5.01-5.22 Solids, Liquids, and Gases
0.0(0)
Card Sorting
1/125
Earn XP
Description and Tags
Last updated 6:04 AM on 1/3/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
126 Terms
1
New cards
degrees Celsius (°C)
Unit using the Celsius scale to measure temperature, which has 0°C based on water’s freezing point and 100°C based on water’s boiling point.
2
New cards
Kelvin (K)
SI unit for measuring temperature with the kelvin (absolute) temperature scale. It has the same magnitude as 1°C, i.e. 1K obtains an equal magnitude to 1°C.
3
New cards
Joule (J)
SI unit for measuring energy.
4
New cards
Kilogram (kg)
SI unit for measuring mass.
5
New cards
Kilograms/meters^3 (kg/㎥)
Unit for measuring density.
6
New cards
Meter (m)
Unit for measuring length.
7
New cards
Meters^2 (㎡)
Unit for measuring area.
8
New cards
Meters^3 (㎥)
Unit for measuring volume.
9
New cards
Meters/second (m/s)
Unit for measuring speed.
10
New cards
Meters/second^2 (㎨)
Unit for measuring acceleration.
11
New cards
Newton (N)
Unit for measuring force.
12
New cards
Pascal (Pa)
SI unit for measuring pressure.
13
New cards
Joules/kilogram degrees Celsius (J/kg°C)
Metric unit for measuring specific heat capacity.
14
New cards
Density definition
Degree of compactness of a substance, measured as the quantity of mass per unit volume of that substance.
15
New cards
Density formula
Density = Mass (kg or g)/Volume (㎥ or ㎤), measured in kg/㎥ or g/㎤
16
New cards
Displacement
Way of measuring the volume of an irregularly-shaped object by:
1. Putting a known volume of water in a measuring cylinder (V1).
2. Putting the irregularly-shaped object inside.
3. Measure the new volume (V2).
4. Object volume is the volume of water displaced, or V2-V1).
You can also use a Eureka can to calculate the volume of irregularly-shaped objects:
1. Fill the eureka can with water until the water level reaches just at/below the spout’s level.
2. Wait for all the excess water to drip out of the spout.
3. Once all the excess water drips out the spout and the water level is just underneath the spout’s hole, place an appropriately-sized graduated measuring cylinder underneath the eureka can so it can collect water that drips out.
4. Gently lower your object into the can (preferably with it suspended onto a thread/string to minimize splashing that will lead to inaccurate results for volume).
5. The volume of water that drips out of the eureka can’s spout should be equal to the volume of the irregularly-shaped object.
The density of the objects can be calculated by dividing their mass (recorded from an electronic balance) by their volume (determined from using any 1 of the above 2 methods).
1. Putting a known volume of water in a measuring cylinder (V1).
2. Putting the irregularly-shaped object inside.
3. Measure the new volume (V2).
4. Object volume is the volume of water displaced, or V2-V1).
You can also use a Eureka can to calculate the volume of irregularly-shaped objects:
1. Fill the eureka can with water until the water level reaches just at/below the spout’s level.
2. Wait for all the excess water to drip out of the spout.
3. Once all the excess water drips out the spout and the water level is just underneath the spout’s hole, place an appropriately-sized graduated measuring cylinder underneath the eureka can so it can collect water that drips out.
4. Gently lower your object into the can (preferably with it suspended onto a thread/string to minimize splashing that will lead to inaccurate results for volume).
5. The volume of water that drips out of the eureka can’s spout should be equal to the volume of the irregularly-shaped object.
The density of the objects can be calculated by dividing their mass (recorded from an electronic balance) by their volume (determined from using any 1 of the above 2 methods).
17
New cards
Water displacement
When an object enters water, it pushes out water to make room for itself. The object pushes out/displaces (change the position of an object) a volume of water that is equal to its own volume.
18
New cards
Buoyancy
Ability of an object to float in a fluid medium.
19
New cards
Object density > Fluid medium density
Object sinks as upthrust < weight
20
New cards
Object density = Fluid medium density
Object is partially submerged or partially floating as upthrust = weight
21
New cards
Object density < Fluid medium density
Object floats as the upthrust > weight
22
New cards
Buoyant force/upthrust
Upward force exerted on an object partially or wholly immersed in a fluid.
23
New cards
Archimedes' principle
A body partially or wholly immersed in a fluid experiences an upthrust (buoyant force) equal to the weight of the fluid displaced by that object.
24
New cards
Directly proportional
When the ratio of 2 variables is constant, resulting in a linear graph that goes through the origin. As 1 variable increases, the other increases, as 1 variable decreases, the other decreases.
25
New cards
Inversely proportional
When the product of 2 variables is constant, resulting in a downward sloping curve graph. As 1 variable increases, the other decreases, as 1 variable decreases, the other increases. We can linearize an inversely proportional relationship by stating that one variable is directly proportional to the reciprocal of the other variable.
26
New cards
Extrapolate
To extend a graph, curve, or range of values by inferring unknown values form trends in the known data.
27
New cards
Interpolate
To estimate unknown values that fall between known values.
28
New cards
Pressure definition
The perpendicular force applied per unit of area.
29
New cards
Pressure formula
Pressure (Pa) = Force exerted onto object(s) (N) / Area of contact between objects (㎡).
P = F/A.
P = F/A.
30
New cards
Pressure is measured in ___________, the SI unit for ____________. 1 _____________ is equal to 1 _________.
Pascals (Pa), pressure, Pascal, Newton/meter squared or N/㎡
31
New cards
Pressure is __________ to force.
proportional.
32
New cards
Pressure is ____________ to area.
Inversely proportional (or proportional to 1/Area).
33
New cards
Gas pressure
Pressure in gases caused by particles exerting forces as they collide with the container walls.
34
New cards
Gas particles continually travel in____________ motion until they _________ into other ___________ and/or ___________________ to _______________. They move ___________ and in every __________ at high _________.
linear, collide, particles, container walls, change direction, randomly, direction, speeds.
35
New cards
Atmospheric pressure > interior air pressure causes
implosion (e.g. aluminum can collapses inside itself or "implodes")
36
New cards
Atmospheric pressure < interior air pressure causes
Explosion (e.g. balloon explodes when there is too much air inside it).
37
New cards
Describe how pressure acts in fluids
Pressure in fluids act equally in all directions, as long as the fluid medium is at rest (not moving).
38
New cards
Fluids
States of matter that can flow, specifically liquids and gases.
39
New cards
Pressure (in fluids) is _____________ to depth.
proportional
40
New cards
Pressure (in fluids) is ______________ to height.
inversely proportional
41
New cards
Explain why pressure in liquids is proportional to depth and inversely proportional to height.
More depth (lower height) means more fluid mass is above this object so more weight/force is acting on this object at its current position, leading to higher pressure.
42
New cards
Pressure is ______________ to gravitational field strength. Explain why.
proportional; higher gravitational field strength means there is more weight and therefore more force acting on an object, hence increasing the pressure.
43
New cards
Pressure is ______________ to density of the fluid medium an object is submerged in. Explain why.
proportional; higher density of the fluid medium means the fluid medium has a larger mass, and therefore a larger weight as well as force acting on it, hence increasing the pressure.
44
New cards
Pressure in a fluid (Stevin's law) formula
Pressure (Pa) = height (m) * density (kg/㎥) * gravitational field strength (N/kg)
P = h𝝆g
P = h𝝆g
45
New cards
Pressure difference in a fluid (Stevin's law) formula
Change in pressure (Pa) = change in height (m) * density (kg/㎥) * gravitational field strength (N/kg)
𝚫P = 𝚫h𝝆g
𝚫P = 𝚫h𝝆g
46
New cards
Atmospheric air pressure
Approximately 100 000Pa (101 325Pa to be exact), however this varies slightly from day to day.
47
New cards
The air pressure in our bodies through ______________ is similar to the _______________ air pressure, so we do not notice it.
ventilation, atmospheric
48
New cards
Water's density =
1000kg/㎥ or 1g/㎤
49
New cards
Total pressure acting on a body in a liquid =
Change in pressure due to being submerged in the liquid body + Atmospheric pressure
50
New cards
Total pressure acting on a body in the air/atmosphere =
height of object * density of atmosphere/air medium * gravitational field strength of object's location
51
New cards
Factors affecting gas pressure in a container
temperature, volume, and particle concentration
52
New cards
Brownian motion
Random motion of atoms/particles suspended in a fluid medium caused by miscellaneous collisions between the particles and atoms/molecules/particles found in their surroundings. It was first observed by Robert Brown when he studied pollen grains' random motion in water.
53
New cards
Kinetic theory of matter states that
1. Gas particles are in constant random motion.
2. The combined volume occupied by gas molecules in a container is negligible compared to the distance or space between them.
3. Particles exert no forces on one another unless they collide.
4. Collisions between particles are completely elastic (i.e. no transfer of kinetic energy occurs, the particles involved in collisions do not gain or lose any kinetic energy).
5. Average kinetic energy of particles is proportional to their temperature in Kelvins.
6. Molecules always have linear motion, they always move in straight lines before colliding with the container walls and/or other particles to change direction.
7. Collision duration is negligible between the time between collisions.
2. The combined volume occupied by gas molecules in a container is negligible compared to the distance or space between them.
3. Particles exert no forces on one another unless they collide.
4. Collisions between particles are completely elastic (i.e. no transfer of kinetic energy occurs, the particles involved in collisions do not gain or lose any kinetic energy).
5. Average kinetic energy of particles is proportional to their temperature in Kelvins.
6. Molecules always have linear motion, they always move in straight lines before colliding with the container walls and/or other particles to change direction.
7. Collision duration is negligible between the time between collisions.
54
New cards
Gas pressure
Pressure exerted by gas particles due to the combined force they exert through their collisions with their container walls.
55
New cards
Particle concentration is ____________ to pressure.
proportional; more particles -> more collisions between particles and container walls -> more force -> more pressure.
56
New cards
Temperature is ______________ to pressure.
proportional; higher temperature -> more average kinetic energy for the particles -> particles move at faster speeds and have more frequent collisions between each other as well as container walls -> More force -> Increased pressure.
57
New cards
Temperature
A measure of average kinetic energy per molecule in a substance, usually measured in degrees Celsius, degrees Fahrenheit, or Kelvins.
58
New cards
Thermal energy
A type of energy, it's the total kinetic energy of the particles in a substance.
59
New cards
Temperature VS thermal energy
1. Temperature is a WAY OF MEASURING energy, but thermal energy is a TYPE of energy.
2. Temperature is a measure of AVERAGE kinetic energy per molecule in a substance whereas thermal energy is a measure of the TOTAL kinetic energy of the particles in a substance.
2. Temperature is a measure of AVERAGE kinetic energy per molecule in a substance whereas thermal energy is a measure of the TOTAL kinetic energy of the particles in a substance.
60
New cards
More particle motion indicates ________________________ and hence higher ____________ as well as higher ___________ of ________________.
more average kinetic energy, temperature, levels, thermal energy.
61
New cards
More atoms (a.k.a. higher ______________________) and higher ________________ leads to higher levels of _________________.
particle concentration, temperature, thermal energy
62
New cards
Gay-Lussac's experiment
1. Independent variable is temperature, dependent variable is pressure, control variables are volume and mass of gas. Investigating effect of temperature on pressure.
2. Set water bath to 90 degrees Celsius.
3. Put thermometer and gas flask in water bath.
4. Wait until the gas reaches equilibrium, or when temperature and pressure readings remain constant.
5. Record the pressure reading from a pressure gauge.
6. Repeat steps 2-5 for 80, 70, 60, 50, 40, 30, and 20 degrees Celsius.
7. Plot the results in a graph.
2. Set water bath to 90 degrees Celsius.
3. Put thermometer and gas flask in water bath.
4. Wait until the gas reaches equilibrium, or when temperature and pressure readings remain constant.
5. Record the pressure reading from a pressure gauge.
6. Repeat steps 2-5 for 80, 70, 60, 50, 40, 30, and 20 degrees Celsius.
7. Plot the results in a graph.
63
New cards
If we _____________ the graph from Gay-Lussac's experiment until the line intersects the x-axis (where pressure is _________kPa), we will find that the temperature is _____________________ degrees Celsius or ________ Kelvin. Pressure, however, is always __________________ as there ___________ be a negative force exerted by particles.
extrapolate, 0, -273.15, 0, nonnegative, cannot
64
New cards
Absolute zero
Lowest temperature theoretically attainable at which pressure is 0, it's equivalent to -273.15 degrees Celsius or zero Kelvin. At this stage, it's impossible to cool a gas further.
65
New cards
Molecular motion _____________ cease at absolute zero, molecules still ___________ with ________________ . They move EXTREMELY slowly at the slowest _______________ speed to an extent where their motion is ________________. The energy at absolute zero is ______________ and close to __________ as no _______ energy from molecular _________ is available for ___________ or __________ to other systems.
does not, vibrate, zero-point energy, possible, unobservable, minimal, nothing, thermal, motion, transfer, conversion
66
New cards
Zero-point energy
Vibrational energy molecules obtain even at absolute zero
67
New cards
Particle kinetic energy infinitesimally nears zero (or is ___________ equal to 0) -> Gas particles _______ colliding with container walls -> ________ Pressure
theoretically, stop, zero
68
New cards
Kelvin scale
Temperature scale using Kelvins (SI unit for temperature). Absolute zero is zero on the Kelvin scale and there are no negative temperatures in the Kelvin scale. It is more mathematically accurate and convenient for absolute temperature.
69
New cards
Don't say "degrees Kelvin" or write "°K", it is just said as "___________" and written as "___"
Kelvin, K
70
New cards
Kelvin scale = _____________ temperature scale because ...
absolute, it measures the absolute value of temperatures (non-negative magnitudes) and absolute zero is equivalent to zero Kelvin.
71
New cards
Celsius (centigrade) scale
Temperature scale using degrees Celsius as a unit. It's based on 0°C for water's freezing point and 100°C for water's boiling point.
72
New cards
The ____________ of each unit in the Celsius and Kelvin scale are ________, meaning that a change of 1°C is ___________ to a change of _______.
magnitude, equal, equal, 1K.
73
New cards
Kelvin to Celsius
Celsius = Kelvin - 273.15 (273 is accurate enough for IGCSE unless stated otherwise by the question).
74
New cards
Celsius to Kelvin
Kelvin = Celsius + 273.15 (273 is accurate enough for IGCSE unless stated otherwise by the question).
75
New cards
Temperature (IN KELVINS) =
Average kinetic energy of particles
76
New cards
Explain the relationship between temperature (IN KELVINS) and pressure.
Directly proportional. Higher temperature -> Higher average kinetic energy of particles -> Higher speed of particle motion -> More frequent collisions between particles and container walls -> More force exerted over the same container wall area -> Higher pressure.
77
New cards
Temperature in ____________ is _____________________ to pressure, when the … of gas is …, so their graph would be _________ and go through the __________ as their ___________ is constant. This means doubling the temperature in _____________ leads to ____________ average kinetic energy of particles (average … of particles also …), ______________ collisions with container walls, _____________ force, and hence also ___________ pressure.
Kelvins, directly proportional, volume, constant, linear, origin, ratio, Kelvins, doubled, speed, doubles, doubled, doubled, doubled
78
New cards
Gay-Lussac's Law
Law stating that the ratio between pressure and absolute temperature (in Kelvins) of a gas (whose volume and particle concentration is kept the same) is constant, because pressure and absolute temperature of a gas are directly proportional. In other words:
P1/T1 (initial pressure and temperature conditions) = P2/T2 (final pressure and temperature conditions).
P1/T1 (initial pressure and temperature conditions) = P2/T2 (final pressure and temperature conditions).
79
New cards
Volume is ________________ to pressure
inversely proportional (pressure is proportional to 1/volume); larger volume -> more space for same amount of particles to move in -> less frequent collisions -> less force -> less pressure, smaller volume -> less space for same amount of particles to move in -> more frequent collisions -> more force -> more pressure
80
New cards
Boyle's law experiment
1. Independent variable = Pressure (kPa), dependent variable = volume (㎤), investigating the effect of pressure on volume.
2. Use air pump to increase pressure to at least 300kPa.
3. Wait until the gas reaches equilibrium, i.e. temperature stays constant.
4. Release the air pump/pressure until the Bourdon gauge reads 300kPa.
5. Record the volume of air in the tube by reading of the scale.
6. Repeat for each pressure reading.
7. Repeat the entire experiment once or twice or to calculate an average volume of air.
7. Plot the results, one graph for pressure against volume, and one for pressure against 1/volume.
2. Use air pump to increase pressure to at least 300kPa.
3. Wait until the gas reaches equilibrium, i.e. temperature stays constant.
4. Release the air pump/pressure until the Bourdon gauge reads 300kPa.
5. Record the volume of air in the tube by reading of the scale.
6. Repeat for each pressure reading.
7. Repeat the entire experiment once or twice or to calculate an average volume of air.
7. Plot the results, one graph for pressure against volume, and one for pressure against 1/volume.
81
New cards
Ways to improve experiment reliability
Take repeats, improves data reliability as averages can be calculated and anomalies are easier to notice.
82
New cards
Anomaly
A piece of data that does not follow the trend formed by other pieces of data, often due to experimental error.
83
New cards
Ways to improve accuracy.
1. Use mm instead of cm scale as there is better precision.
2. Have a pump to hold pressure to give more time to make a reading (Boyle's law experiment)
3. Use a spirit level to ensure your eye is level when the scale when reading it to avoid parallax error.
4. Read from the bottom of the meniscus.
2. Have a pump to hold pressure to give more time to make a reading (Boyle's law experiment)
3. Use a spirit level to ensure your eye is level when the scale when reading it to avoid parallax error.
4. Read from the bottom of the meniscus.
84
New cards
Precision VS Accuracy
Accuracy refers to how close a measurement is to the true or accepted value. Precision refers to how close measurements of the same item are to each other, it reflects how reproducible measurements are, even if they are far from the accepted value and inaccurate.
85
New cards
Spirit level
Device made from a sealed glass tube partially filled with alcohol or another liquid, it contains an air bubble whose positionn reveals if a surface is perfectly level.
86
New cards
Parallax error
Incorrect reading on a measuring instrument by viewing it from different angles to create a perspective of the object's positional shift.
87
New cards
Systemic error
Consistent, repeatable error associated with faulty equipment or a flawed experiment design.
88
New cards
Meniscus
Curve in surface of molecular substance (especially water) when it touches another material.
89
New cards
Boyle's law
Law stating that under constant temperature and mass of gas, the volume of a gas and its pressure are inversely proportional, meaning they have a constant product. In other wards P1V1 (initial pressure and volume situation) = P2V2 (final pressure and volume situation).
90
New cards
In Boyle's law, because temperature is ___________, particles have the ________ average kinetic energy and move at the same __________________. Gas compression therefore leads to _________ space and __________ for particles to move in, so particles hit ____________ walls _________ hard but ___________ often due to _________ collisions. This __________ force and __________ area of contact leads to _________ gas pressure.
constant, same, average speed, less, area, container, equally, more, more, increased, decreased, increased
91
New cards
Atmospheres(atm)
Unit of pressure equal to air/atmospheric pressure at sea level
92
New cards
1 atm =
101325 Pa
93
New cards
Energy efficiency equation
Efficiency = (Useful energy output/Total energy output) * 100%
94
New cards
Specific heat capacity
Amount of heat/thermal energy required to change the temperature of the unit mass of a given substance (usually 1kg) by a given amount (usually 1°C or 1K).
95
New cards
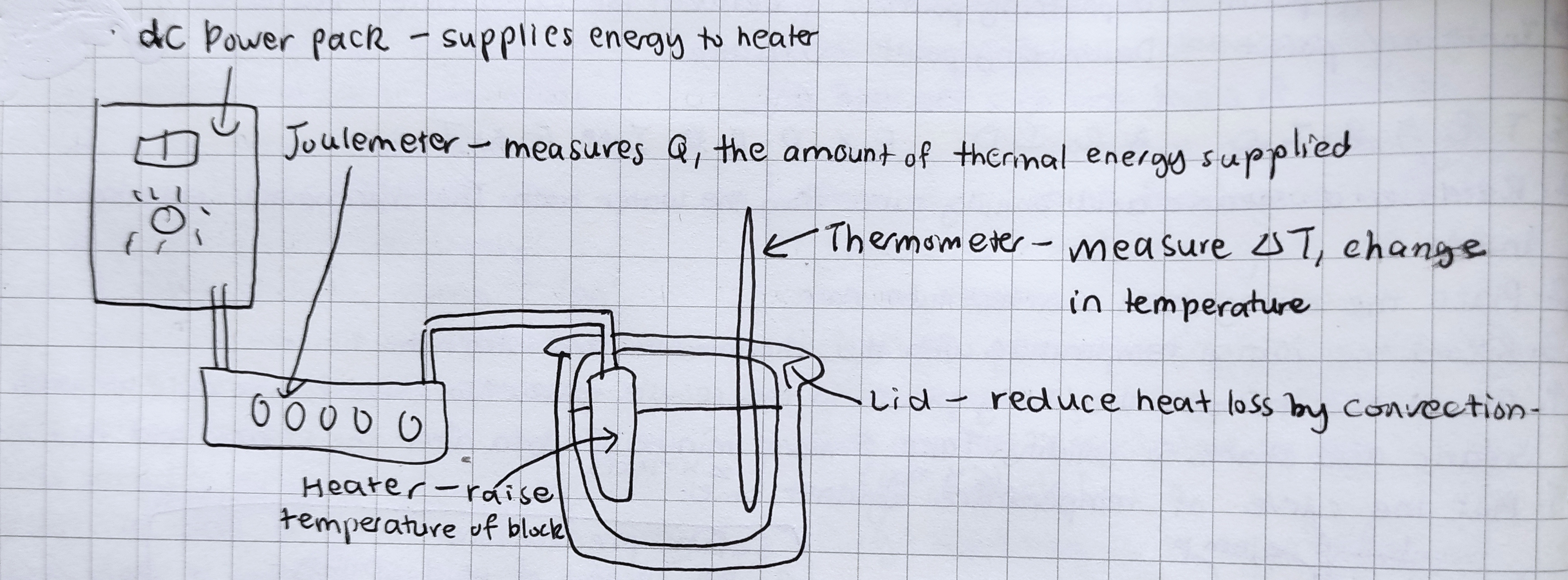
Specific heat capacity practical
1. Use scale to find mass of block/object.
2. Insert thermometer and immersion heater into respective holes in the block. You can optionally drop a small amount of oil or vaseline into the thermometer hole to improve the thermal contact between the thermometer and block.
3. Allow thermometer to reach thermal equilibrium (no temperature difference/changes between the system and their surroundings), then write down the initial temperature (T1).
4. Set up the circuit to measure energy input into the heater.
5. Turn on the power and allow the block to heat up until the Joulemeter reaches its maximum reading of 9999J, then turn off the power.
6. Even though the heater is switched off, heat will still be coming from the inside of the heater to the heated object/block. You’ll therefore notice that the temperature continues to rise for a short time. Record the highest temperature reached (T2).
7. Calculate the material’s specific heat capacity:
𝚫Q = mc𝚫T → c = (𝚫Q)/(m𝚫T)
96
New cards
Water’s specific heat capacity
4200J/kg°C or 4.2J/g°C
97
New cards
Change in thermal energy formula
Change in thermal energy (J) = mass (kg) * specific heat capacity (J*kg/°C or J*kg/K) * change in temperature (°C or K)
𝚫Q = mc𝚫T or E = mc𝚫T
𝚫Q = mc𝚫T or E = mc𝚫T
98
New cards
Matter
Substance made from various types of particles that occupies physical space and has inertia.
99
New cards
Inertia
Physical force that keeps something stationary in the same position, or causes something moving to move in the same direction, unless an external force is applied.
100
New cards
Phase (a.k.a. state)
A physically distinctive form of matter, e.g. solids, liquids, gases, plasma.