8.1 group 2 and 8.2 group 7
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
what can group 2 metals sometimes be named?
the alkaline earth metals, coming from the alkaline properties of metal hydroxides
do the metals occur in their elemental form naturally?
no
how are they found?
in stable compounds, eg calcium carbonate
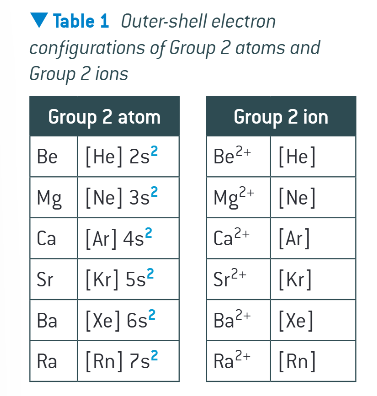
how many outer electrons are in group 2 metals?
2, in out s subshell
what are the most common type of reaction of group 2 elements?
redox reactions
what happens in these redox reactions?
each metal atom is oxidised, losing 2 electrons to form a 2+ ion
another species will gain these 2 electrons and be reduced
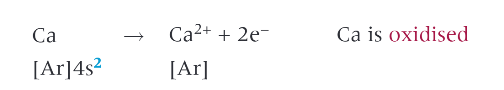
why are group 2 elements called reducing agents?
it reduces other species
electron configurations of group 2 atoms vs ions
group 2 metals react with oxygen to form what ?
metal oxide, general formula MO
examples: magnesium with oxygen:
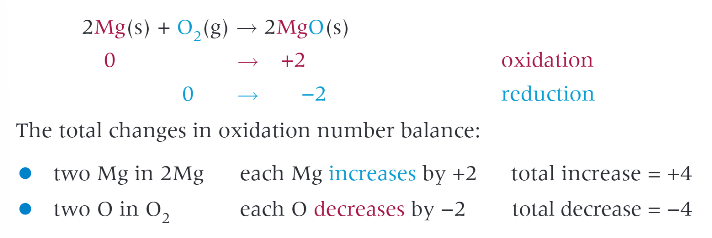
group 2 metals react with water to form what?
an alkaline hydroxide, general formula M(OH)2 and H2
example:
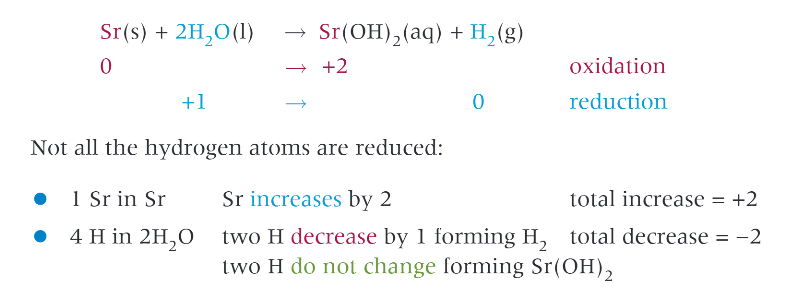
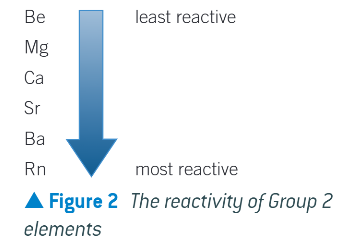
what happens to reactivity down the group?
increases
group 2 metal + acid → ?
salt and hydrogen
example: magnesium with HCl:
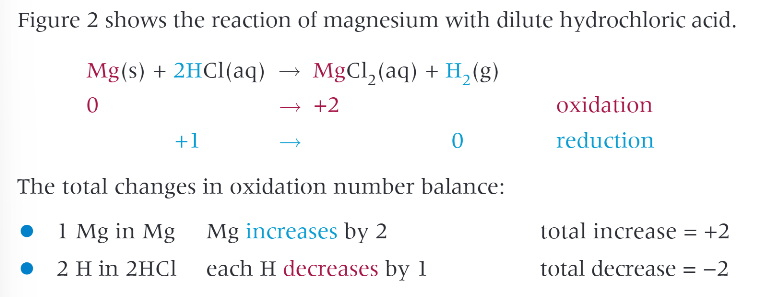
why does the reactivity increase down group 2?
the atoms of group 2 elements react by losing electrons to form +2 ions. The formation of +2 ions from gaseous atoms require the input of 2 ionisation energies, to lose 2 electrons
example of this:

first and second ionisation energies of group 2 elements:
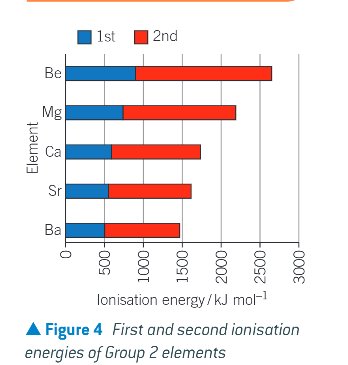
why do ionisation energies decrease down the group?
the attraction between the nucleus and the outer electrons decreases as a result of increasing atomic radius and increasing shielding
what do the 1st and 2nd ionisation energies make most of?
the energy input of reacting
group 2 oxides reacting with water:
produces hydroxide ions and alkaline solutions of the metal hydroxide
example:
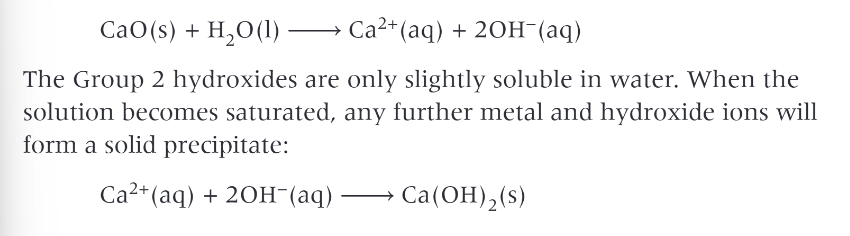
what happens to the solubility of the hydroxides down the group?
it increases
what does this mean?
the resulting solutions contain more OH- ions and are more alkaline
trend in alkalinity:
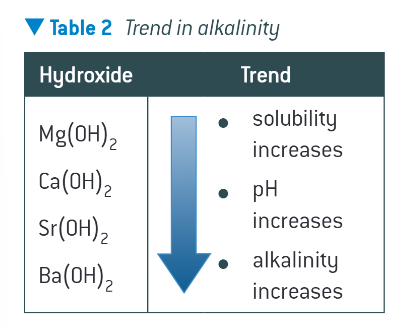
what experiment can you do to show this trend?
add a spatula of each group 2 oxide to water in a test tube
shake the mixture. on this scale, there is insufficient water to dissolve all of the metal hydroxide that forms. you will have a saturated solution of each metal hydroxide with some white solid undissolved at the bottom of the test tube
measure the pH of each solution. the alkalinity will be seen to increase down the group
how can group 2 compounds be used in agriculture?
calcium hydroxide is added to fields as lime by farmers to increase the pH of acidic soils. the calcium hydroxide neutralises acid in the soil, forming neutral water
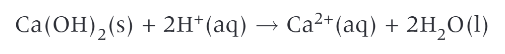
how can group 2 compounds be used in medicine?
group 2 bases are often used as antacids for treating acid indigestion. many indigestion tablets use magnesium and calcium carbonates as the main ingredients, while “milk of magnesia” is a suspension of white magnesium hydroxide, in water
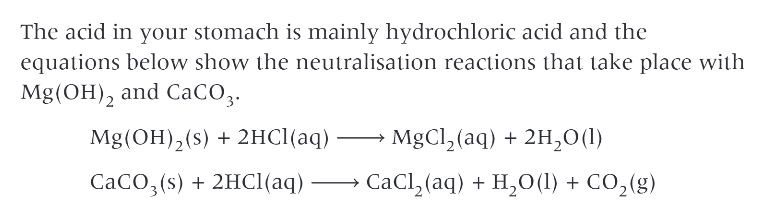
GROUP 7
what are group 7 elements?
most reactive non metallic group
do non metals exist in their elemental form in nature?
no
how do they occur on earth?
as stable halide ions (eg. Cl-) dissolved in sea water, or combined with sodium/ potassium as solid deposits, like in salt mines (eg. NaCl)
how do halogens exist at room temp and pressure?
diatomic molecules (X2)
what state are halogens in room temp?
can exist as all 3 depending on the halogen
what do halogens form in their solid states?
simple molecular structures
tends in boiling points of the 5 halogens:
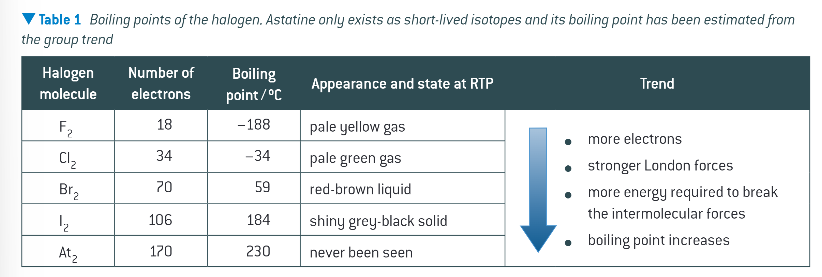
how many outer shell electrons are in halogens?
7 (2 in s subshell and 5 in p subshell)
what are the most common type of reaction for halogens?
redox reactions
what happens in these redox reactions?
each halogen atom is reduced, gaining one electron to form a 1- halide ion with the electron configuration of the nearest noble gas
another species loses electrons to halogen atoms, being oxidised
example of halogen being reduced:
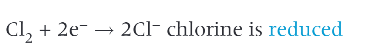
why is a halogen called an oxidising agent?
it has oxidised another species
comparison of outer shell electron configuration of halogen atoms vs halide ions:
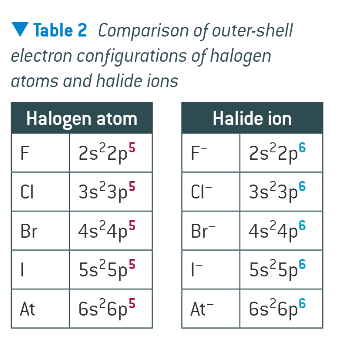
what do halogen halide displacement reactions show?
the reactivity of halogens decreases down the group
how does a displacement reaction happen?
a solution of each halogen is added to aqueous solutions of the other halides
if the halogen added is more reactive than the halide present then a reaction takes place and the halogen displaces the halide, changing the colour
what halogens might appear similar in water?
iodine and bromine, both being brown/ orange
what can you do to tell these apart?
an organic non polar solvent, like cyclohexane, can be added and shaken. the non polar halogens dissolve more readily in cyclohexane than in water, changing the colour better
colour differences:

results and conclusions from displacement reactions of aqueous solution of halogens and halides:
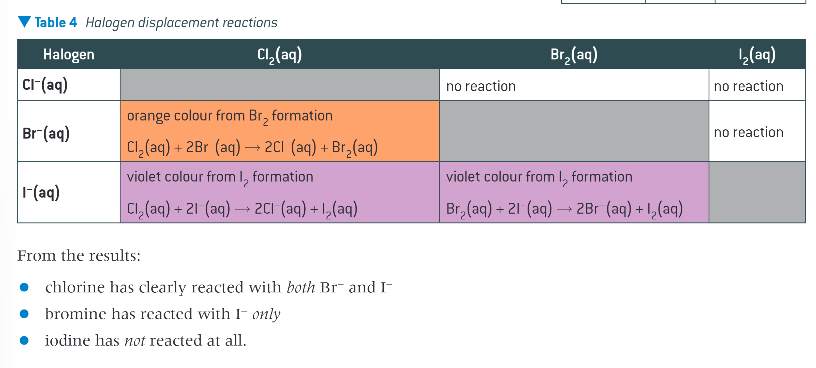
order of reactivity of chlorine, bromine and iodine:
reaction of chlorine with bromide ions:
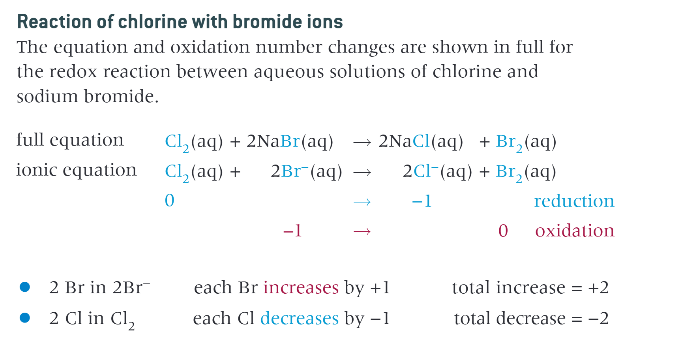
fluorine properties:
pale yellow gas
reacts with almost any substance that it comes in contact with
astatine properties:
radioactive
rare
decays rapidly
least reactive halogen
chlorine properties:
pale green gas
bromine properties:
red liquid
extremely toxic
vaporises readily at room temp
iodine properties:
solid
grey black crystals
trend in reactivity of the halogens table:

what is disproportionation?
a redox reaction in which the same element is both oxidised and reduced
examples of disproportionation reactions:
chlorine with water
chlorine with sodium hydroxide
reaction of chlorine with water:
chlorine used in water purification
widely used for disinfection of water
2 products are both acids
chloric acid can act as both a weak bleach, and kill bacteria for water drinking
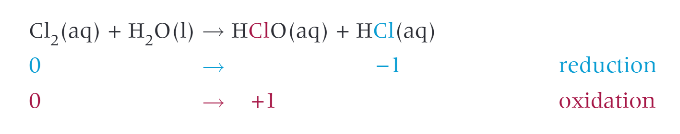
how to demonstrate chlorine acid as acting as a weak bleach:
add indicator to solution of chlorine in water. indicator first goes red (from acid) , then disappears and bleaches white
the reaction of chlorine with cold, dilute aqueous sodium hydroxide:
lots of chlorine dissolves and another disproportionation reaction takes place
large concentration of chlorate ions from the sodium chlorate formed
household bleach
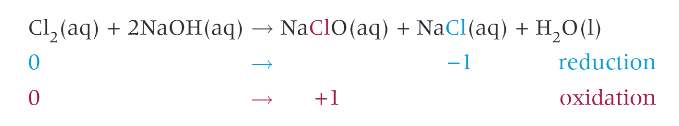
pros of chlorine use:
ensures water is fit to drink and bacteria is killed
better than not drinking water
typhoid and cholera might break out otherwise
cons of chlorine use:
toxic gas
respiratory irritant in small concentrations (let alone large concs)
chlorine in drinking water can react with organic hydrocarbons, eg. methane, formed from decaying vegetation, forming chlorinated hydrocarbons, which are suspected of causing cancer
eg chlorine tablets in pools:

test for halide ions:
aqueous halide ions and aqueous silver ions to form precipitates of silver halides
basic test for the presence of halides
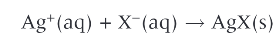
extra info: halide ions as reducing agents
