5. Quantum Numbers
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
what do quantum numbers descirb
the e-s in an atom
what are the 2 things the pricniple quantum number deescribes
describes shells or energy levels or orbitals
1. size of a shell
2. E of a shell
ex: for Li, for 2nd shell > it is bigger than n=1 and has more E than n=1
what letter reperesnts the secondary quantum number + 2 other names for it
letter 'l'
angular momentum quantum #
the azimuthal quantum #
what was later discovered abt the emission spectrum and why was this initally missed
discoevered that EACH bright line was actually a SERIES of lines (w slightly diff frequencies therefore slightly diff Energies)
missed at first bcs the lines were so close tgt and couldnt be distinguished when tech was not so advanced
what did the presence fo multiple lines in emission spectrum mean + who theorized
1915, arnold sommerfeld theorized that each main Energy level/shell (n value) consisted of subshells
what does the secondary quantum # (l) describe + what range does its values have
l describes the SHAPE of each SUBSHELL
- the # of subshells in a shell range from 0 to n-1
ie) if n=3, number of subshells: 0,1,2 so 3 subshells
how are the subshells (l) numbered + lettered and each general shape
l = 0 = s (sharp)
l = 1 = p (principle)
l = 2 = d (diffuse)
l = 3 = f (fundamental)
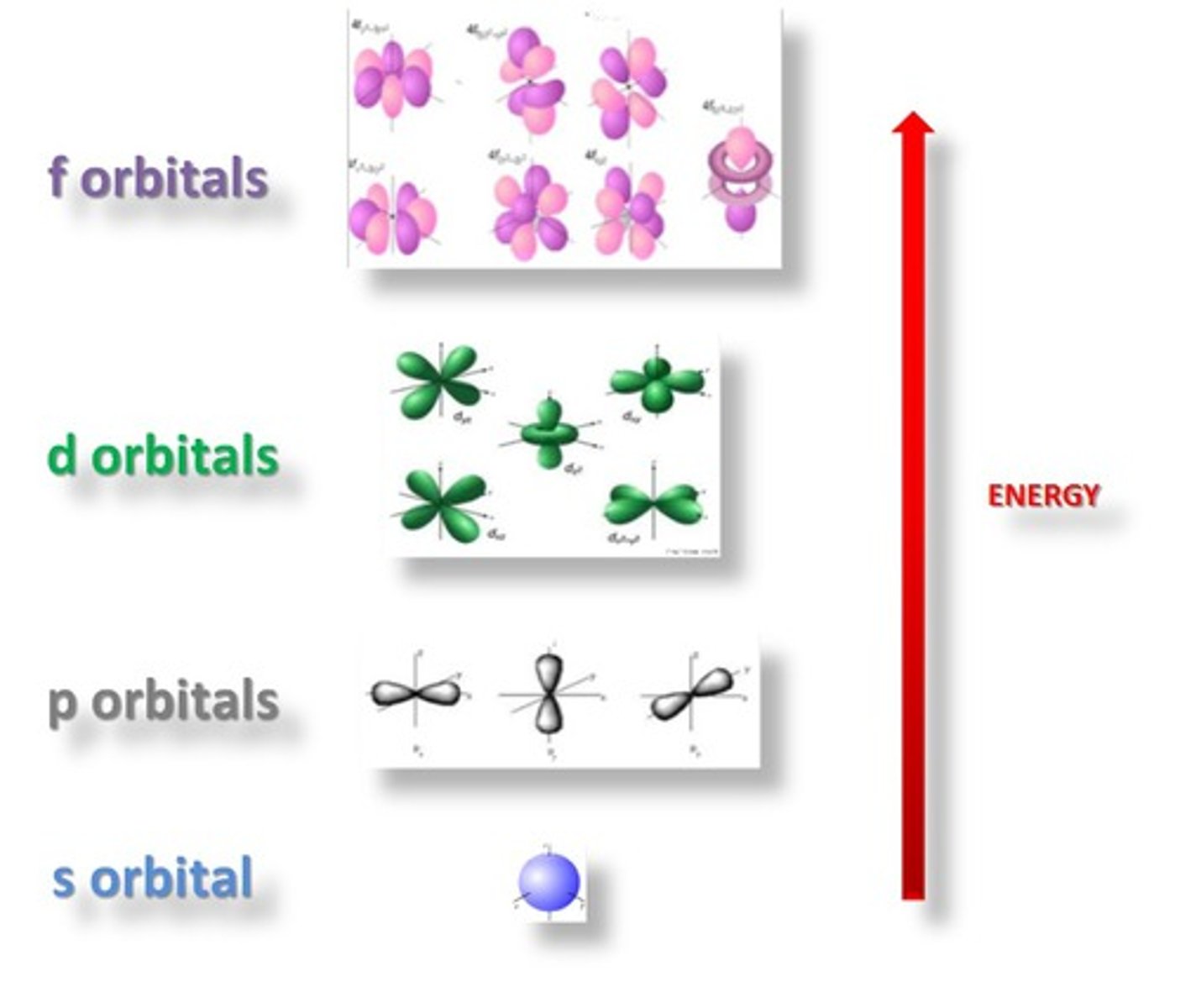
s > sphere
p > dumbbell
d > 4 clover leaf
f > bunch of eggs *dont need to know
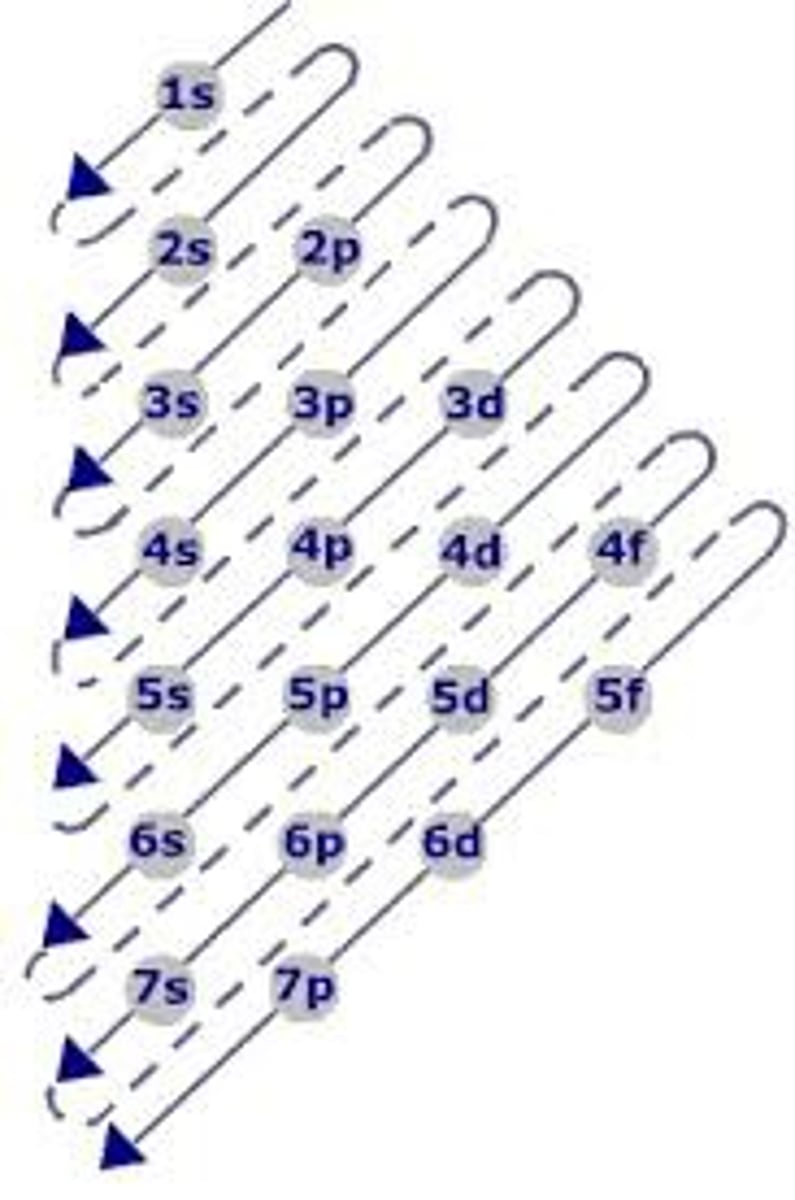
how to know how mnahy subshells each shell/orbital will have
for each shell, there are 'n' kinds of subshells
so if n=3, 3 diff types of subshells (s, p, d corresponfing to 0, 1, 2)
what is m(subscript l)? and what it describes
mₗ = magnetic quantum number
sommerfeld explained that the diff shaped subshells could exist in diff positions of orientations > known as suborbitals
> magnetic quantum # describes the orientation of one suborbital, relative to another
what is the range for the values of mₗ aand how does this correspond to the diff orientations for s,p, d, f
range for mₗ = -l to +l where the # of values possible in total correspods to the # of orientations for that subshell)
~ for s: l = 0 so mₗ can only be 0 > only 1 value means only 1 orientation (only 1 way to draw a sphere)
~ for p: l = 1 so mₗ for 1 can be -1,0,1 so 3 values = 3 orientations
~ for d: l= 2 so mₗ for 2 can be -2,-1,0,1,2 so 5 values = 5 orientations
~ for f: l =3 so mₗ for 3 casn be -3, -2, -1, 0, 1, 2, 3 so 7 values = 7 orientations
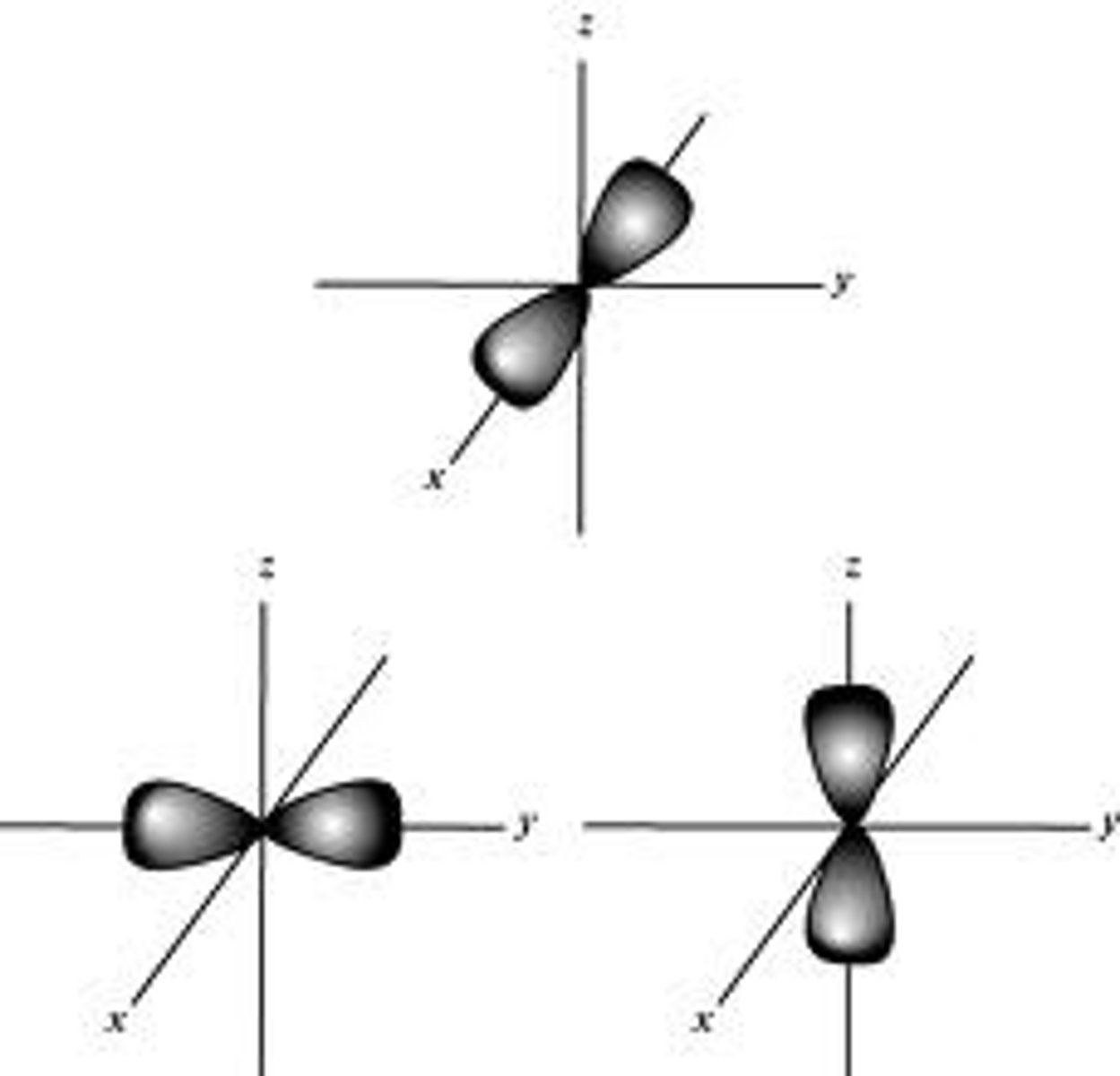
name and draw the 3 orientations for p subshell
(pₓ, pᵧ, pz)
each subscript shows which axes the balloons are aligned on top of
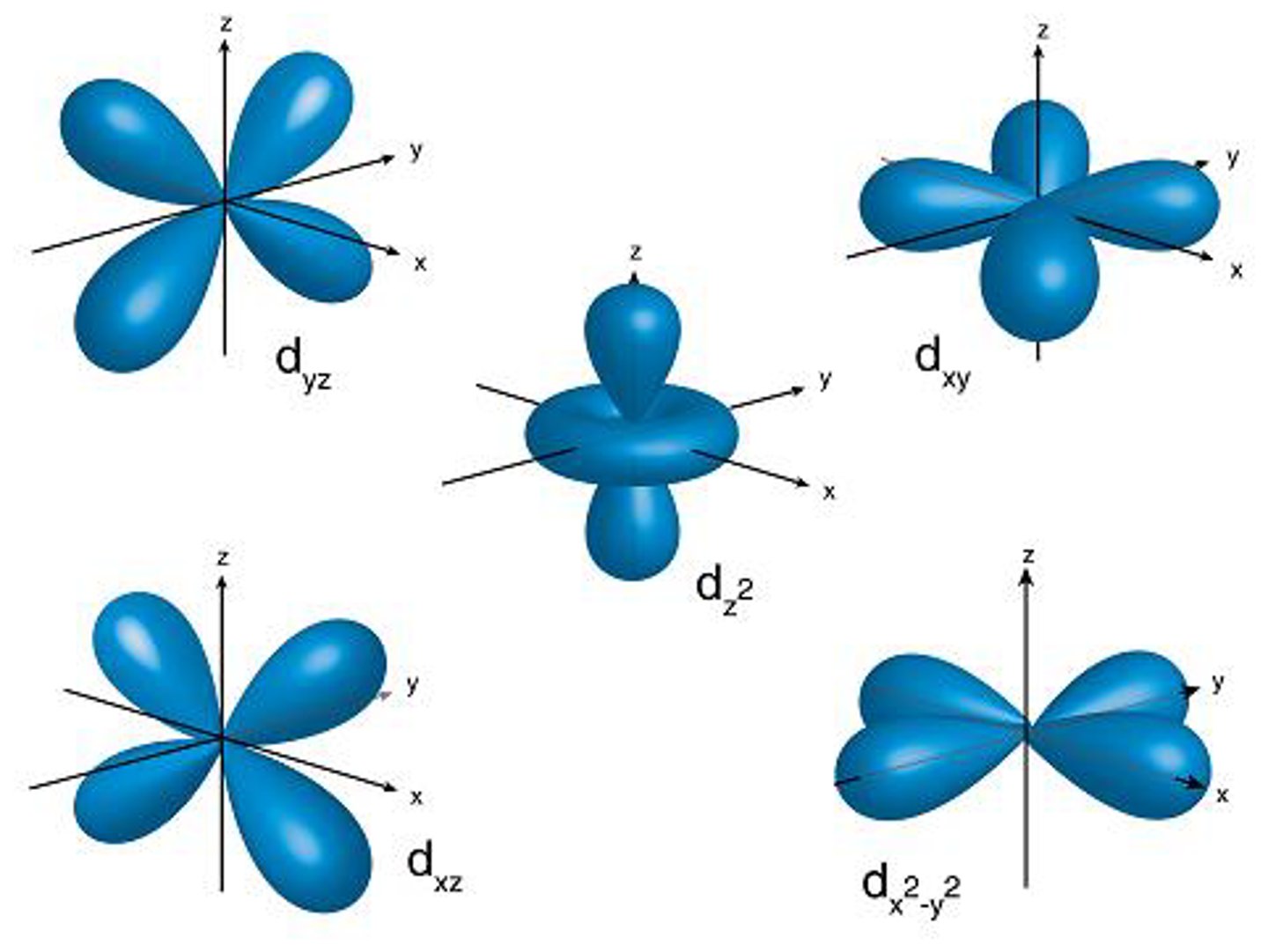
name the 5 orienattaions for d subshell + what each looks like
dₓᵧ
dᵧz
dₓz
dₓ² - ᵧ²
dz² (ring around the middle)
subscripts correspond to the planes where the balloons are in
unless name is squared, the balloons are in the space BETWEEN the 2 axes
if squared, the balloons are ON the axes
formula for the total number of suborbitals in a shell and how many e-s in eah suborbital
n^2 = the total # of suborbitals in a shell (number of values possible for mₗ)
2e-s in each suborbital
what is mₛ
magnetic spin quantum number
if e-s are negative and like charges repel, why do they pair up & who theorized the reasoning
1925, wolfgang pauli theorized that e- are constantly spinning
when they pair up, they rotate with equal magnitude but opposite direction
these opposite spins create oppste electrical force
therefore the attraction of the opp electrical forces is GREATER than the repulsion of the identical charges

Hund's Rule
Hund's rule states that:
Every level in a subshell is singly occupied before any level is doubly occupied.
All of the electrons in singly occupied sublevels have the same spin (to maximize total spin).
aka we fill up sublevels with 1 electron first before doubling them up bcs as the negatively charged electrons fill sublevels, they first try to get as far as possible from each other before having to pair up.
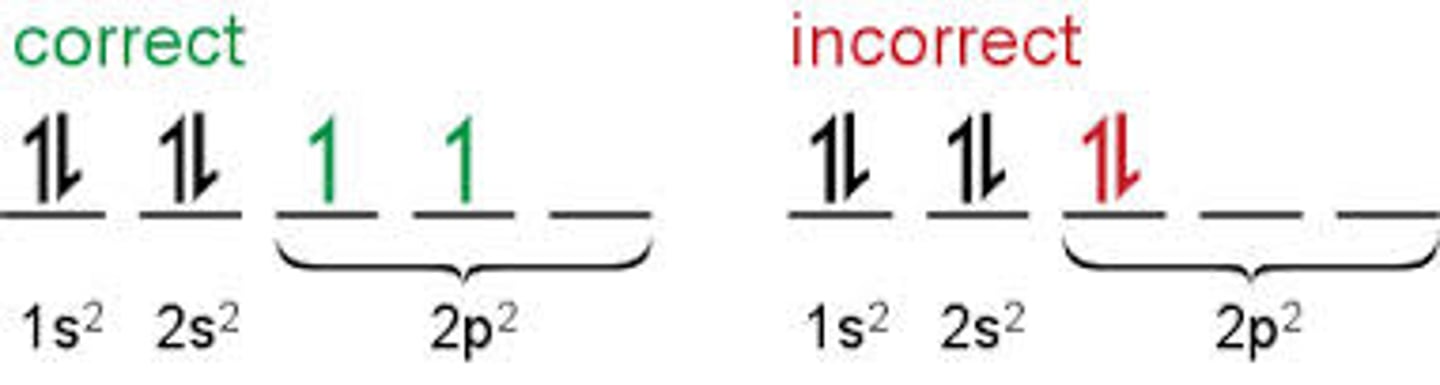
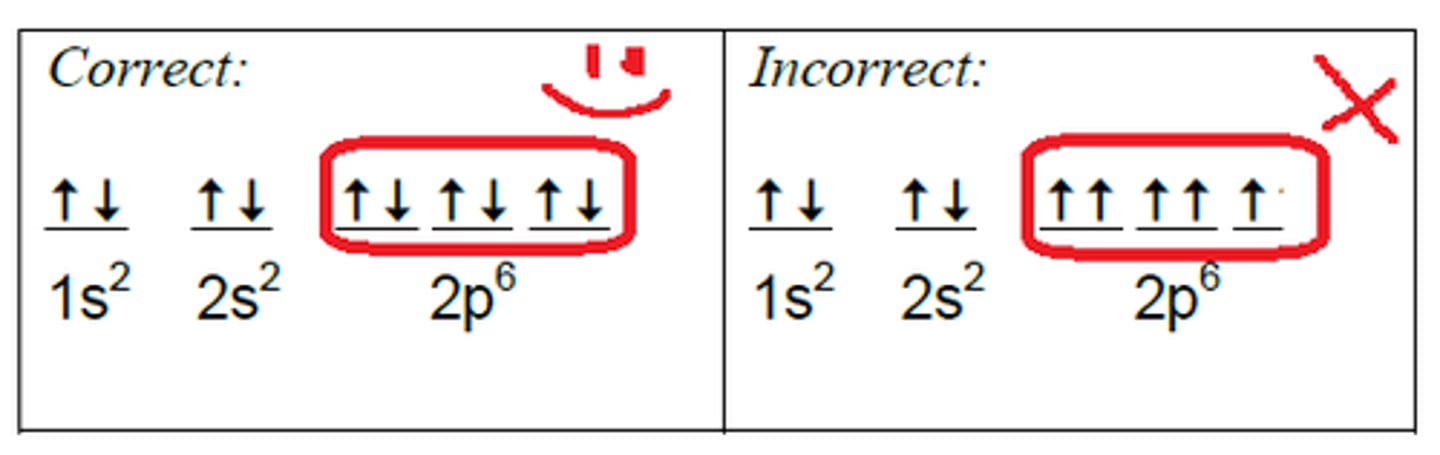
Pauli Exclusion Principle
Pauli Exclusion Principle states that, in an atom or molecule, no two electrons can have the same four electronic quantum numbers.
As an orbital can contain a maximum of only two electrons, the two electrons must have opposing spins. This means if one electron is assigned as a spin up (+1/2) electron, the other electron must be spin-down (-1/2) electron.
understand why pauli exclusion principle must alwasy be true
bcs if 2 e-s had all the same quantum numbers then they would have the same directional electric feild (no opp spin if both are +1/2 etc) so they will both repel eo completely as there is no electric fweild to overpower the repuslion of their negative charges
The Aufbau Principle + what visual tool helps follow the principle
The Aufbau principle states that an electron occupies orbitals in order from lowest energy to highest.
- we must fill up e-s in the lower orbitals (n=2) before starting higher orbitals (n=3)
can also use the diagonal rule of electron filling order (in diagram)