
Leading to Enlargement of Hypersecreting Foci
Formation of Large Nodules in Thyroid Gland
Cold Nodules: Nodules that cannot trap iodine or make thyroid hormone.
Toxic Multinodular Goiter
This occurs when large nodules form in the thyroid gland, autonomously secreting thyroid hormone.