3.1.3.4 - Bonding and physical properties
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
36 Terms
Are ionic compound boiling + melting points high/low?
High
What is the state of ionic compounds at room temp + pressure?
Solid
Do ionic compounds conduct electricity when solid?
No (ions held in place)
Do ionic compounds conduct electricity when liquid?
Yes (ions free to move)
Are ionic compounds soluble in water?
Yes
Are simple covalent compound boiling + melting points high/low?
Low (involves breaking intermolecular forces but not covalent bonds)
What is the state of simple covalent compounds at room temp + pressure?
Usually liquid/gas (may be solid like I₂)
Do simple covalent compounds conduct electricity when solid?
No
Do simple covalent compounds conduct electricity when liquid?
No
Are simple covalent compounds soluble in water?
Depends on how polarised molecule is
Are giant covalent compound boiling + melting points high/low?
High
What is the state of giant covalent compounds at room temp + pressure?
Solid
Do giant covalent compounds conduct electricity when solid?
No (except graphite)
Do giant covalent compounds conduct electricity when liquid?
N/A (sublimes rather than melting)
Are giant covalent compounds soluble in water?
No
Are metallic compound boiling + melting points high/low?
High
What is the state of metallic compounds at room temp + pressure?
Solid
Do metallic compounds conduct electricity when solid?
Yes (delocalised electrons)
Do metallic compounds conduct electricity when liquid?
Yes (delocalised electrons)
Are metallic compounds soluble in water?
No
Diamond structure
GIANT COVALENT (macromolecular)
Each C atom covalently bonded to 4 other C atoms, in tetrahedral shape
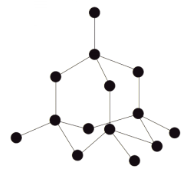
Diamond properties
Because of strong covalent bonds:
Very high melting point
Extremely hard
Good thermal conductor - vibrations travel easily through stiff lattice
Can’t conduct electricity - all outer electrons held in localised bonds
Insoluble in any solvent
Graphite structure
GIANT COVALENT (macromolecular)
C atoms covalently bonded with 3 other C atoms, arranged in sheets of flat hexagons
4th outer electron of each C atom is delocalised
Hexagon sheets bonded together by weak VdW forces
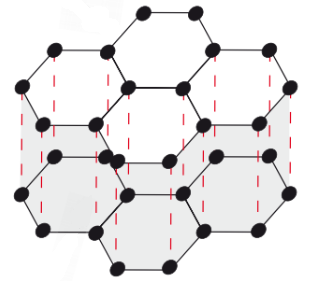
Graphite slipperiness
Slippery (used as dry lubricant; in pencils)
Weak bonds between layers are easily broken, so sheets slide over each other
Graphite electrical conductivity
Good electrical conductor
Delocalised electrons free to move along sheets, carrying charge
Graphite density
Low density (used for strong, lightweight sports equipment)
Layers far apart compared to length of covalent bonds
Graphite melting point
High melting point
Strong covalent bonds
Graphite solubility
Insoluble in any solvent
Covalent bonds too strong to break
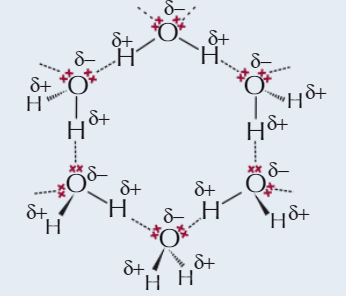
Ice
SIMPLE MOLECULAR
As liquid water cools to form ice, molecules make more H bonds and arrange themselves into regular lattice structure
In regular structure, H₂O molecules are further apart than molecules in liquid water
→ ice less dense than water
Iodine
SIMPLE MOLECULAR
Solid at room temp.
VdW forces between iodine molecules hold them together in lattice
Iodine atoms held together in pairs by strong covalent bonds to form I₂ molecules
Molecules held in molecular lattice by weak VdW attractions
Magnesium structure
METALLIC
Outer shell is delocalised - electrons free to move around metal
→ leaves +ve metal ion, e.g. Mg²⁺+ve metal ions attracted to delocalised sea of electrons, forming lattice
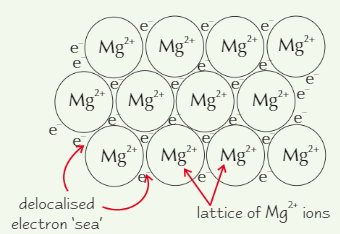
Metal melting points
High melting points
Strong electrostatic attraction between +ve metal ions + delocalised sea of electrons
More delocalised electrons per atom → stronger bonding → higher melting point
Metal thermal conductivity
Good thermal conductors
Delocalised electrons can pass KE to each other
Metal solubility
Insoluble
Metallic bonds are strong
NaCl ionic lattice
Na⁺ + Cl⁻ ions packed together in cube shape
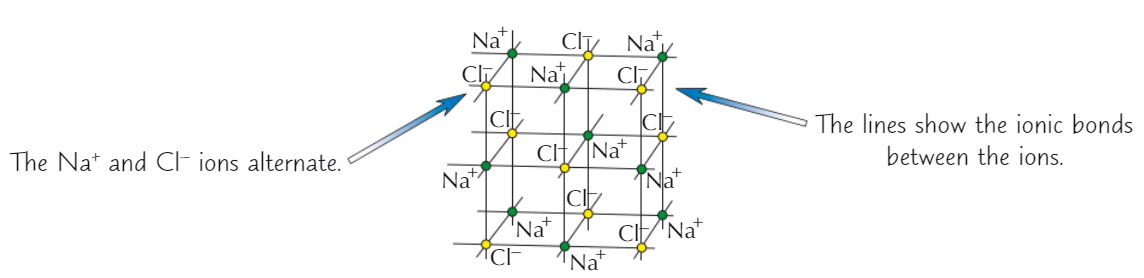
Ionic compound solubility in water
Dissolve in water
Water molecules are polar - one part has slight -ve charge, one part has slight +ve charge
Charged parts pull ions away from lattice, causing it to dissolve