Ethics - Lecture 5 - Medical ethics
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
hippocratic oath
2500 years ago
Hippocratic ethical standards:
Non-maleficence (do no harm)
Duty of confidentiality
positivism
19th century → medicine evolved into science → positivism
a philosophical system recognizing only that which can be scientifically verified or which is capable of logical or mathematical proof, and therefore rejecting metaphysics and theism
An age in which the idea of reflection and interaction was not so valuable, instead authorary empirical observation and mastering nature was considered valuable. In this age there were different medical developments taking place: like vaccination
Neurenberg code
voluntary consent is essential
experiment should yield beneficial results
evidence informed approach / experiment must be based on prior research
avoid unnecessary suffering and injury
no experiment if death or disability is foreseeable
balance of risks for the individual and benefits for society / risk should not exceed the potential benefits
adequate preparations to protect participants
qualified personnel only
participants should be free to withdraw
researcher must be prepared to terminate the experiment
Declaration of Helsinki (1964)
sectoral self-regulation → declaration not binding under international law
Proxy consent: Proxy consent can be given by legal guardians of those unable to consent. E.g. the parent decides what happens to the baby (which treatment) -
The interests of the subject “take precedence over the interests of science and society” (article 5)
Research protocols for human experimentation must be evaluated by a research ethics review committee → institutionalization of ethics review
Vulnerable populations require special protections
Subjects should not be withheld standard of care
Tusekegee studies
600 black men were told that they were treated against bad blood (fatigue/anemia) but were actually researching what happens when you do not treat syphilis
not one of them received treatment of syphilis (penicillin) even though they thought they would get it → oncourse of the disease, some of them died
there was no consent
there was deception on the part of the US government
went on for 40 years → distrust of medical care
HIV / AIDS epidemic
men were dying of pneumonia in big numbers
lead to a lot of controversies
the HIV/AIDS activists were primarily gay
civil rights movement for equal rights → experience they made in this movement they used them again when HIV epidemic started
gays had huge impact
ethics in medical research
in order to be successful the [RCT] study required that a sufficient number of patients die: only by pointing to deaths in the placebo group could researchers establish that those receiving the active treatment did comparatively better’ (Epstein 1996)
but gays participated in the study even thought the drug was not approved (compassionate use)
the use of an unapproved drug or medical device by people with serious conditions who do not meet enrolment criteria for clinical trials.
AIDS has forced us to recognise that respecting individual rights is a critical safeguard for the health of the community, as well as for the person.
when is placebo acceptable
when there is no proven effective treatment
Withholding treatment poses negligible risks
Compelling methodolocigal reasons and no risk of serious harm
compelling methodological reasons and no interference with other interventions
compelling methodological resend and interventions intended for the participants population and no forgoing of treatment participants would otherwise receive
principles of biomedical ethics
Bauchamp and Childress (1985)
respect for autonomy → providing information (enables the patients as autonomous beings), consent, always have the right to back out, privacy, confidentiality
non maleficence (do not harm) → stop the study if harmful - harm bigger than the benefits - psychological and physiological integrity - triggering trauma / offenses
beneficence (do good) → curing people should be the goal - you have to contribute to well being - risk benefit ratio - Who is benefitting? is it the patient or the pharmaceutical company
justice → fair selection participants, limited placebo use, equal distribution of resources, equity
four models of the physician patient relationship
paternalistic → (physicians asks the patient what they want) the physician-patient interaction ensures that patients receive the interventions that best promote their health and well-being
informative → the objective of the physician-patient interaction is for the physician to provide the patient with all relevant information, for the patient to select the medical interventions he or she wants, and for the physician to execute the selected interventions
interpretive → The aim of the physician-patient interaction is to elucidate the patient’s values and what he or she actually wants, and to help the patient select the available medical interventions that realize these values
deliberative → The aim of the physician-patient interaction is to help the patient determine and choose the best health-related values that can be realized in the clinical situation
principlism
Principlism is a framework in biomedical ethics that outlines four mid level principles to guide ethical decision-making. These principles provide a structured approach to make decisions in moral dilemmas, especially in healthcare settings. Each principle is considered prima facie binding and none is to be considered primary.
autonomy
beneficence
non-maleficence
justice
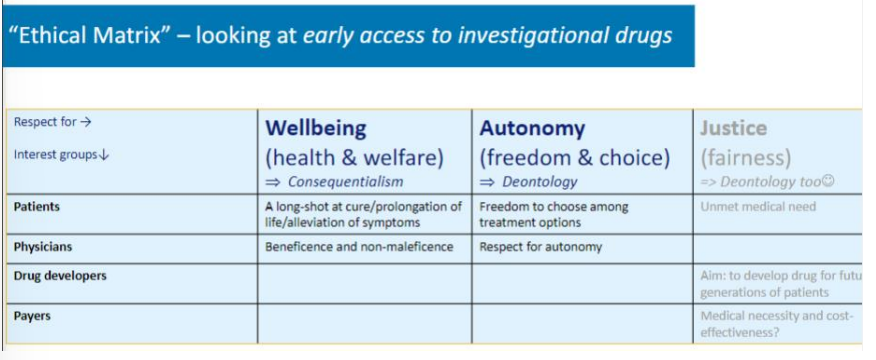
ethical matrix
Developed by Ben Mepham
Used for systemizing ethical reflection so everyone can engage in ethical reflection.
Table: interest groups vs. respect for (values)
→ who are all the groups of actors that are affected by the consequences of doing one or another things
→ whether it is distributed fairly through society
you need the justification / explanations for why and what you have filled into the table
dogmatism
there is only one truth, you do not agree with any other arguments; open ethical reflection is a waste of time; (you think you are objective)
immediate self justification
no self reflection, just follow your first impression; everything that counters your argument is definitely not right. Results in a heated debate with people who do not listen to eachother.
relativism
each value is good as another one, you are so open for everything that all is good, absence of universal moral values; everyone is equally right or wrong. Why bother thinking about values if they all weigh the same?