Chemical Reactions - Types of Chemical Reactions
0.0(0)
Card Sorting
1/33
Last updated 2:06 PM on 12/12/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
1
New cards
What is the general equation for a synthesis reaction?
A + B = AB
2
New cards
List the three TYPES of synthesis reactions:
1. Two elements forming a binary compound
2. An element and a compound forming a new compound
3. Two compounds forming a new compound
3
New cards
What is the PRODUCT of a non-metal oxide reacting with water?
An acid
4
New cards
What is the PRODUCT of a metal oxide reacting with water?
A base
5
New cards
What is the general equation for a decomposition reaction?
AB = A + B
6
New cards
Electrolysis
A process that uses electricity to cause a chemical reaction.
Electrolysis equation: 2H2O → O2 + 2H2
Electrolysis equation: 2H2O → O2 + 2H2
7
New cards
How do metal nitrates behave in a reaction?
Being made up of >2 elements, these compounds generally do not completely decompose into their INDIVIDUAL elements.
8
New cards
How do metal carbonates behave in a reaction?
Metal carbonates generally decompose to form a metal oxide and carbon dioxide.
9
New cards
Quicklime
Quicklime (calcium oxide - CaO) is a component of cement that helps to bind the different components of cement (water, sand, gravel) together.
10
New cards
Common usage of the decomposition of sodium azide
The rapid decomposition of sodium azide is used to quickly inflate air bags in cars during a collision.
Equation: 2NaN3 → 2Na + 3N2
Equation: 2NaN3 → 2Na + 3N2
11
New cards
What is the general equation for a single displacement (SD) reaction?
A + BC → B + AC
12
New cards
List the two TYPES of decomposition:
1. Decomposition of metal nitrates
2. Decomposition of metal carbonates
13
New cards
What is the general equation for a double displacement (DD) reaction?
AB + CD → AD + CB
14
New cards
List the three TYPES of SD reactions?
1. Cationic SD
2. Anionic SD
3. A metal displacing hydrogen from an acid or water molecule
15
New cards
Cationic SD
Metal displacing another metal in an ionic compound
16
New cards
Anionic SD
Non-metal displacing another non-metal in an ionic compound
17
New cards
List the three TYPES of DD reactions
1. Reaction that forms a precipitate
2. Reaction that forms a gas
3. Reaction that forms water (neutralization reaction)
18
New cards
How do you use a solubility chart for DD reactions?
Check whether or not a product is soluble in water (REMEMBER TO SPECIFY STATES WHILE WRITING EQUATIONS)
19
New cards
Vinegar + baking soda
CH3COOH + NaHCO3 → NaCH3COO + H2CO3
H2CO3 is not stable, so it further decomposes:
H2CO3 → H2O + CO2
H2CO3 is not stable, so it further decomposes:
H2CO3 → H2O + CO2
20
New cards
Ammonium hydroxide reaction
Ca(OH)2 + NH2NO3 → Ca(NO3)2 + NH4OH
NH4OH → H2O + NH3
NH4OH → H2O + NH3
21
New cards
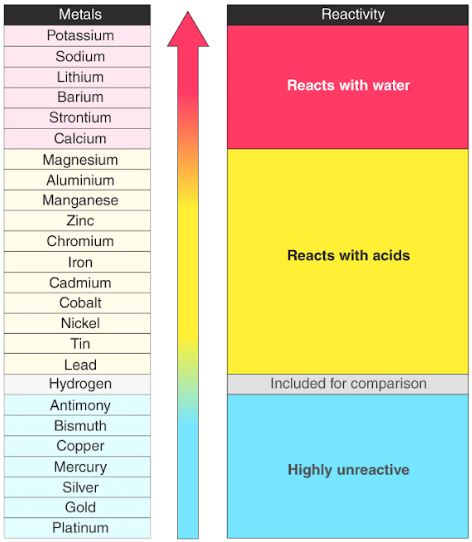
What is this chart called and what is it used for?
Activity series of metals; for predicting whether a metal is reactive enough to replace another metal in a SD reaction.
22
New cards
Reactivity of non-metals increases _________________
left to right, bottom to up
23
New cards
Exothermic
Produces energy
24
New cards
Endothermic
Absorbs energy
25
New cards
Combustion
The reaction of a substance with oxygen, producing one or more oxides, heat, and light.
* Occurs when you burn something
* Occurs when you burn something
26
New cards
27
New cards
Hydrocarbons
Compounds only composed of carbon and hydrogen
28
New cards
What are the two TYPES of combustion reactions?
1. Complete combustion
2. Incomplete combustion
29
New cards
Complete combustion
* Occurs when oxygen is available in sufficient amounts.
* Reactions of hydrocarbons with oxygen always produce carbon dioxide and water.
* Creates a blue-coloured flame.
* Reactions of hydrocarbons with oxygen always produce carbon dioxide and water.
* Creates a blue-coloured flame.
30
New cards
Incomplete reaction
* Occurs when oxygen is NOT available in sufficient amounts.
* May form additional products such as carbon (soot) and carbon monoxide.
* Creates a yellow/orange flame
* Produces an abundance of smoke
* May form additional products such as carbon (soot) and carbon monoxide.
* Creates a yellow/orange flame
* Produces an abundance of smoke
31
New cards
What is the main example for a COMPLETE COMBUSTION REACTION?
Cellular respiration
32
New cards
What is the general equation for a complete combustion reaction?
CXHY + O2 → CO2 + H2O
33
New cards
Cellular respiration chemical equation
C6H12O6 + 6O2 → 6H2O + 6CO2 + energy
(glucose + oxygen → water + carbon dioxide + energy)
(glucose + oxygen → water + carbon dioxide + energy)
34
New cards
Give two examples of an INCOMPLETE COMBUSTION REACTION?
1. Burning wood
2. Burning coal