Acids Bases and Salts
0.0(0)
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Last updated 12:30 PM on 1/9/26
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
1
New cards
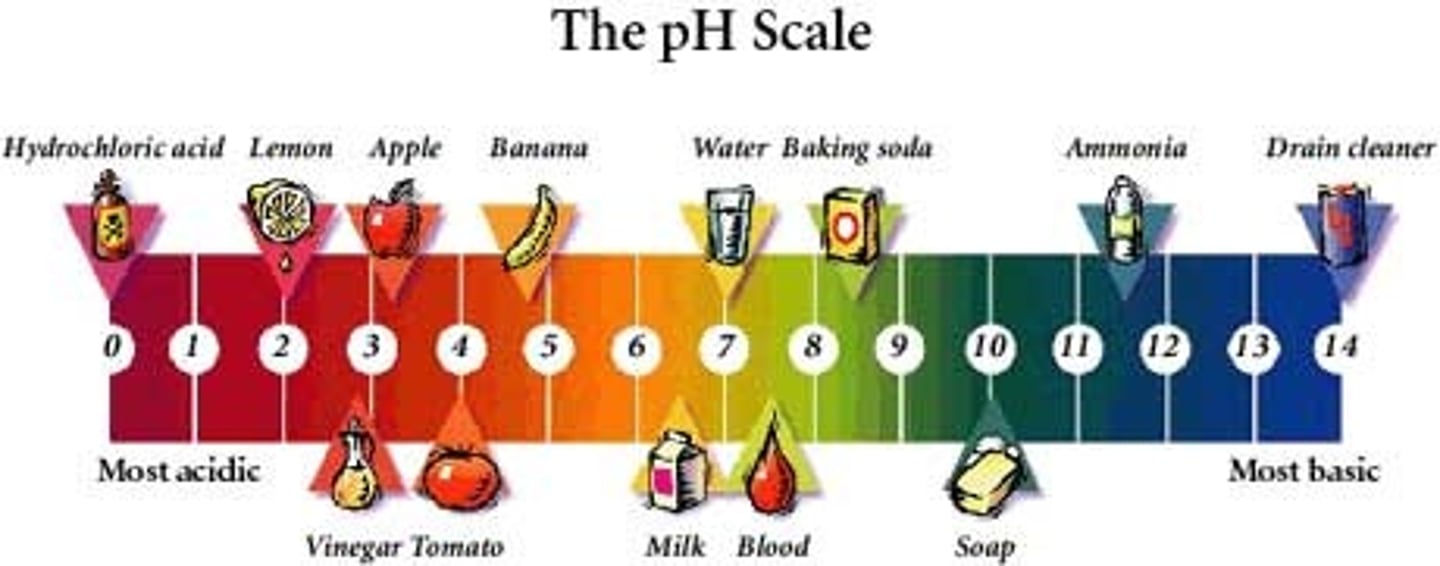
What is the pH of an acid?
1-6
2
New cards
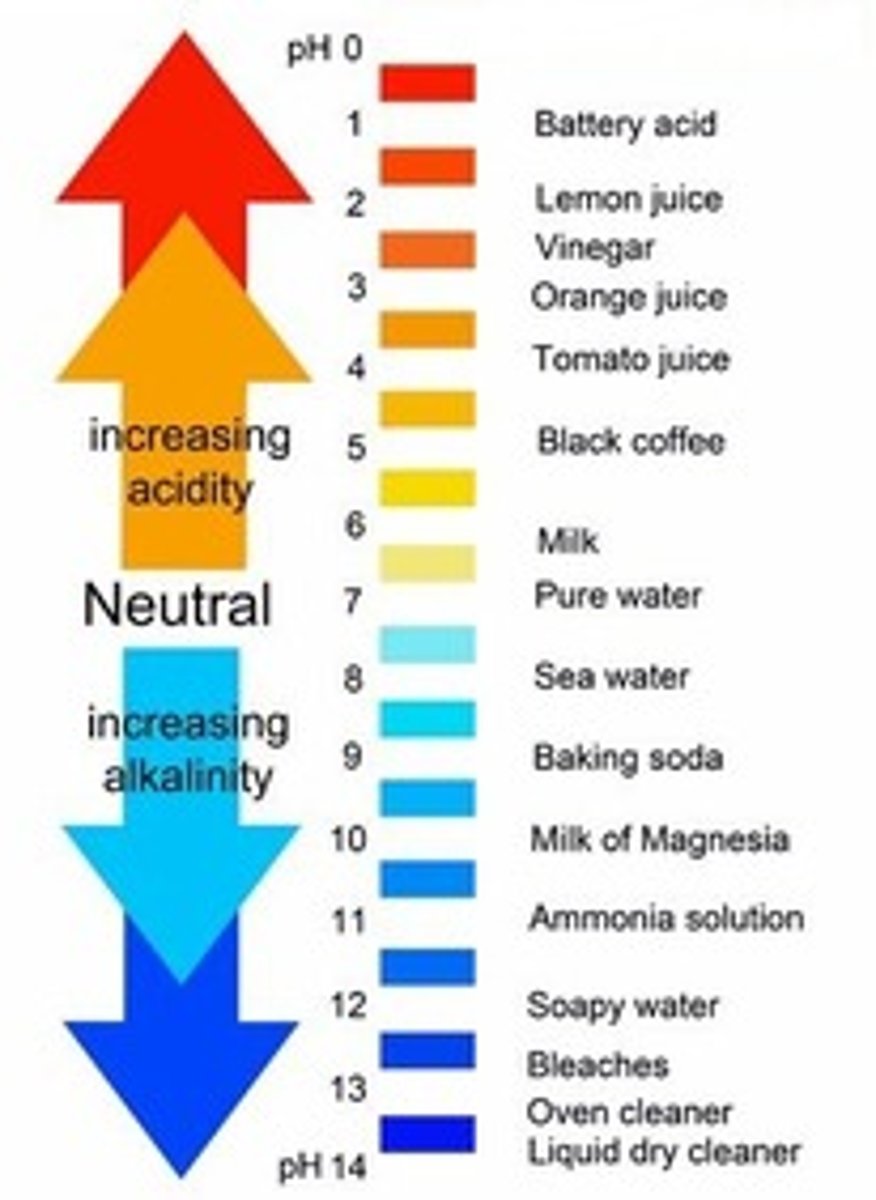
What is the pH of an alkali or base?
8-14
3
New cards
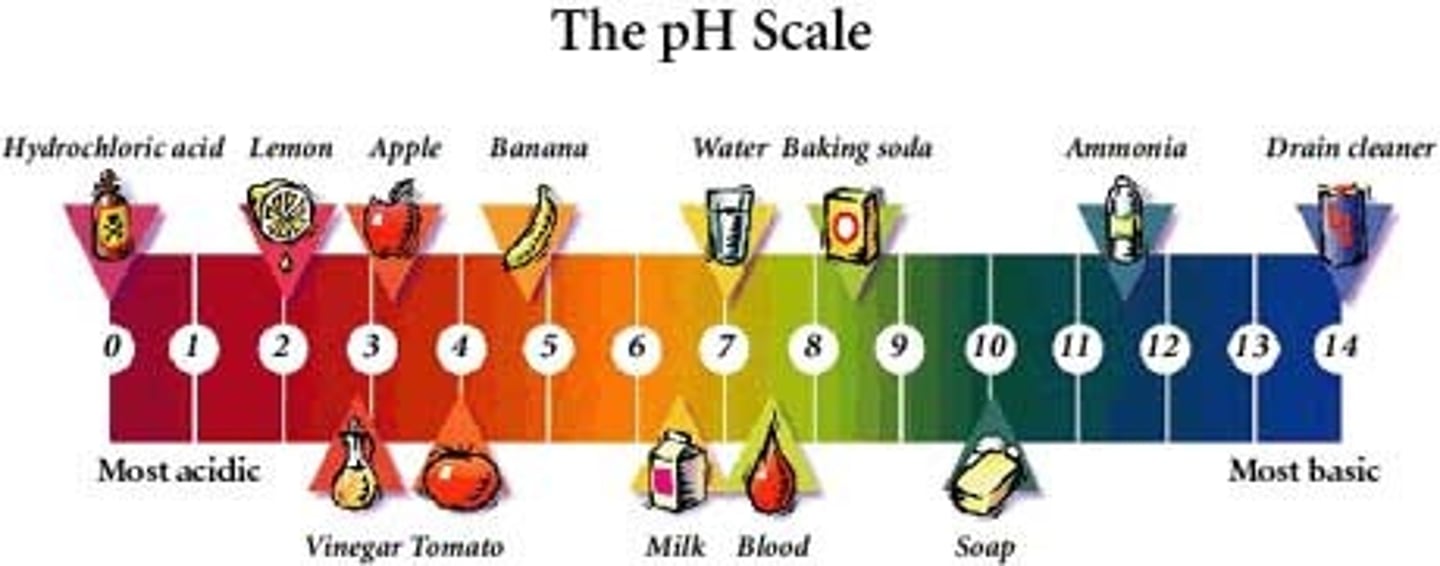
What pH is neutral?
7
4
New cards
What does a pH of 1-3 indicate
a strong acid
5
New cards
What type of chemical can you use to tell if something is acid or alkali?
Indicator.

6
New cards
Acid + alkali (or base) →
salt + water
called a neutralization reaction

7
New cards
Examples of alkali or bases
soluble base
Sodium Hydroxide, Potassium Hydroxide,
Calcium Hydroxide & Ammonium Hydroxide.
8
New cards
Salt formed from Hydrochloric acid and Sodium Hydroxide
HCl + NaOH
Sodium chloride (NaCl)
9
New cards
Acidic ion
H⁺
10
New cards
Alkaline ion
OH⁻