Chapter 16 and 17 cell bio
1/102
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
103 Terms
Chapter 16: Define signal transduction and list the basic components involved in this process in cells.
signals that pass between cells
the signaling cell produces a particular type of extracellular signal molecule that is detected by the target cell.
Target cells possess proteins called receptors that recognize and respond specifically to the signal.
signal transduction begins when the receptor on a target cell receives an incoming extracellular signal and then produces intracellular signalling molecules that alter cell behavior.
Distinguish the main types of signal-mediated cell-cell communication and identify the type of extracellular signal molecule involved in each
signal molecules can be proteins, peptides, amino acids, nucleotides, steroids, fatty acids, derivatives or even dissolved gases.
the most "public" style cell-cell communication: endocrine signaling
some less public signaling is paracrine signaling
Endocrine signaling
secretes the signal into the animals bloodstream, made by endocrine cells in the pancreas
Explain how the same signal molecule can induce different responses in different target cells
a cells response depends on the set of intracellular signaling molecules each receptor produces or how these molecules alter the activity of the effector proteins
Differentiate the types of cell responses that occur rapidly with those that take minutes or hours to execute
response times corresponds to the complexity of the response
for ex: cell growth and division can take a long time to execute since the response requires changes in gene expression and production of new proteins
transmembrane receptors
detect a signal on the outside and relay the message, in a new form across the membrane into the interior cell
steps of an intracellular signaling pathway
signal is relayed onward
the signal received is amplify
detect signals from more than one pathway and integrate them before relaying a signal onward
signal is distributed to more than one effector protein
response to the signal is regulated through feedback
Compare positive and negative feedback and contrast the types of responses produced by each
Positive: a component that lies downstream in the pathway acts on an earlier component in the pathway to enhance the response to the initial signal
Negative: a downstream component acts to inhibit an earlier component in the pathway to diminish the response to the initial signal
Summarize how phosphorylation can act as a molecular switch, and identify the types of proteins that add and remove this chemical modification
protein kinase covalently attaches to a phosphate group onto the switch protein
protein phosphatase takes the phosphate off again
Distinguish the two main types of GTP-binding proteins
trimeric GTP-binding proteins: AKA G-proteins that relay messages from G-protein coupled receptors
monomeric GTPases: help to hydrolyze GTP
Guanine nucleotide exchange factors (GEFs) activate the protein by exchange of GDP for GTP
GTPases-activating proteins (GAPs) turn them off by promoting GTP hydrolysis
Describe how monomeric GTPases toggle between active and inactive form
depends on whether they have GTP or GDP bound to them respectively
once activated by GTP binding many of these proteins have intrinsic GTP hydrolyzing (GTPase) activity, and they shut themselves off by hydrolyzing their bound GTP to GDP
Differentiate the three main classes of cell-surface receptors and provide an example of each.
-all are coupled receptors
1) ion-channel: change the permeability of the plasma membrane to select ions thereby altering the membrane potential and if the conditions are right producing an electrical current
2) g-protein: activate membrane-bound trimeric GTP-binding protein, which then activate/inhibit an enzyme or an ion channel in the plasma membrane, initiating an intracellular cascade
3) enzyme: either act as enzymes or associate with enzymes inside the cell. when stimulated the enzymes can activate a wide variety of intracellular signaling pathways
List some foreign substances that alter physiology by interacting with cell-surface receptors.
heroin, nicotine, tranqs and chili peppers
these substances either block or overstimulate the receptor's natural activity
Describe the type of signal transduction carried out by ion-channel-coupled receptors.
rapid transmissions of signals across the synapses in the nervous system
convert a chemical signal, a neurotransmitter, into an electrical chemical signal, voltage
when the neurotransmitter binds to ion channel-coupled receptors on the surface of a target cell, the receptor alters its conformation so as to open a channel in the target cell's membrane, making it permeable.
ex. Na+, K+, Ca 2+
structure of G-protein-coupled receptors (GPCRs)
each is made of a single polypeptide chain that threads back and forth across the lipid bilayer seven times
specific for a particular set of receptors and for a particular set of target enzymes or channels in the plasma membrane
the general structure of a G protein and how the protein responds when activated by a GPCR
composed of three subunits (alpha beta and Y) two of which are tethered to the plasma membrane by short lipid tails.
in the unstimulated state, the alpha subunit is GDP bound and the g-protein is idle.
when an extracellular signal binds to its receptor the altered receptor activates a G-protein by causing the alpha subunit to decrease its affinity for GDP which is exchanged for GTP.
alpha subunit and the beta(y) can each interact directly with target proteins in the plasma membrane, which in turn may relay the signal to other destinations in the cell.
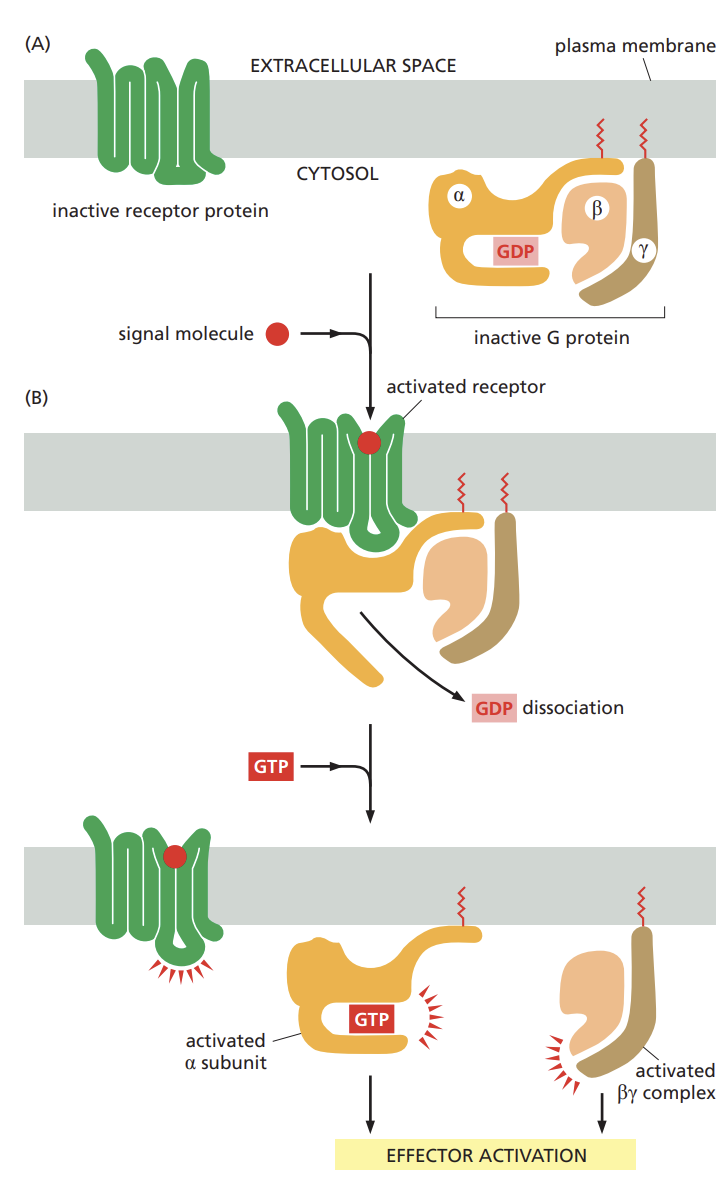
Summarize the factors that determine the duration of a GPCR-stimulated response
the longer the target proteins remain bound to an alpha subunit or beta(Y) complex the more prolonged the relayed signal will be.
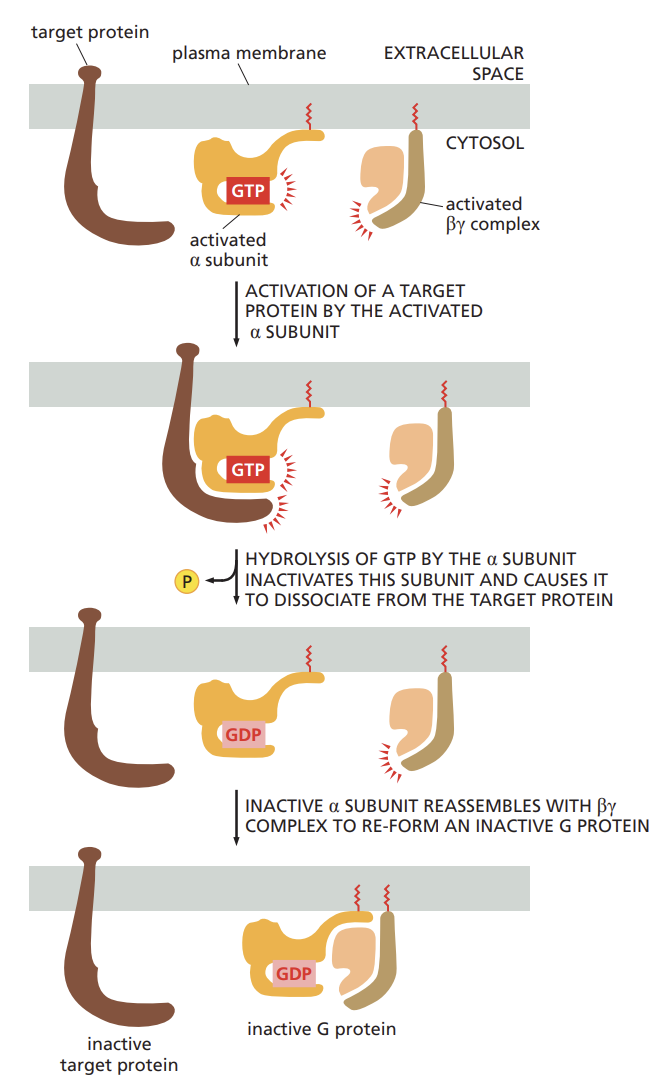
cholera toxin:
the protein cholera toxin enters the cell that line the intestine and modifies the alpha subunit of g-protein called Gs
this modification prevents Gs from hydrolyzing when its bound to GTP, thus locking the g-protein in an active state
results in diarrhea and dehydration
Relate the speeds of the responses produced by G proteins activating an ion channel versus activating a membrane-bound enzyme.
when g-protein interact with ion channels they cause an immediate change in the state and behavior of the cell
the interaction of activated g-proteins with enzymes are less rapid and more complex as they lead to the production of additional intracellular signalling molecules
Name the classes of enzymes that are the most frequent targets of G proteins, and list the second messenger molecules they produce
adenylyl cyclase and phospholipase C (PLC)
adenylyl cyclase produces a small molecule called cyclic AMP
PLC produces inositol trisphosphate and diacylglycerol.
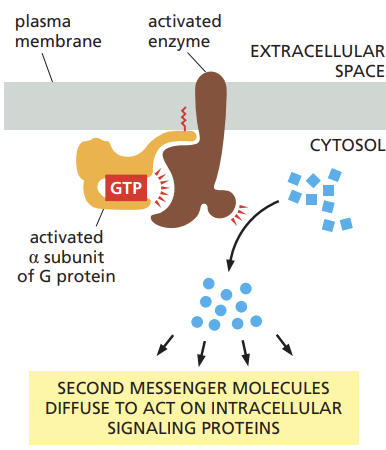
Outline how cyclic AMP is produced in response to G protein activation, and recall how caffeine can potentiate this response.
activated G protein alpha subunit switches on the adenylyl cyclase, causing an increase in the synthesis of cyclic AMP from ATP
to help terminate the signal, a second enzyme called cyclic AMP phosphodiesterase, rapidly converts cyclic AMP to ordinary AMP
caffeine: inhibits phosphodiesterase in the nervous system which blocks cyclic AMP degradation and keeps the concentration of this second messenger high.
Compare a signaling pathway in which cyclic AMP produces a response within seconds to one in which the response takes minutes or hours to develop.
cyclic AMP phosphodiesterase is continuously active inside the cell
eliminates cyclic AMP quickly
so the cytosolic concentration of this second messenger can change rapidly in response to extracellular signals rising or falling
Cyclic AMP is water soluble so it can carry the signal throughout the cell traveling from the site on the membrane where it is synthesized to interact with proteins located in the cytosol, nucleus, or on other organelles
Recall the location and action of the second messenger molecules produced by activated phospholipase C.
phospholipase C (PLC) activated by GCPRs or RTKs
once activated PLC propagates the signal by cleaving a lipid molecule that is a component of the plasma membrane
this cleavage produces two second messenger molecules: inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG)
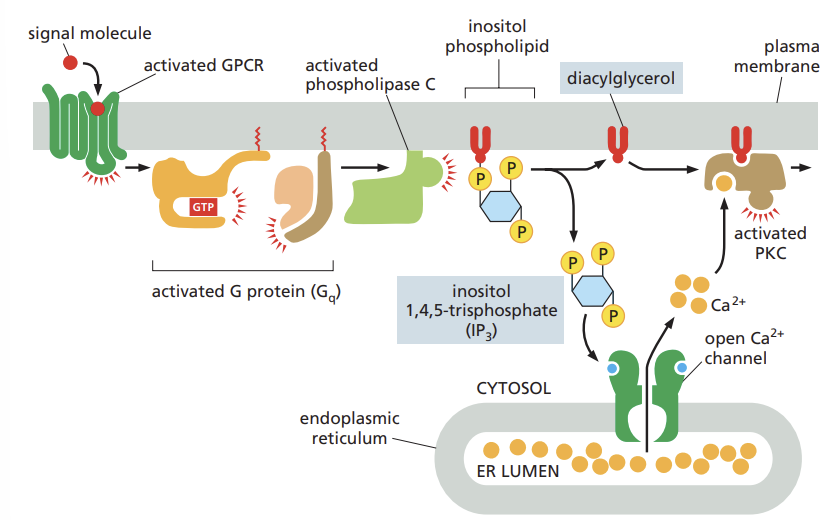
List several biological processes triggered by calcium ions.
when a sperm fertilizes an egg , Ca2+ channels open
a rise in cytosolic Ca 2+ triggers the egg to start development for muscles cells, a signal from nerve triggers a rise in cytosolic Ca 2+ that initiates muscle contraction.
in many secretory cell including nerve cells Ca 2+ triggers secretion
Explain how cells keep the concentration of calcium ions in the cytosol low and how they terminate a calcium ion signal
membrane-embedded Ca pumps actively remove Ca from the cytosol sending it either into the ER or across the plasma membrane and out of the cell
As a result a steep electrochemical gradient of Ca exist across both ER membrane and the plasma membrane.
Review how calcium-responsive proteins such as calmodulin propagate a calcium ion signal.
calmodulin is present in the cytosol
When Ca binds to calmodulin the protein undergoes a conformational change that enables it to interact with a wide range of target proteins in the cell altering their activity
target is the Ca/calmodulin-dependent protein kinases (CaM-kinases)
when these kinases are activated by binding to calmodulin complexes with Ca, they influence other processes in the cell by phosphorylating selected proteins
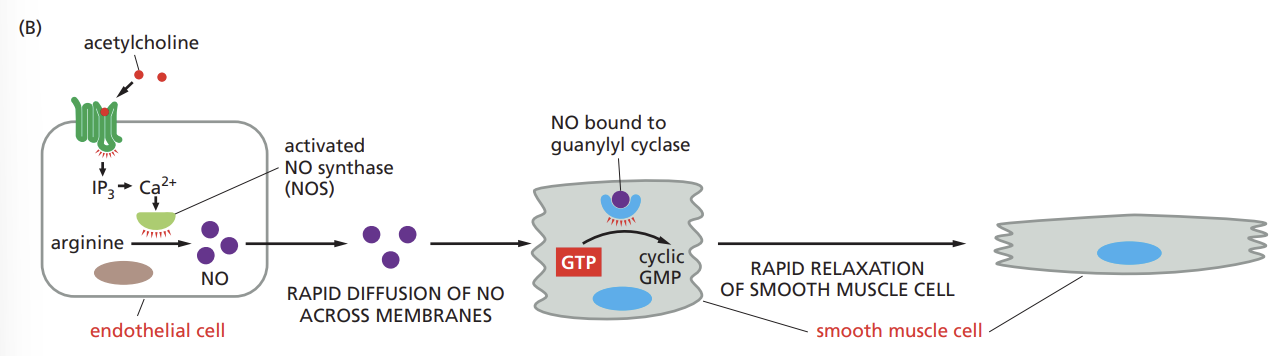
Outline how the gas nitric oxide (NO) can act as a signaling molecule to trigger the relaxation of smooth muscle cells.
nitric oxide diffuses readily from its site of synthesis and slips into neighboring cells,
the distance the gas diffuses is limited by its reaction with oxygen and water in the extracellular environment, which converts NO into nitrates and nitrites within seconds.
Recall why nitric oxide acts as a paracrine signal only on cells near its site of synthesis.
highly reactive gas molecule with a very short half-life, so it quickly breaks down, limiting its ability to reach distant cells
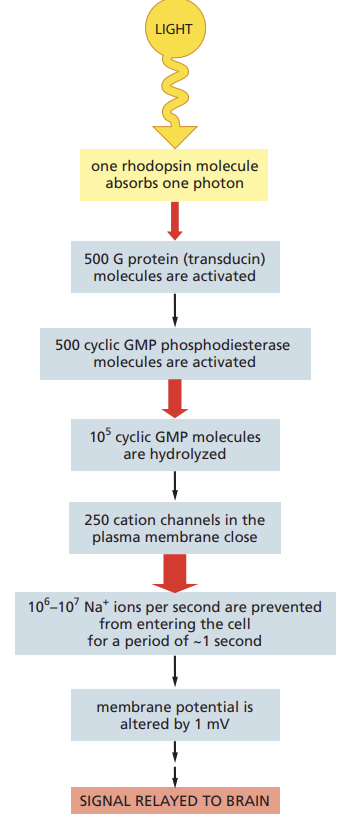
Outline how GPCRs in the photoreceptors of the retina transmit an extremely rapid signal in response to stimulation by light.
But photoreceptors also provide a beautiful illustration of the advantage of intracellular signaling cascades
in particular, such cascades allow spectacular amplification of the incoming signal and allow cells to adapt so as to be able to detect signals of widely varying intensity.
Summarize how adaptation in the intracellular signaling cascade of photoreceptors allows the eye to respond to dim or bright light.
adaptation occurs in intracellular signaling pathways that respond to extracellular signal molecules
allows cells to respond to fluctuations in the concentrations
positive and negative feedback mechanisms allow cells to respond equally well to the signaling equivalents of shouts and whispers.
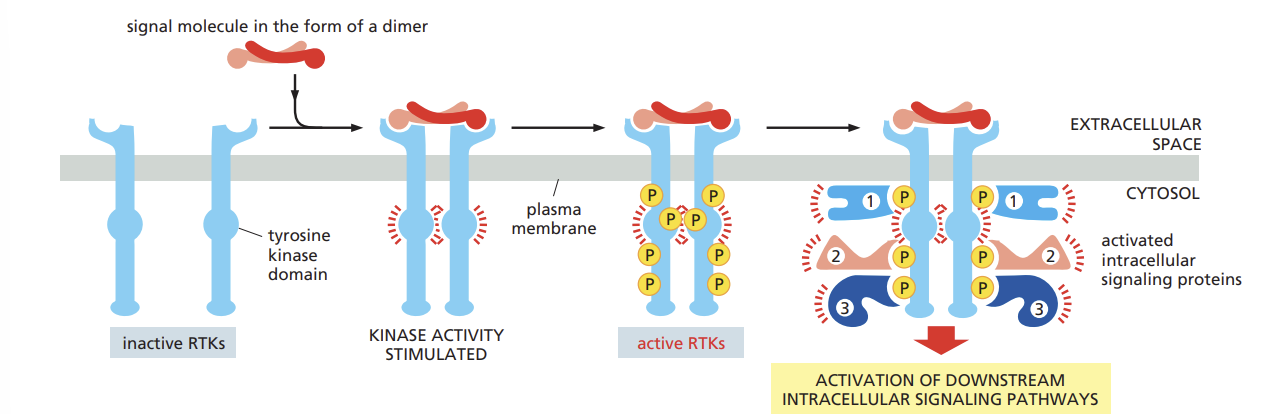
enzyme-coupled receptors
transmembrane proteins that display their ligand-binding domains on the outer surface of the plasma membrane
instead of associating with a G-protein however the cytoplasmic domain of the receptor either acts as an enzyme itself or forms a complex with another protein that acts as an enzyme.
Review how the binding of a signal molecule activates RTKs to trigger the assembly of an intracellular signaling complex
phosphorylation of tyrosines on the receptor tails triggers the assembly of an intracellular signaling complex on the tails
The newly phosphorylated tyrosines serve as binding sites for a variety of signaling proteins that then pass the message on to yet other proteins
the binding of a signaling molecule with an RTK activates tyrosine kinase in the cytoplasmic tail of the receptor
Recall how signals transmitted by RTKs can be terminated.
tyrosine phosphorylation is reversed by tyrosine phosphatases which remove the phosphates that were added to the tyrosine of both the RTKs and other intracellular signaling proteins in response to the extracellular signal
in some cases activated RTKs are inactivated in a more brutal way: they are dragged into the interior of the cell by endocytosis and then destroyed by digestion in the lysosomes.
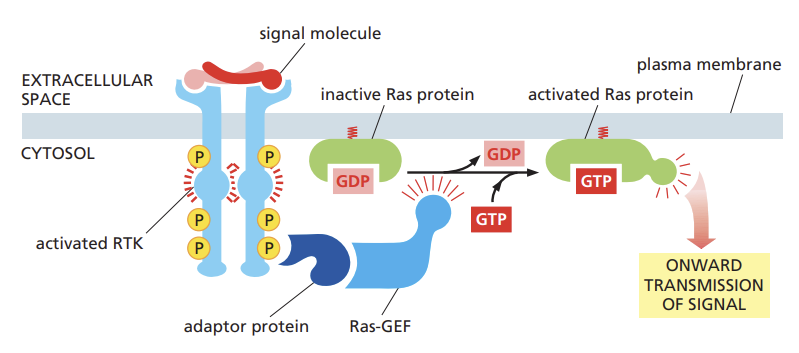
outline how intracellular signaling proteins activate RTKs and travel
A signal molecule activates the RTK, which then phosphorylates itself
Adaptor proteins bind to the phosphorylated RTK and recruit SOS, a Ras-GEF, which activates Ras by exchanging GDP for GTP
Active Ras then transmits the signal downstream, leading to cellular responses.
Outline how RTKs activate the MAP kinase signaling module.
When Ras is activated (by binding GTP), it initiates a phosphorylation cascade
This cascade involves a series of serine/theronine kinases being activated in sequence
Phosphorylation can change the activity, localization, or interactions of proteins, leading to cellular responses like gene expression or changes in cellular behavio
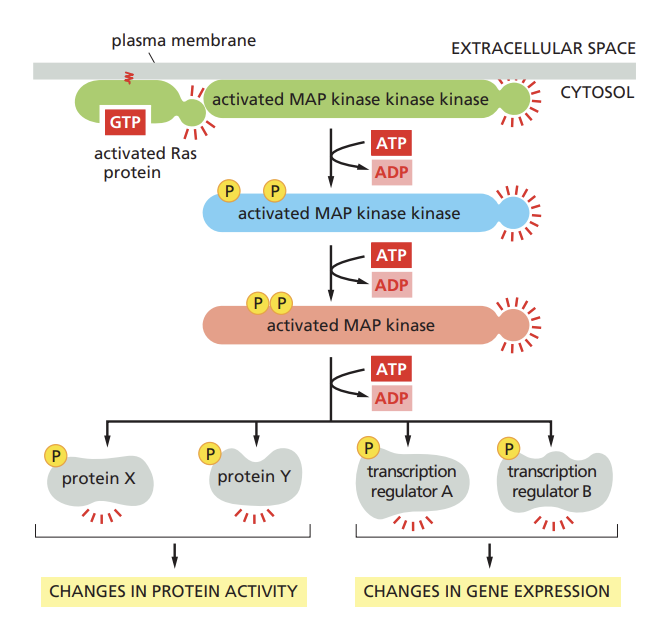
Indicate how Ras can fuel uncontrolled proliferation in cancer.
a mutant form of Ras inactivates the GTPase activity of Ras
so that the protein cannot shut itself off
promotes uncontrolled cell proliferation and the development of cancer
Review how extracellular signals that promote cell growth and survival activate PI-3-kinase signaling pathways.
one important signaling pathway that these RTKs activate to promote cell growth and survival involves the enzymes phosphoinositide 3-kinase(PI 3-Kinase) which phosphorylates inositol phospholipids in the plasma membrane
these phosphorylated lipids serve as docking sites for specific intracellular signaling proteins, which relocate from the cytosol to the plasma membrane, where they can activate one another.
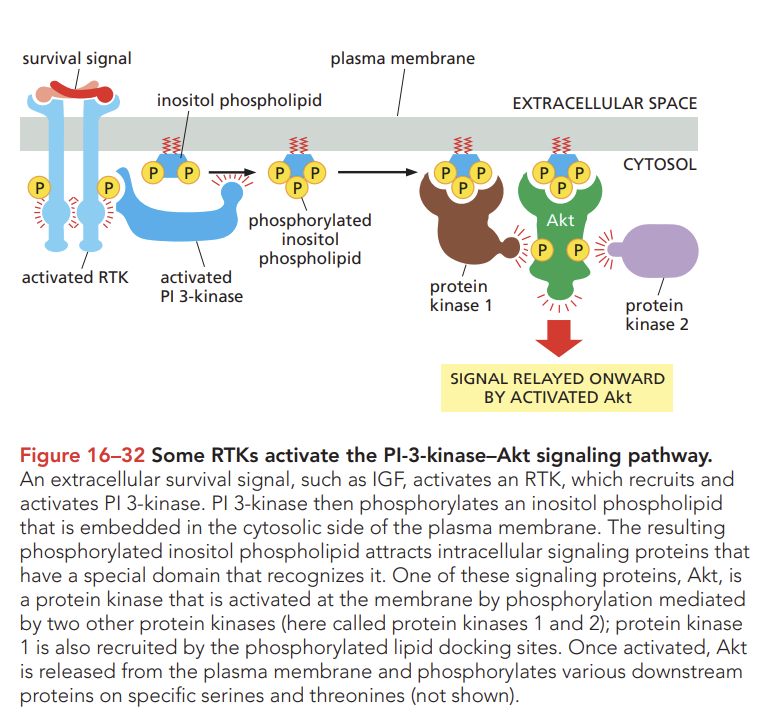
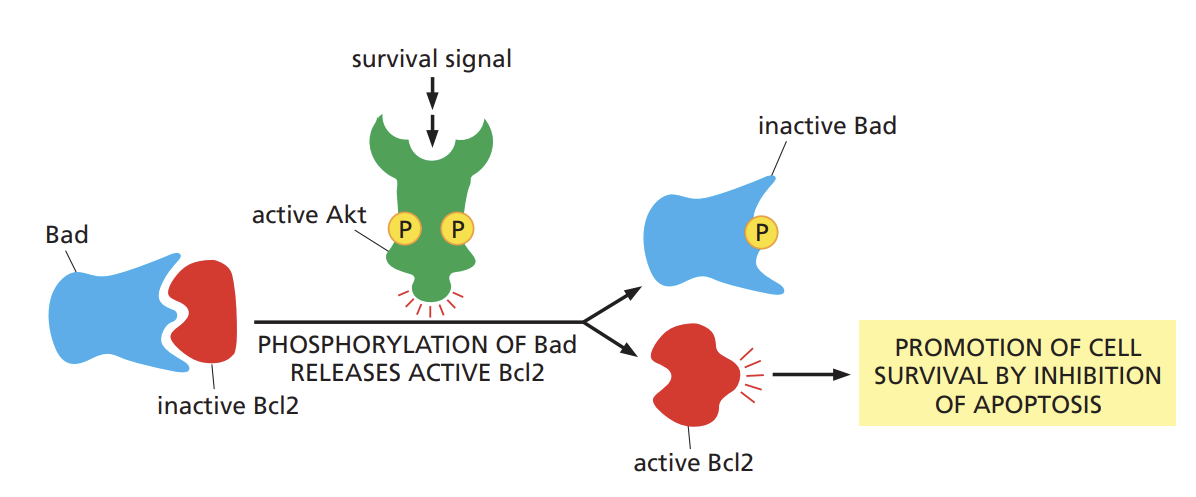
Compare how Akt promotes cell survival via Bad and stimulates cell growth via Tor.
akt (AKA serine/threonine kinase or protien kinase b) promotes cell growth and survival of many cell types often by inactivating the signaling proteins it phosphorylates
Akt phosphorylates and inactivates a cytosolic protein called bad
in its active state, Bad encourages the cell to kill itself by indirectly activating a cell-suicide programed called apoptosis
phosphorylation by Akt thus promotes cell survival by inactivating a protein that otherwise promotes cell death.
Review how different types of receptors can trigger a rise in the cytosolic concentration of calcium ions.
intracellular Ca2+ stores and external Ca2+ entering across the plasma membrane
intracellular Ca2+ release mechanisms involves the phosphoinositide specific phospholipase C (PI-PLC)-derived second messenger IP3, which acts by binding to a specific receptor on the endoplasmic reticulum and releases Ca 2+
Describe a method to identify proteins that interact in response to stimulation by an extracellular signal.
one involves using a protein as "bait" isolating the receptor that binds to insulin, one could attach insulin a chromatography column
cells that respond to the hormone are broken open with detergents that disrupt their membrane, releasing the transmembrane receptor proteins
protein-protein interactions in a signal pathway can also be identified by co-immunoprecipitation.
Outline how a set of mutant RTKs can be used to determine which tyrosines serve as docking sites for the intracellular signaling proteins that propagate the signal.
a series of mutant receptors can be constructed, each missing a different from its cytoplasmic domain
in this way, the specific tyrosine required for binding can be determined
similarly one can determine whether this phosphotyrosine docking site is required for the receptor to transmit a signal to the cell
Review how a technology such as RNA interference or CRISPR can be used to assess the importance of a particular protein in a signaling pathway.
the activity of a specific signaling protein can be inhibited or eliminated
in this case of Ras, one could shut down the expression of the Ras gene in cells by RNA interference or CRISPER
such cells do not proliferate in response to extracellular mitogens, indicating the importance of normal Ras signaling in the proliferative response
Explain how mutant proteins can be used to determine the order in which proteins participate in a signaling pathway.
by examining other mutant animals, many of the genes that encode the protein involved in a signaling pathway can be identified
to determine whether these proteins lie upstream or downstream of Ras one could create cells that express an inactive mutant to form of each protein and then ask whether these mutant cells can be "rescued" by the addition of a continuously active form of Ras.
Outline how steroid hormones trigger the transcription of different sets of target genes.
cortisol, estradiol, and testosterone and the thyroid hormones such as thyroxine are hydrophobic molecules that pass through the plasma membrane of the target cell and bind to receptor proteins located in either the cytosol or the nucleus
regardless of their initial location, these intracellular receptor proteins are referred to as nuclear receptors because when activated by hormone binding they enter the nucleus, where they regulate transcription.
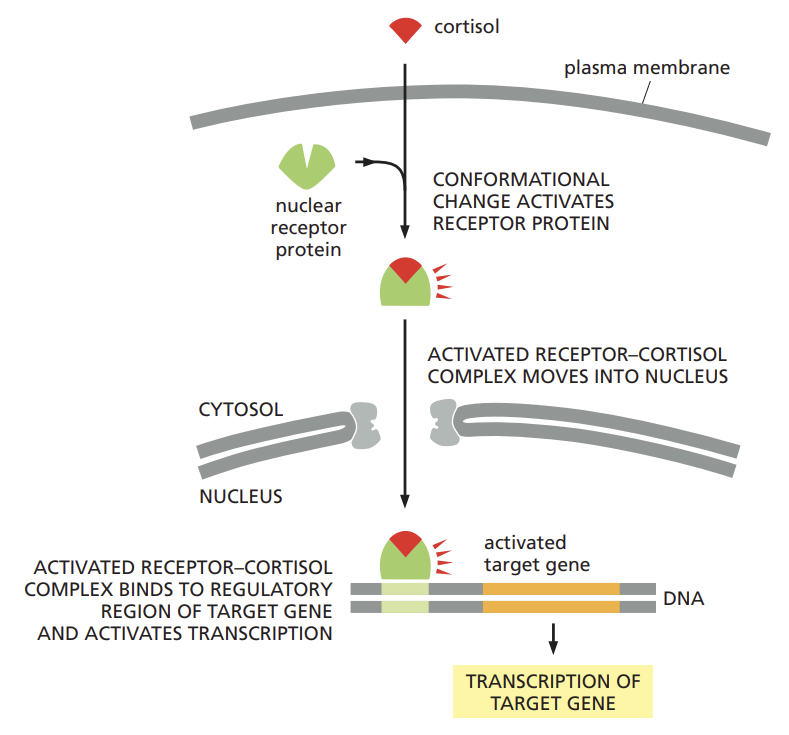
Contrast the cell signaling systems used by plants and animals.
plants have hundreds of genes encoding receptor serine and threonine kinases
but they are structurally different then the receptors found in animals
the plant receptors are thought to play an important part in a large variety of cell signaling processes including those governing plant growth, development and disease resistant
in contrast to animal cells, plant cells seem not to use RTKs, steroid hormones type nuclear receptors or cyclic AMP they seem to use few GPCRs.
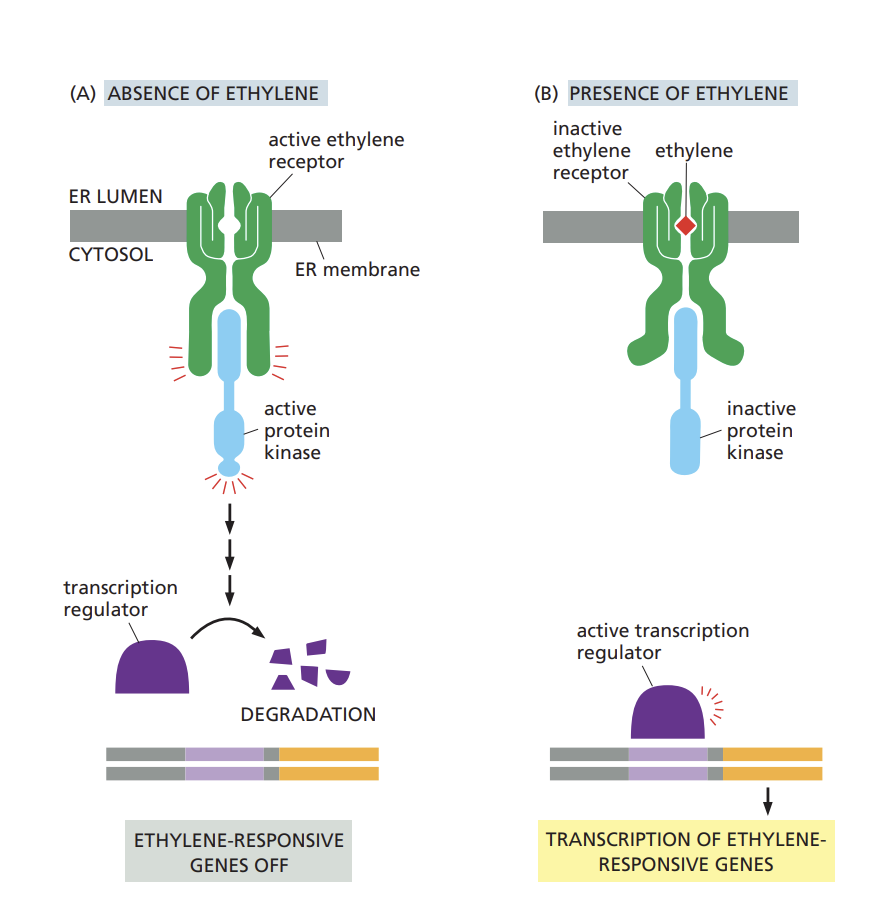
Describe how the ethylene signaling pathway regulates ripening of fruits.
ethylene receptor is located in ER
it is active in the absence of ethylene, and inactive when ethylene is present
presence of ethylene:
ethylene binds to the receptor and causes a conformational change in the receptor, which activates the signaling pathway
the protein kinase is deactivated and the the transcription regulator is activated
absence of ethylene:
the receptor interacts with and activates a protein kinase which inhibits downstream signaling
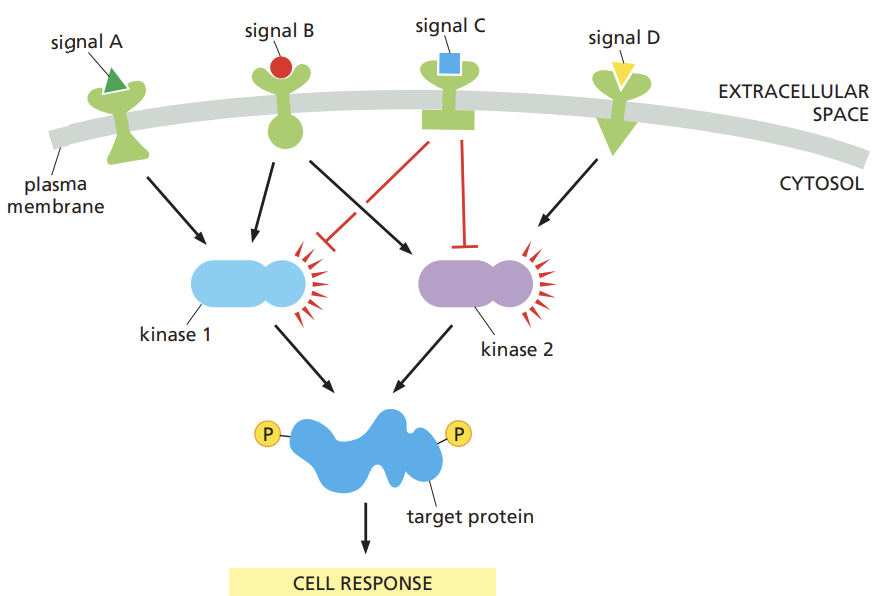
Explain how multiple signaling pathways can integrate information to produce a coordinated cell response.
connection and interactions that occur between different signaling pathways
the central rod domains of different intermediate filaments proteins are all similar in size and amino acid sequence so that when they pack together they always form filaments of similar diameter and internal structure. by contrast, the terminal head and tail domains vary greatly in both size and amino acid sequence from one type of intermediate filament protein to another. these unstructured domains are exposed on the surface of the filaments, where they allow it to interact with specific components in the cytoplasm
1) keratin filaments in epithelial cells
2) vimentin and vimentin related filaments in connective tissue cells, muscle cells, and supporting cells of the nervous system
3) neurofilaments in nerve cells
4) nuclear lamins which strengthen the nuclear envelope
keratin genes mutation can form a rare human disease called epidermolysis bullosa simplex where the skin is highly vulnerable to mechanical injury, and even gentle pressure can rupture its cells, causing the skin to blister.
neurofilaments can form neurodegenerative disease amyotrophic lateral sclerosis. axon degeneration and muscle weakness and muscle weakness seen in patients
colchicine: which binds tightly to free tubulin dimers and prevents their polymerizations into microtubules, the mitotic spindle rapidly disappears the cell stalls in the middle of mitosis unable to partition the chromosomes into two groups. the mitotic spindle is normally maintained by a balanced addition and loss of tubulin subunits; when tubulin addition is blocked by colchicine tubulin loss continues until the spindle disappears
taxol: its binds tightly to microtubules and prevents them from losing subunits. because new subunits can still be added the microtubules can grow but cannot shrink.
-cilia are hairlike structures covered in plasma membrane, that extend from the surface of many kinds of eukaryotic cells. each cilium contains a core stable microtubules arranged in a bundle, that grow from a cytoplasmic basal body which serves as an organization center. motile cilia beat in a whiplike fashion either to move fluid over the surface of a cell or to propel single cells through a fluid.
-flagella that propel sperm and many protozoa are usually much longer than cilia are. they are designed to move the entire cell rather than moving fluid across the cell surface. flagella propagate regular waves along their length, propelling the attached cell along.
each cilium contains a core stable microtubules arranged in a bundle, that grow from a cytoplasmic basal body which serves as an organization center.
the movement of a cilium or flagellum is produced by bending that takes place as its microtubules slide against each other. the microtubules are associated with numerous accessory proteins which project a regular positions along the length of the microtubule bundle.
depending on which of these proteins they associate with actin filaments can form stiff and stable structures such as:
microvilli on the epithelial cells lining the intestine
small contractile bundles that contract and act like tiny muscles in most animal cells. they also can form temporary structures such as dynamic protrusions formed at the leading edge of a crawling cell or the contractile ring that pinches the cytoplasm in two when an animal cell divides
cells contain small proteins such thymosin and profilin that bind to actin monomers in the cytosol, preventing them from adding to the ends of the actin filaments. by keeping actin monomers in reserve until required these proteins play crucial role in regulating actin polymerization. when actin filaments are needed other actin binding proteins such as forminins and actin related proteins to promote actin polymerization.
-Myosins are a large super-family of motor proteins that move along actin filaments, while hydrolyzing ATP to forms of mechanical energy that can be used for a variety of functions such as muscle movement and contraction
myosin-1 molecules which are present in all cell types, have a head domain and a tail. the head domain binds to an actin filament and has the ATP hydrolyzing motor activity that enables it to move along the filament in a repetitive cycle of binding, detachment and rebinding. the tail varies among the different types of myosin-1 and determines what type of cargo the myosin will carry.
myosin 2: structurally and mechanistically more complex, muscle cells use of its specialized form to drive muscle contraction. these are proteins who are dimers with two globular ATPases heads at one end and a single coiled-tail at the other. they bind to each other to form a bipolar myosin filament, which smaller contractile muscle contractions
List tissues in which actin and myosin filaments are organized in contractile bundles
myosin filament: double headed arrow, with two sets of myosin heads pointing outwards
one set binds to actin filaments in one orientation and moves the filaments one way; the other set binds to other actin filaments in the opposite orientation and moves the filaments in the opposite direction
the myosin filaments slides sets of oppositely orientated actin filaments past one another
if actin filament and myosin filaments are organized together in a bundle, the bundle can generate a strong contractile force
clearly seen in muscle contraction, but it also occurs in the much smaller contractile bundles of actin filaments and myosin-2 filaments that assemble transiently in nonmuscle cells, and in the contractile ring that pinches dividing cells into two.
skeletal muscles are huge, multinucleated individual cells formed by the fusion of many smaller cells. the nuclei of the contributing cells are retained in the muscle fiber and lie just beneath the plasma membrane.
-the bulk of the cytoplasm is made up of myofibrils, the contractile elements of the muscle cell. these cylindrical structures are 1-2 um in diameter and maybe as long as a muscle cell itself. each consists of a chain of identical tiny contractile units or sarcomeres.
paracrine signaling
signal diffuses locally through the extracellular fluid, local mediators on to nearby cells
neuronal signaling:
signaling that is delivering messages quickly/ specifically to individual target cells through private lines, extracellular signal is called a neurotransmitter
contact dependent signaling
allows adjacent cells that initially similar to become specialized to form different cell types
receptor protein
performs the primary step in signal transduction
recognizes the extracellular signal generates new intracellular signals in response.
RTKs (receptor tyrosine kinases):
activated in response to extracellular signals
consists of receptors with a cytoplasmic domain that functions as a tyrosine kinase, which phosphorylates tyrosines on specific intracellular signaling proteins