Chemistry Unit 3 (1/2)
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
Physical Processes
atomic-scale structure of the substance remains before and after
Chemical processes
processes that change one (or more) substance into one (or more) new substance; atoms are neither created nor destroyed
Oxidation-reduction reactions
involve the movement of electrons
Acid-base reaction
involve the redistribution of lone pairs of elections and breaking/forming covalent bonds
Dynamic chemical equilibrium
Where the rate of the forward reaction and reverse reaction are equal; results in the concentrations of the reactants and products remaining constant even though the reactions continue to occur
Chemical equilibrium characteristics
Forward and reverse reactions occur at the same rate, concentrations remain constant; The same eq. constant can be achieved by starting with only reactants, only products, or a mixture of both
Equilibrium constant (K)
A ratio of equilibrium partial pressures or concentrations of products and reactants that has a specific value for a given reaction at a given temperature
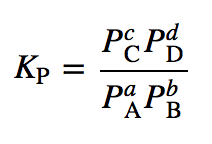
This represents:
Equilibrium equation for a gas-phase reaction
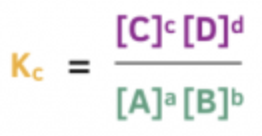
This represents:
Equilibrium equation for a solution-phase reaction
T/F: K expressions contain terms for pure solids or pure liquids
False
T/F: For dilute solutions, the solvent is not included in the K expression
True
Homogenous equilibrium
all of the reactants and products are present in a single phase
T/F: In aqueous equilibrium systems, the solvent (H2O) is included in the equilibrium expression
False
Heterogeneous equilibrium
Reactants and products are found in two or more phases
Product-favored reaction
A reaction that proceeds spontaneously in the forward direction when all concentrations (or partial pressures) have the value 1M (or 1 bar)
Reactant-favored reaction
A reaction that proceeds spontaneously in the reverse direction when all concentrations (or partial pressures) have the value 1M (or 1 bar)
If K > 1, the reaction is ________ favored at equilibrium
Product
If K < 1, the reaction is ________ favored at equilibrium
Reactant
If K = 1
Neither the products nor reactants are favored at equilibrium
Does the ratio of products to reactants depend on initial concentrations?
No
Spontaneous (in the forward direction)
When reactants change to products
Not spontaneous (in the reverse direction)
When products change to reactants
Standard state
A commonly accepted set of conditions used as a reference point; For chemists: Pressure = 1 bar (0.987 atm); Concentration = 1M; Pure solids; Pure liquids
Product favored
When all substances are at the standard-state conditions, reactants change to products
Reactant Favored
If products change to reactants under standard-state conditions
ICE Table
Describes initial concentration/partial p., change in concentration/partial p., and equilibrium concentration/partial p.O
If a reaction starts with only reactants present…
Reaction must initially be spontaneous in the forward direction
If a reaction starts with only products present…
Reaction must proceed in the reverse direction
Reaction Quotient (Q)
Used when determining which direction a reaction proceeds when both reactants and products are present
Q = 0 when…
Only reactants are present
When Q = K
Equilibrium has been reached because Q no longer changes over time
When Q < K
the reaction proceeds spontaneously in the forward direction (from left to right; to the product side)
When Q > K
the reaction proceeds spontaneously in the reverse direction (from right to left; to the reactant side)
To calculate equilibrium concentrations…
Use an equilibrium constant and ICE tables
Le Chatelier’s Principle helps…
Enable prediction of which direction an equilibrium will shift when conditions change; Used when we don’t know exact concentration/partial pressure of reactants and products
Le Chatelier’s Principle states:
When a chemical system is at equilibrium and conditions are changed so that the reaction is no longer at equilibrium, the chemical system reacts to achieve new equilibrium concentrations or partial pressures in a way that partially counteracts the change in conditions
If a product concentration is increased and all other concentrations remain the same, the reaction equilibrium shifts _____ (toward/away from) the reactant side
toward
Two general rules for predicting whether a chemical reaction releases energy:
If there are more bonds in the product molecules than in the reactant molecules and the bonds has about the same strength, the reaction is likely exothermic; If there are stronger bonds in the product molecules than in the reactant molecules and the number of bonds is the same in reactants and molecules, the reaction is likely exothermic
Thermochemical expression
A balanced chemical equation together with the value of ΔrH°, the standard-state reaction enthalpy change, and a temperature
Standard-state reaction enthalpy change, ΔrH°
The standard enthalpy of pure, unmixed products minus the standard state enthalpy of pure, unmixed reactants; that is, the enthalpy change for a reaction under standard-state conditions; doesn’t specify temperature; units (kJ/mol and J/mol)
A negative ΔrH° indicates
an exothermic reaction
A positive ΔrH° indicates
an endothermic reaction
Hess’s law
If a process can be written as the sum of several stepwise processes, the enthalpy change of the total process equals the sum of the enthalpy changes of the various steps; reaction enthalpy change is the same whether the reaction occurs in one or two steps
Standard formation of enthalpy, ΔfH°
The enthalpy change for a reaction in which exactly one mole of a pure substance in specified state is formed from free elements in their most stable states under standard conditions; Equals zero for an element in its most stable form under standard conditions (kJ/mol)
Entropy
How energy is distributed; Better understood as a measure of energy dispersal (how energy is spread across the various motions and configurations available to particles in a system)
Systems with higher energy are…
more probable, making them more stable
List the three fundamental ways a molecule can move
Translational, rotational, vibrational motion
Translational motion
involves whole molecules moving through space(dominant form of motion in gases)
Rotational motion
involves molecules spinning around their axes
Vibrational motion
more localized: atoms within a molecule stretch and bend the bonds the connect them. Larger, more complex molecules have more _______ nodes than smaller or more rigid ones
Solids mostly allow what type of motion?
Vibrational
Liquids mostly allow what type of motion?
Vibrational, rotational and limited transitional
Gases mostly allow what type of motion?
Vibrational, rotational, and translational
Microstates
Unique combination of the three motions; as substances move from solid to liquid to gas, the number of accessible _________ increases dramatically (why entropy increases across phase changes)
Entropy change during dissolution depends on…
the dispersal of the solute and the restructuring of the solvent
Third Law of Thermodynamics
Defines the entropy of a perfect crystal at absolute zero (0K) as exactly zero; ΔrS° = ∑S°(products) − ∑S°(reactants)
Spontaneous Process
One that, once started, can occur as written without continuous inputs of energy from the surroundings
Second Law of Thermodynamics
In any spontaneous process, the total entropy of the universe increases; ΔSuniverse = ΔSsystem + ΔSsurroundings > 0; ΔSsurroundings = Quniverse/T (the heat lost by the system becomes the entropy gaines by the environment)