Family History Pedigree Analysis and Patient Evaluation
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
95 Terms
What are pedigrees defined as in relation to genetic risk?
Pedigrees (a chart) are cost-effective tools for genetic diagnosis and risk assessment.
What are pedigrees a tool for besides diagnosis and risk assessment?
Establishing the pattern of inheritance; identifying at-risk relatives; calculating disease risks; determining reproductive options; distinguishing genetic from other risk factors.
What is another essential function of the pedigree as a diagnostic tool?
Making decisions on medical management and surveillance.
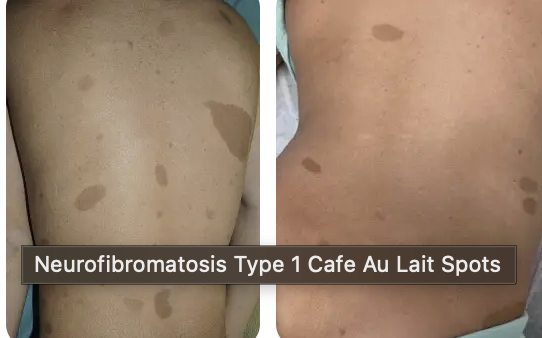
How is a pedigree useful in the diagnosis of Neurofibromatosis type 1 (NF1)
A positive family history plus one other feature (e.g., café-au-lait spots) can establish the diagnosis.
When is molecular genetic testing for Birt-Hogg-Dube syndrome (BHD) indicated in individuals
individuals with specific renal tumors and a family history of renal cancer, or
a family history of autosomal dominant primary spontaneous pneumothorax (unrelated to smoking/COPD).
A man with congenital profound bilateral hearing loss is planning children; what are the various potential inheritance patterns that must be considered?
Hearing loss can be autosomal recessive; autosomal dominant; X-linked; have a mitochondrial inheritance pattern; or be associated with maternal teratogenic exposure.
Why is knowing the inheritance pattern essential when calculating recurrence risk?
Impossible to provide recurrence risk estimates without knowing the inheritance pattern.
When assessing genetic risk; what is equally important to a positive family history?
Negative family histories are just as important as positive ones.
What key information related to affected family members is determined by taking a pedigree that is often missed when only asking about family history?
Whether affected family members are vertical (multiple generations) or horizontal (same generation).
According to NCCN guidelines; when does an individual qualify for genetic testing for Lynch syndrome based on one first-degree relative with CRC/EC?
Has 1 or more first-degree relatives with colorectal or endometrial cancer (CRC/EC) diagnosed < 50y.
According to NCCN guidelines; when does an individual qualify for genetic testing for Lynch syndrome based on a first-degree relative with multiple cancer types?
Has 1 or more first-degree relatives with a CRC/EC and a synchronous or metachronous Lynch syndrome-related cancer.
According to NCCN guidelines; when does an individual qualify for genetic testing for Lynch syndrome based on second-degree relatives diagnosed before 50y?
Has 2 or more first-degree or second-degree relatives with Lynch syndrome-related cancers; including at least one diagnosed before 50y.
According to NCCN guidelines; what is the threshold for genetic testing qualification based on Lynch syndrome-related cancers at any age?
Has 3 or more first-degree or second-degree relatives with Lynch syndrome-related cancers at any age.
What are examples of Lynch syndrome-related cancers?
Colorectal; endometrial; gastric; urinary tract; biliary tract; pancreatic; prostate; brain; et al.
What are the two major categories of inheritance patterns?
Mendelian inheritance and Non-Mendelian inheritance.
What are the types of Mendelian inheritance discussed?
Autosomal (dominant/recessive); X-linked.
What are the types of Non-Mendelian inheritance discussed?
Mitochondrial; Imprinting; Multifactorial.
How is vertical transmission defined when evaluating a pedigree?
Phenotype seen in generation after generation.
How is horizontal transmission defined when evaluating a pedigree?
Phenotype seen in siblings but not previous generations.
In evaluating a pedigree; what question is asked regarding sex ratio?
Is the number of males and females displaying the phenotype equal or unequal?.
In evaluating a pedigree; what question is asked regarding segregation?
Do fathers or mothers appear to pass the trait on to sons or daughters? Is there a pattern?
Is there a pattern in how the trait is passed from parent to child? (e.g., Do fathers pass it to all their daughters? Do mothers pass it to half their sons?).
What quantitative metric is evaluated in a pedigree analysis regarding affected family members?
% of children affected.
How common is Autosomal Dominant inheritance in populations? What is typical regarding the parents of affected offspring in Autosomal Dominant inheritance?
Rare in populations. Affected offspring usually have one affected parent and one unaffected parent.
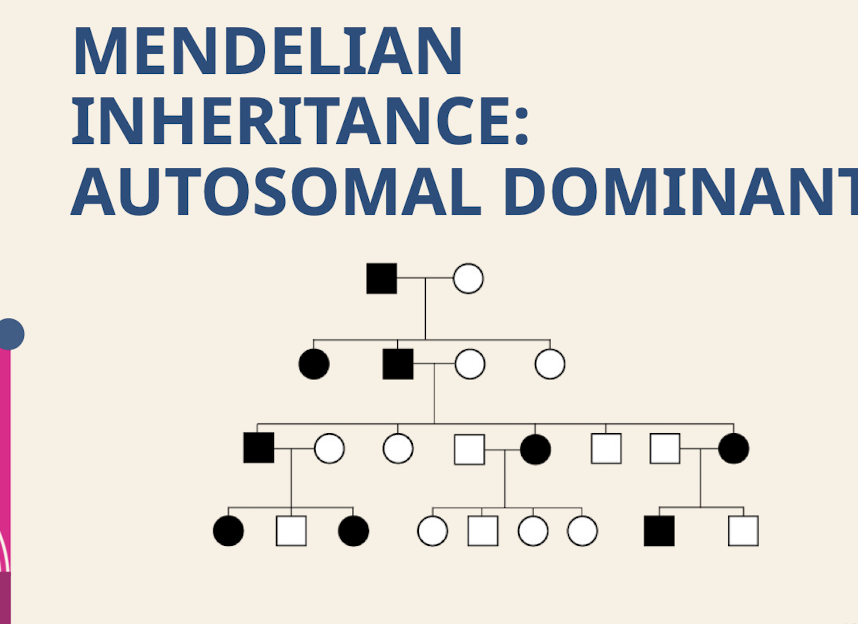
What are the characteristic transmission; sex ratio, segregation patterns, and recurrence risk for Autosomal Dominant inheritance?
Transmission – Vertical; Sex ratio – Equal; Segregation – Either parent passes on the trait to sons and daughters.
50% expected recurrence

How common is Autosomal Recessive inheritance in populations? What is typical regarding the parents of affected individuals in Autosomal Recessive inheritance?
Rare in populations. Parents of affected individuals are both heterozygotes (carriers).
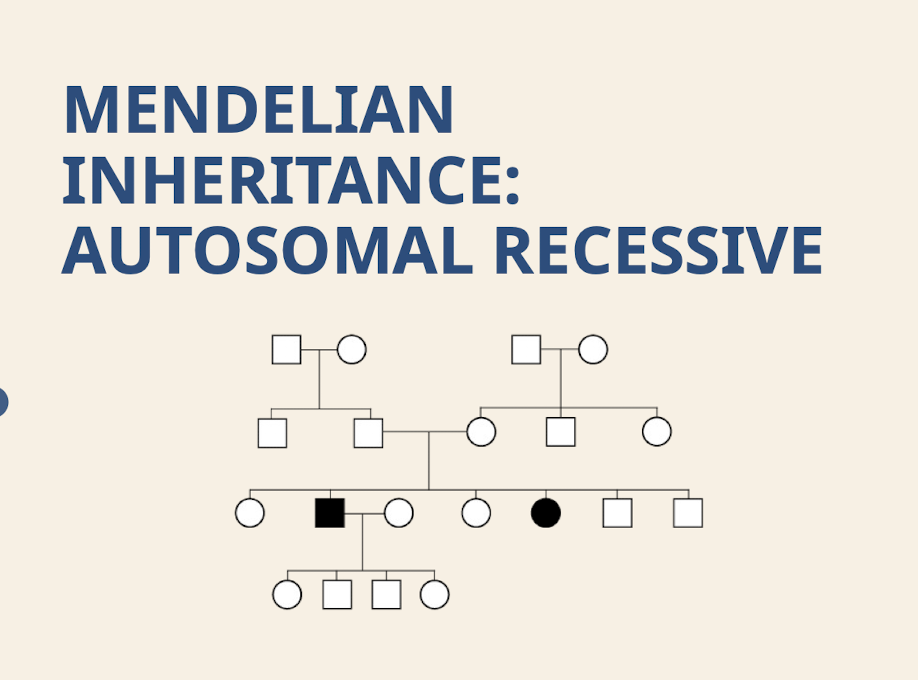
What are the characteristic transmission; sex ratio; and segregation patterns for Autosomal Recessive inheritance? What is the expected recurrence risk for an Autosomal Recessive trait?
Transmission – Horizontal; Sex ratio – Equal; Segregation – Either parent passes on the trait to sons and daughters. 25%.

What type of transmission pattern is characteristic of X-linked inheritance? What is the expected sex ratio in X-linked inheritance?
Vertical. Variable; expected that males are affected more than females.
How does an affected mother pass on an X-linked trait?
Affected mother can pass on to half of both sons and daughters.
How does an affected father pass on an X-linked trait?
Affected father passes on to all daughters and no sons (dad passes Y chromosome).
What mechanism determines the expression of X-linked traits?
Expression of X-linked traits is based on X-inactivation patterns.
So Inheritance of most X-linked traits is not dominant or recessive. Inactivation is completely random in EVERY tissue.
Why do males always show symptoms if they inherit an X-linked affected allele?
Males only have one X chromosome so will always show symptoms.
Why does symptom severity vary widely in carrier females of X-linked traits?
Females have two X chromosomes; but one is inactivated in every cell so it depends on the X-inactivation pattern!.
How is Mitochondrial disease passed through inheritance? Where does all mitochondrial DNA originate?
Disease is passed through mitochondrial DNA. All our mitochondrial DNA comes from our mothers.
What is Heteroplasmy.
Each cell contains many mitochondria, some with the disease mutation and some without. The proportion of mitochondria carrying the mutation is known as heteroplasmy. Higher heteroplasmy increases the chance of disease. This leads to extreme variability in presentation, even within the same family.
Why can mitochondrial disorders exhibit extreme differences in severity within the same family?
Because individuals will have different % heteroplasmy.
When a cell split its random which mitochondria goes into which cell
What is Imprinting?
Certain portions of our DNA are turned “on” or “off” via methylation based on the parent of origin.
What structures within the DNA instruct cells how to turn a section “on” or “off” in imprinting?
Imprinting centers within the DNA.
If a deletion in the 15q11-13 region is inherited from the mother; what disorder results?
Angelman syndrome.
If a deletion in the 15q11-13 region is inherited from the father; what disorder results?
Prader-Willi syndrome. (eating a lot)
What defines a multifactorial trait or disorder? What are examples of multifactorial diseases?
A trait or disorder caused by a combination of genetic and environmental factors. Most diseases out there. Psychiatric disease; autoimmune disease; hypertension; osteoarthritis.
What is reduced penetrance? What does reduced penetrance imply regarding the offspring of apparently unaffected individuals?
Individuals with the genotype may not exhibit the phenotype. Offspring of apparently unaffected individuals can still be at risk.
What percentage of families with hereditary retinoblastoma show a low-penetrance phenotype?
~10% of families with hereditary retinoblastoma exhibit a low-penetrance phenotype.
What is age-dependent penetrance? Why might individuals with age-dependent penetrance pass on the disease before they are symptomatic?
Delay of onset of symptoms (“adult-onset”). Individuals may have children before the age of onset.
What is a classic example of age-dependent penetrance?
Huntington disease.
What is variable expressivity?
Severity of phenotype varies; even within a family.
What condition serves as an example of variable expressivity due to X-inactivation; where carrier females can range from severe hyperammonemia to asymptomatic?
OTC deficiency.
What is a subtle presentation noted in some carrier females of OTC deficiency?
Some carrier females acquire an aversion to meat as only presentation.
What is anticipation?
More severe expression and/or earlier age of onset in more recent generations.
What genetic mechanism causes anticipation in Huntington disease?
Due to CAG trinucleotide repeat – during gamete formation; repeat size can increase due to “slippage” of the DNA polymerase.
Longer CAG repeats is associated with earlier onset disease.
How does the length of the CAG repeat correlate with disease onset?
Longer CAG repeats is associated with earlier onset disease.
What is a de novo (new) mutation?
Affected proband with no history of disease in family.
In what inheritance pattern are de novo mutations common?
Common in autosomal dominant conditions. If other germ cells do not have the mutation, the risk to their siblings is not increased above the general population risk.
What is germline mosaicism?
Genetic change is only present in some; but not all germline cells.
What unique presentation can germline mosaicism cause regarding siblings and parents?
Can see two siblings with the same autosomal dominant condition without an affected parent.
Why can germline mosaicism never be ruled out with a negative parental genetic test?
Because the mosaicism only exists in gametes!. Rare phenomenon
What is consanguinity?
Relatives share genes that are identical by descent (share a common ancestor).
Mating between related individuals (e.g., cousins).
Consanguinity increases the likelihood of what type of disorder in offspring?
Offspring are more likely to be affected with a recessive disorder because relatives are more likely to carry the same recessive alleles.
Do consanguineous grandparents increase genetic risk for their grandchildren if the parents are unrelated?
No. Grandparent consanguinity alone does not increase risk for grandchildren. Risk only increases if the parents themselves are related (carrier pairing).
Why are small families complicating factors in pedigree evaluation?
Pattern of inheritance may not be evident in small families due to small sample sizes.
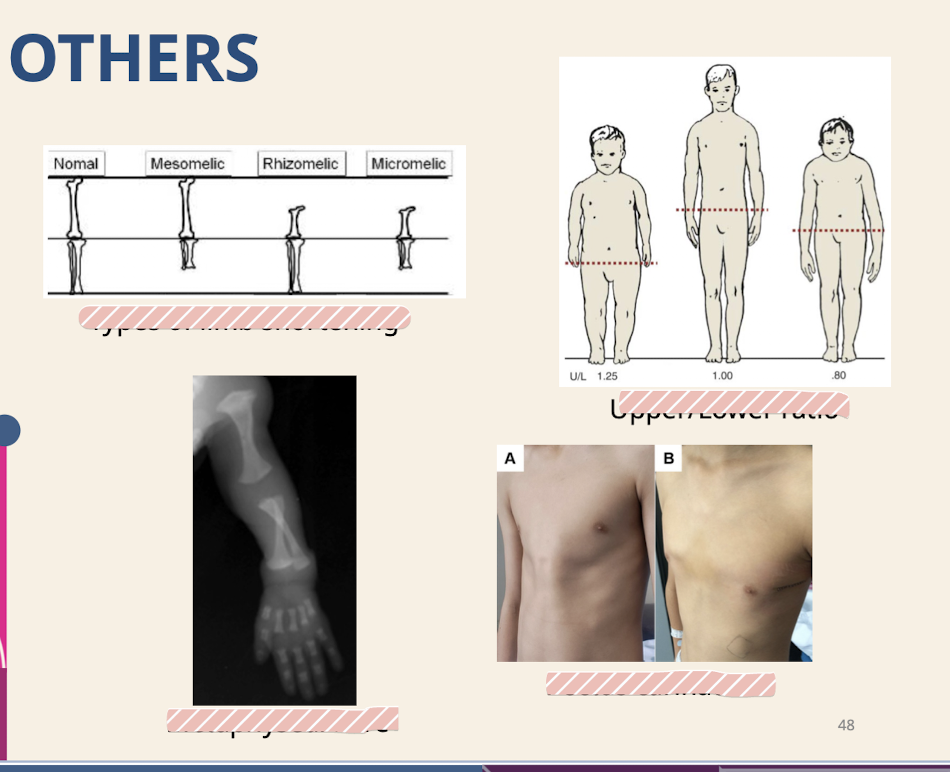
What measurements are part of the basic genetics evaluation?
Height; weight; head circumference; Arm length; leg length; upper:lower body ratio; Eyes; ears; Chest circumference and inter-nipple distance; Palm; finger; foot length.
What are examples of minor malformations evaluated in a genetic physical exam?
Cranial shape; facial features; Mouth; teeth; palatal shape; Neck and chest shape; Extremity (particularly finger and toe) formation; Malformations of genitalia; Skin pigmentation.
What is dysmorphology?
The study and interpretation of patterns of human growth and structural defects.
What is a malformation and what are examples of it?
Structural difference arising from a primary localized error in morphogenesis; Congenital heart defects; polydactyly.
What is a deformation and what is its typical cause?
Alteration in the shape or structure of a body part that has differentiated normally – caused by nondisruptive mechanical forces.
Potter sequence; characterized by recessed chin; short extremities; and contractures secondary to oligohydramnios; is an example of what type of structural defect?
Deformation.
What is a disruption and what is an example of its cause?
Structural defect resulting from the destruction of a body part that has differentiated normally; Missing limb due to amniotic band (fibrous band within the amniotic sac that gets tangled around the developing fetus).
What is a dysplasia?
Abnormal organization of cells into tissue because of a generalized defect in differentiation or growth.
Achondroplasia; characterized by shortened limbs due to abnormal cell signaling during cartilage differentiation; is an example of what type of structural defect?
Dysplasia.
What is a syndrome?
The constellation of findings repeatedly observed in unrelated individuals due to a single underlying cause.
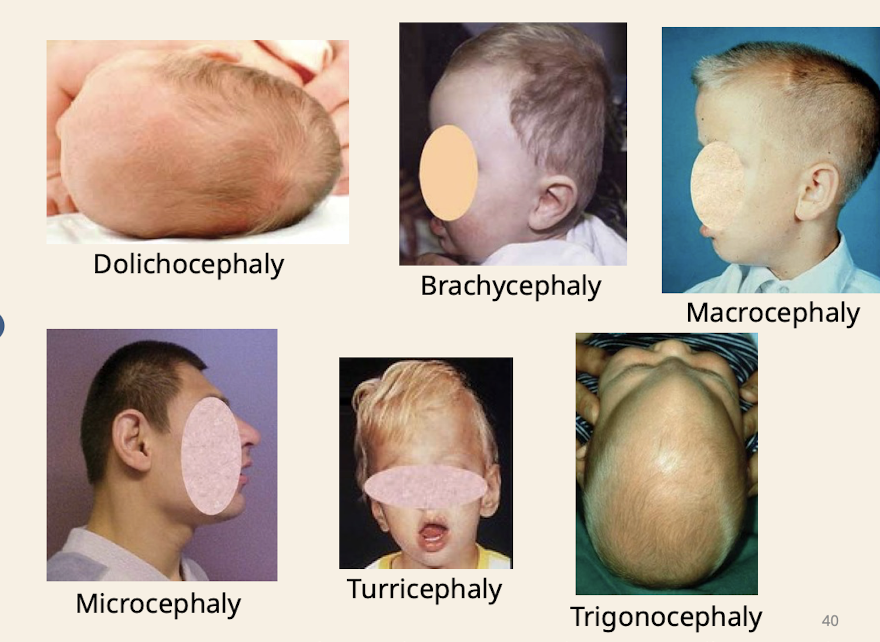
What are common head shapes noted in dysmorphology assessment
Dolichocephaly (increased anterioro posterior length compared to width); Brachycephaly (short head) ; Macrocephaly; Microcephaly; Turricephaly (tall head) trigonocephaly (wedge shaped/triangle shaped head)
.
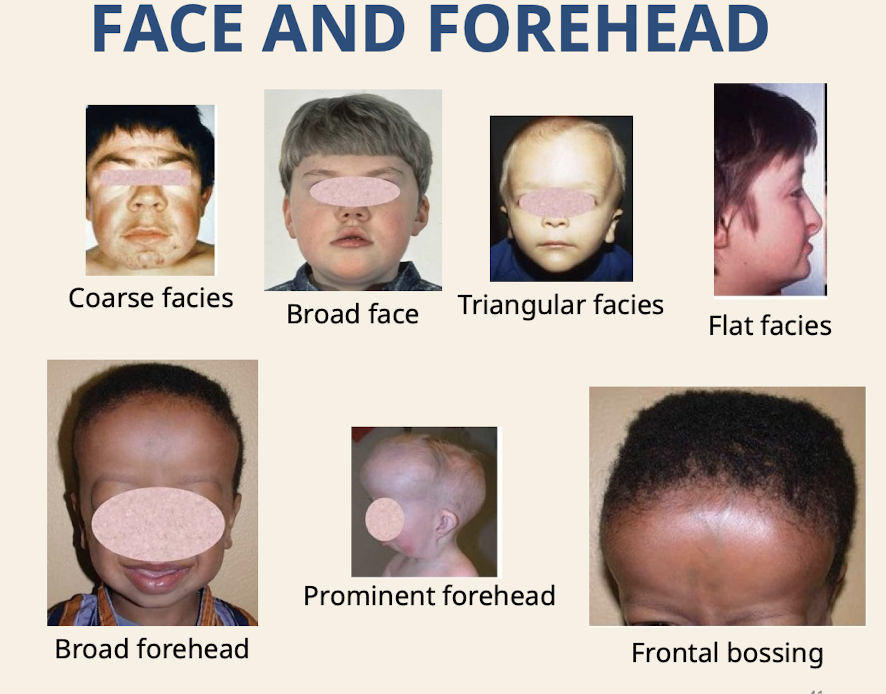
What are common dysmorphologies of the face and forehead
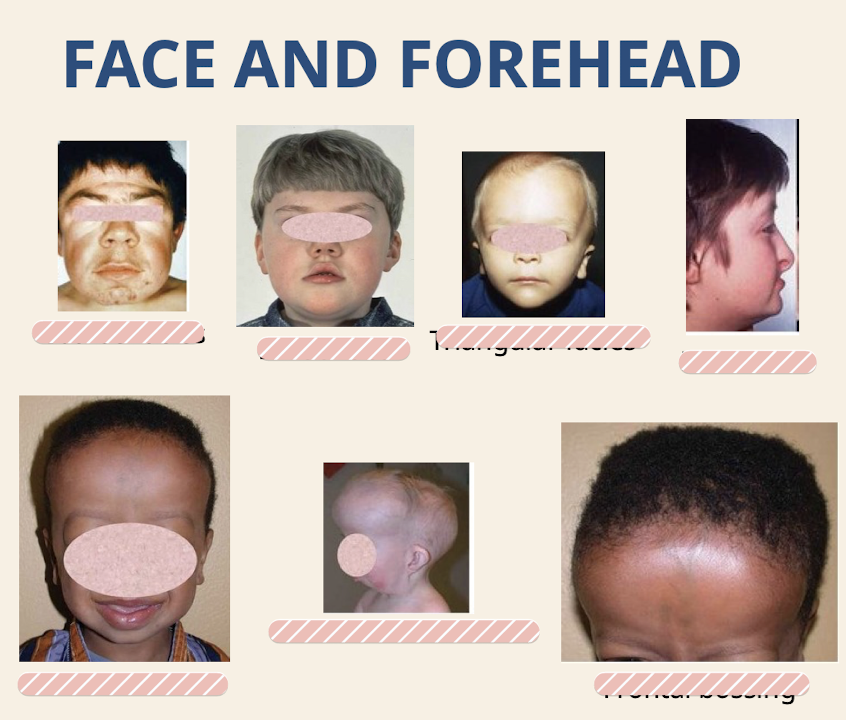
Coarse facies (absence of fine and sharp lines; due to thickened skin) Broad face; Triangular facies (brain growing faster than rest of face); Flat facies; Broad forehead; Prominent forehead; Frontal bossing (lateral frontal bone prominences but spares the center)
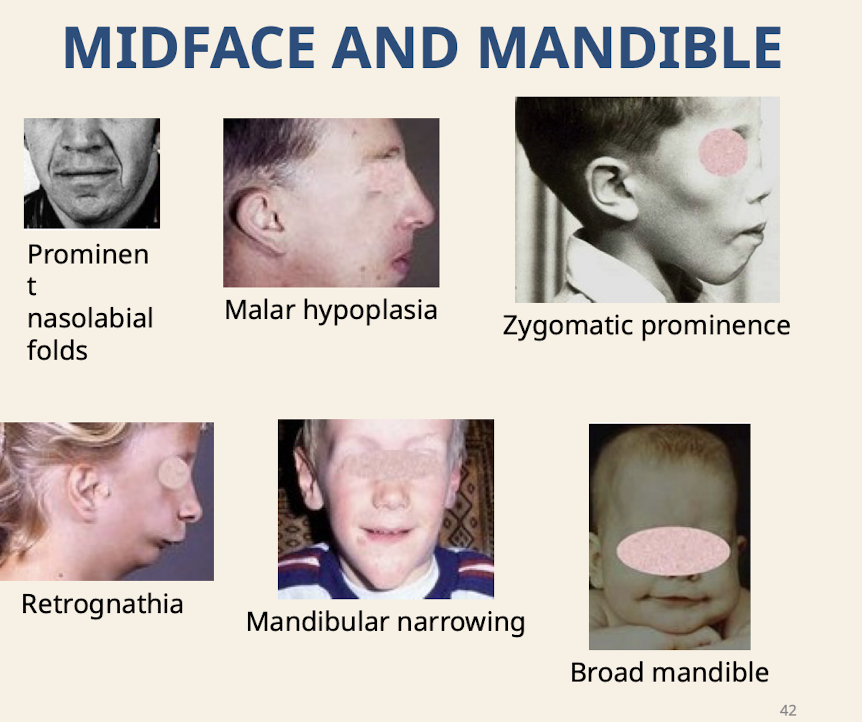
What are common dysmorphologies of the midface and mandible
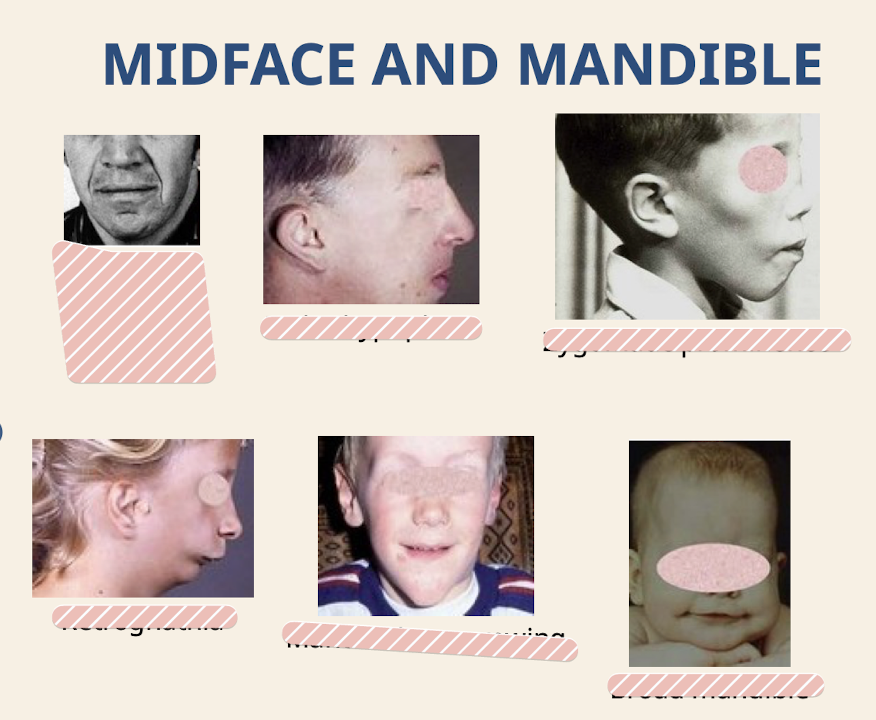
Prominent nasolabial folds; Malar hypoplasia (underdevelopment of malar process of maxilla); Zygomatic prominence; Retrognathia; Mandibular narrowing; Broad mandible
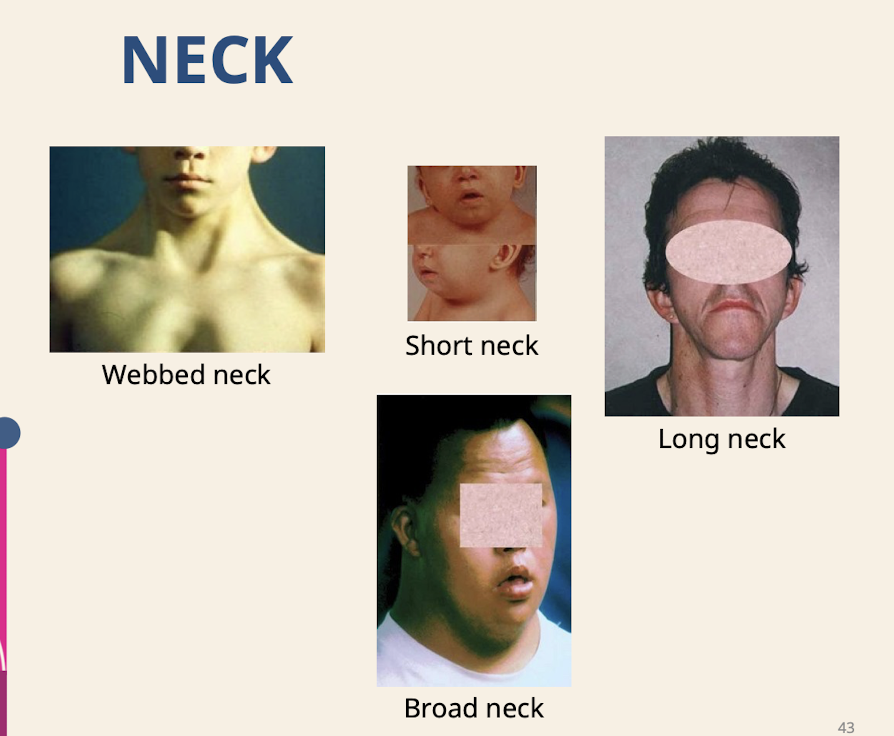
What are common neck dysmorphologies?
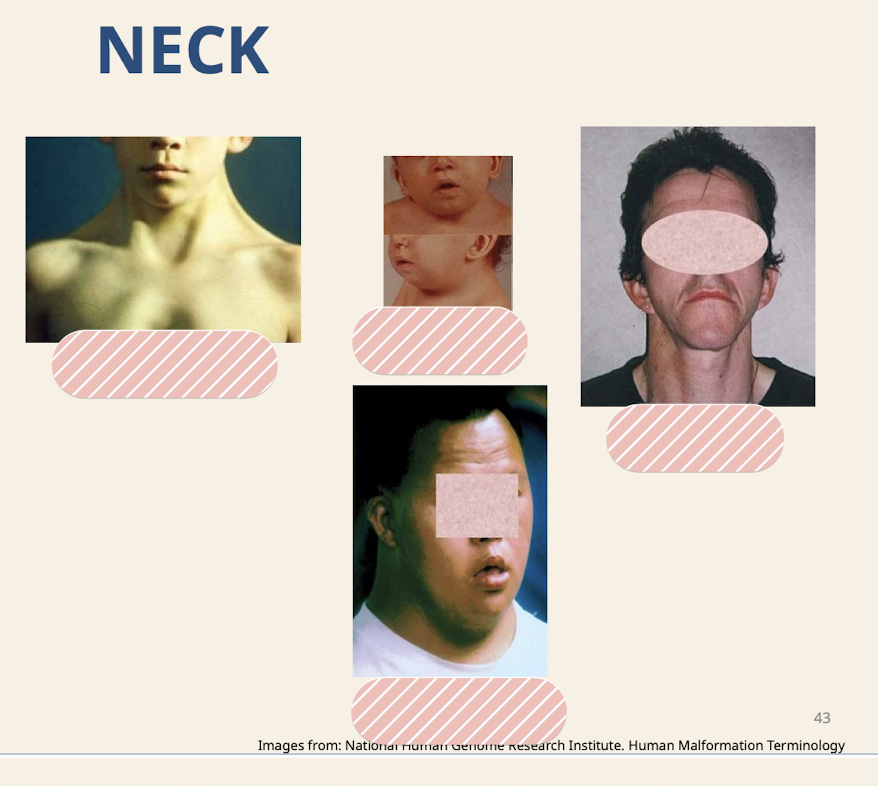
Webbed neck (seen with large nuchal translucency, lymph fluid builds up behind the neck so skin builds up); Short neck; Long neck; Broad neck
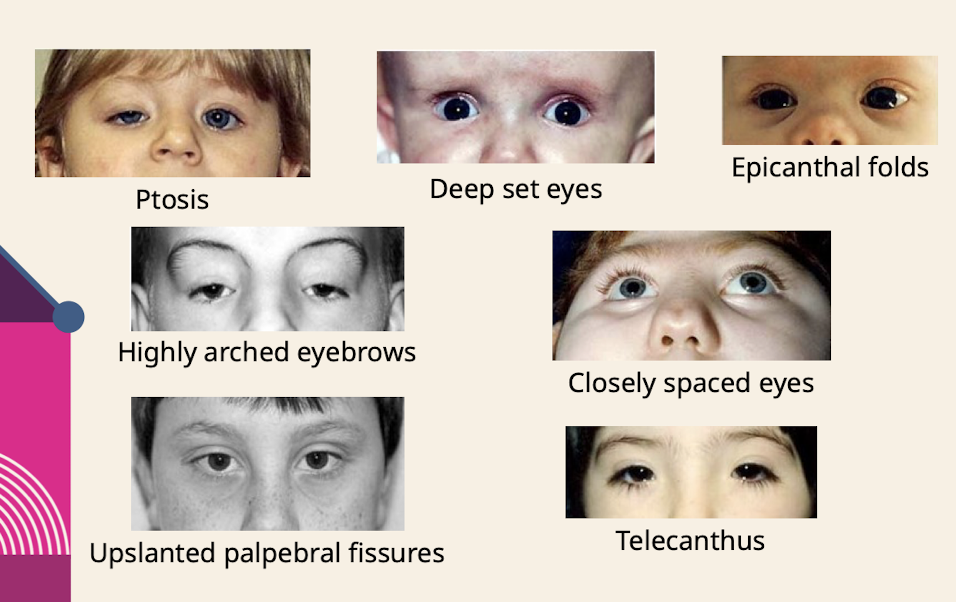
What are common eye dysmorphologies noted in the physical exam
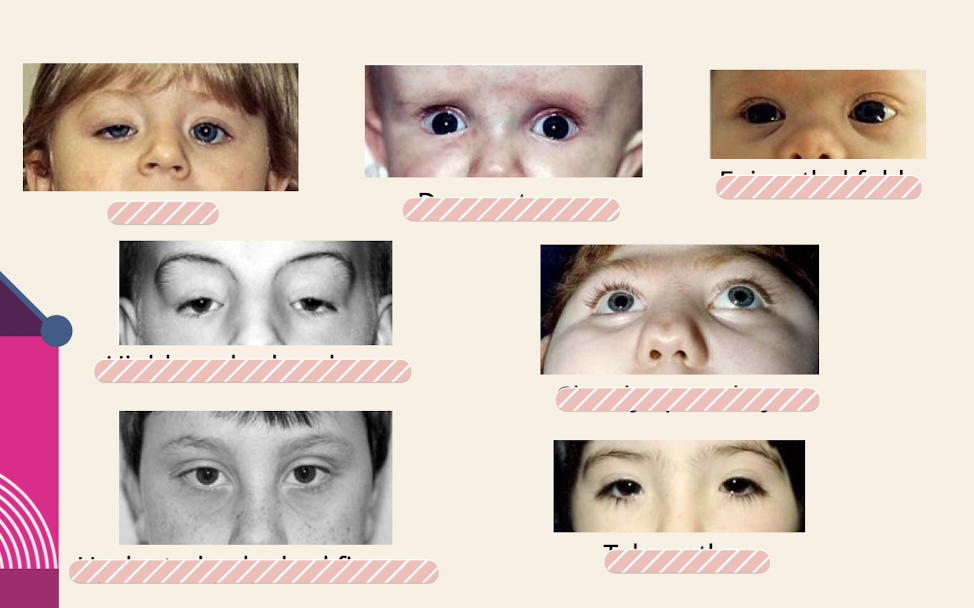
Ptosis; Deep set eyes; Epicanthal folds( skin folds of the upper eyelid that cover the inner corner (medial canthus) of the eye) Highly arched eyebrows; Closely spaced eyes; Upslanted palpebral fissures; Telecanthus (increased distance between the inner corners (medial canthi) of the eyes)
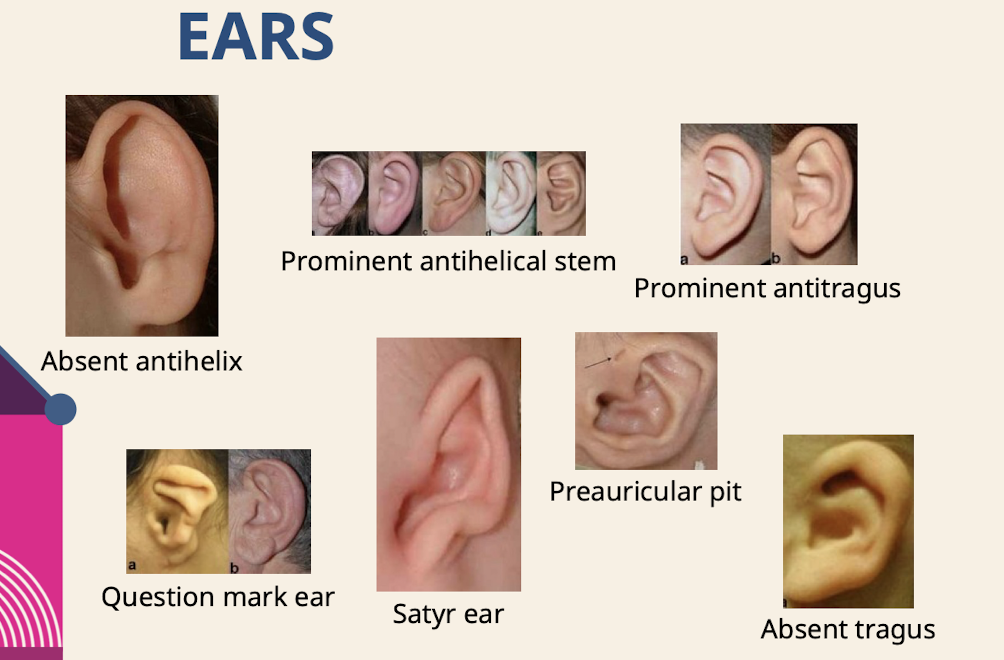
What are common ear dysmorphologies noted in the physical exam
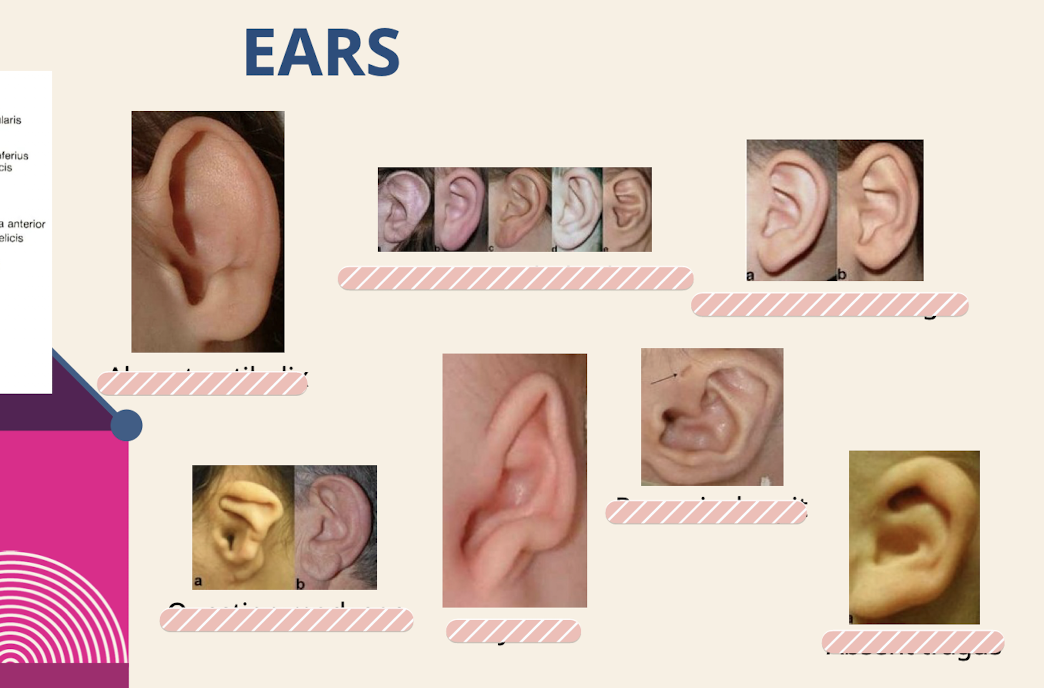
Absent antihelix; Prominent antihelical stem; Prominent antitragus; Question mark ear; Satyr ear; Preauricular pit; Absent tragus
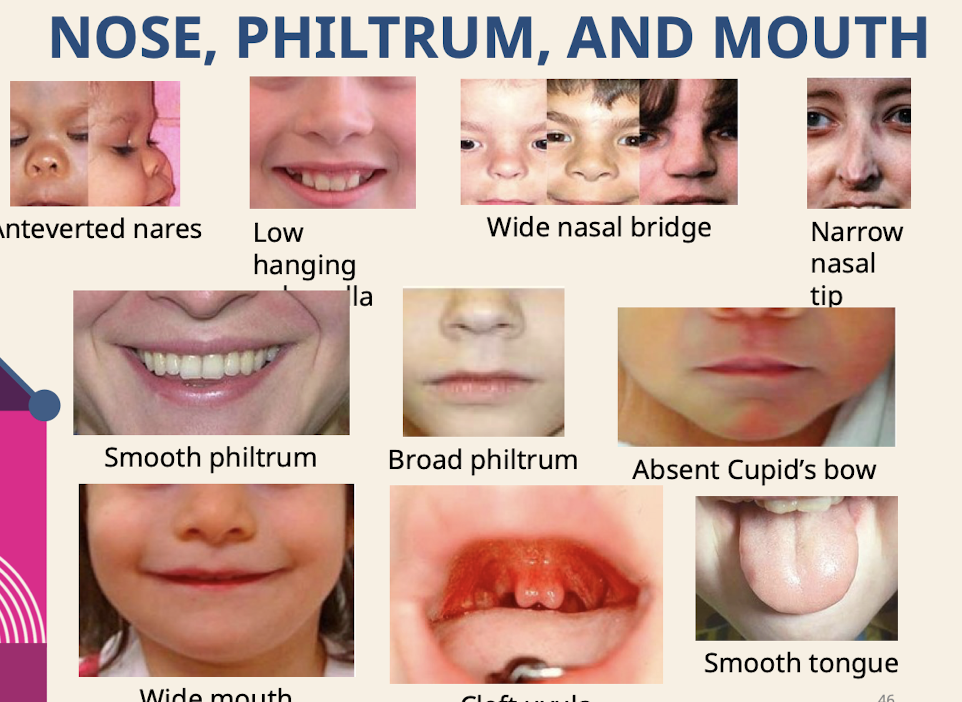
What are common dysmorphologies of the nose; philtrum; and mouth?
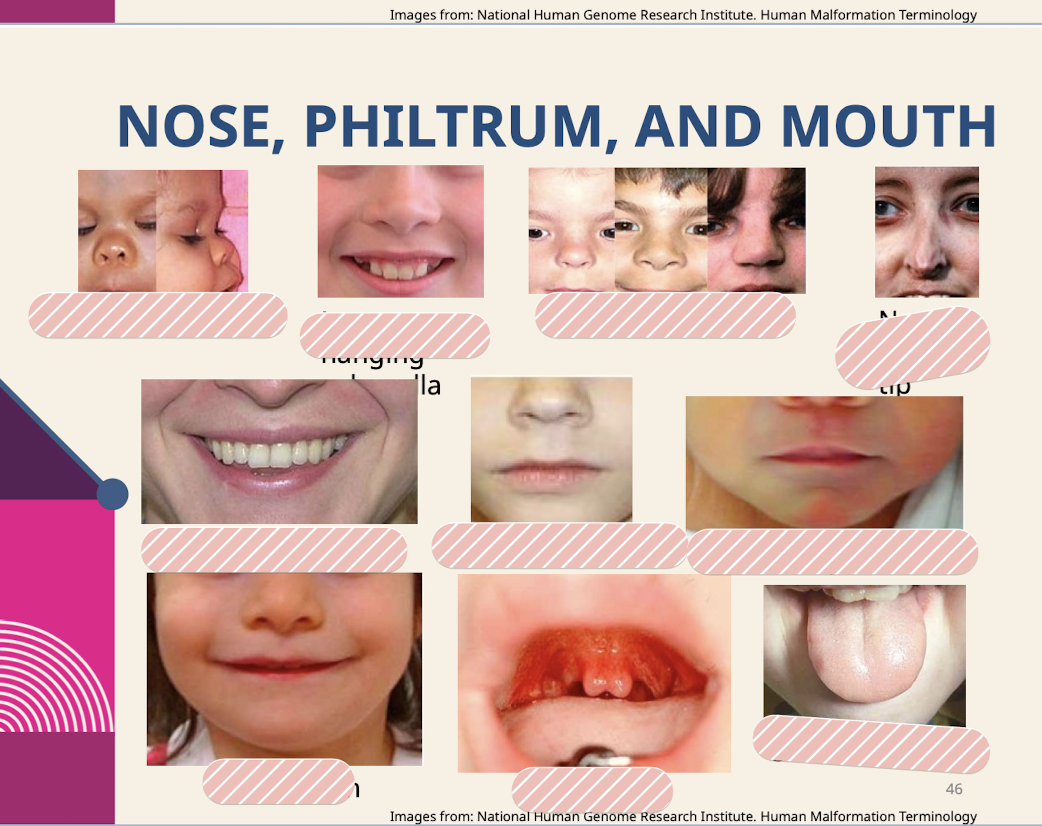
Anteverted nares; Low hanging columella; Wide nasal bridge; Narrow nasal tip; Smooth philtrum; Broad philtrum; Absent Cupid’s bow; Smooth tongue
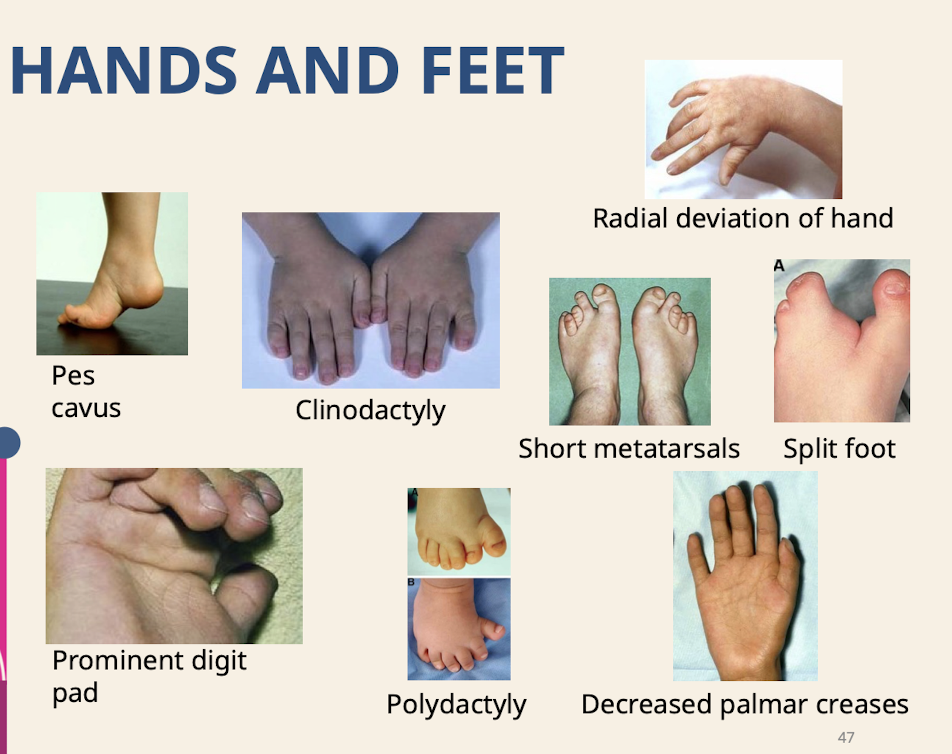
What are common dysmorphologies of the hands and feet
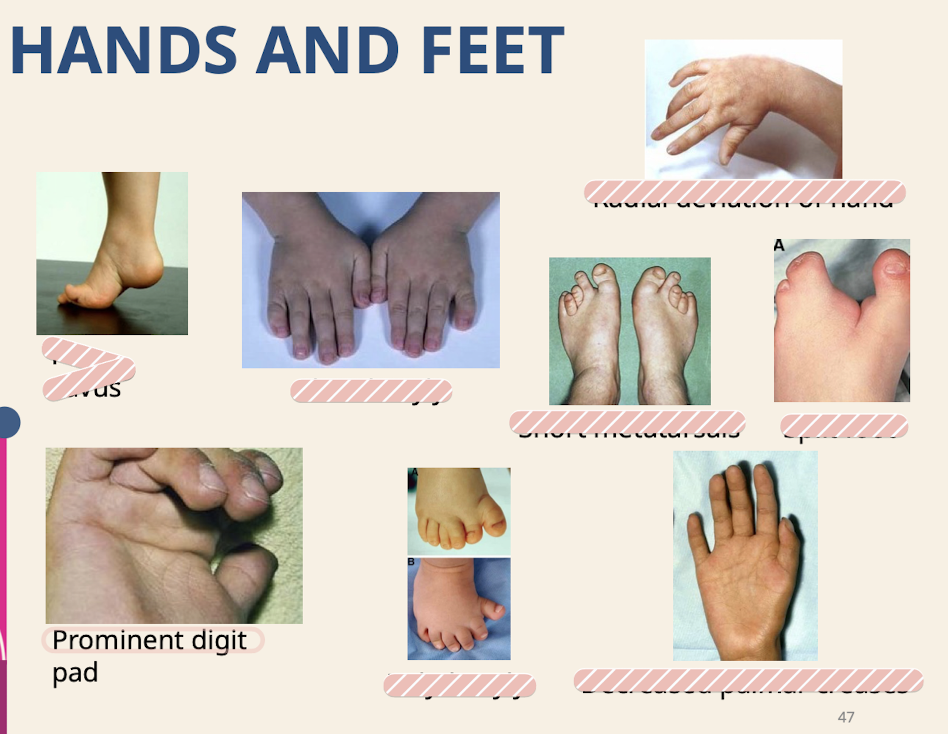
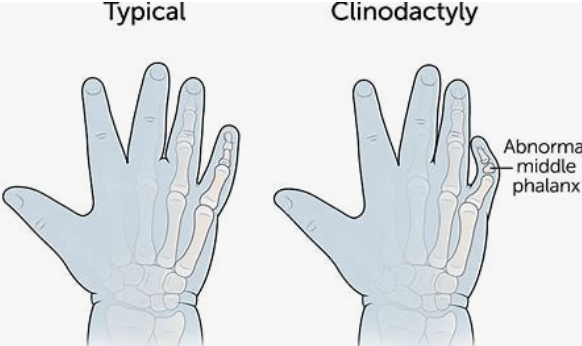
Pes cavus (High-arched foot.) Clinodactyly; Radial deviation of hand; Prominent digit pad; Short metatarsals; Split foot; Decreased palmar creases; Polydactyly
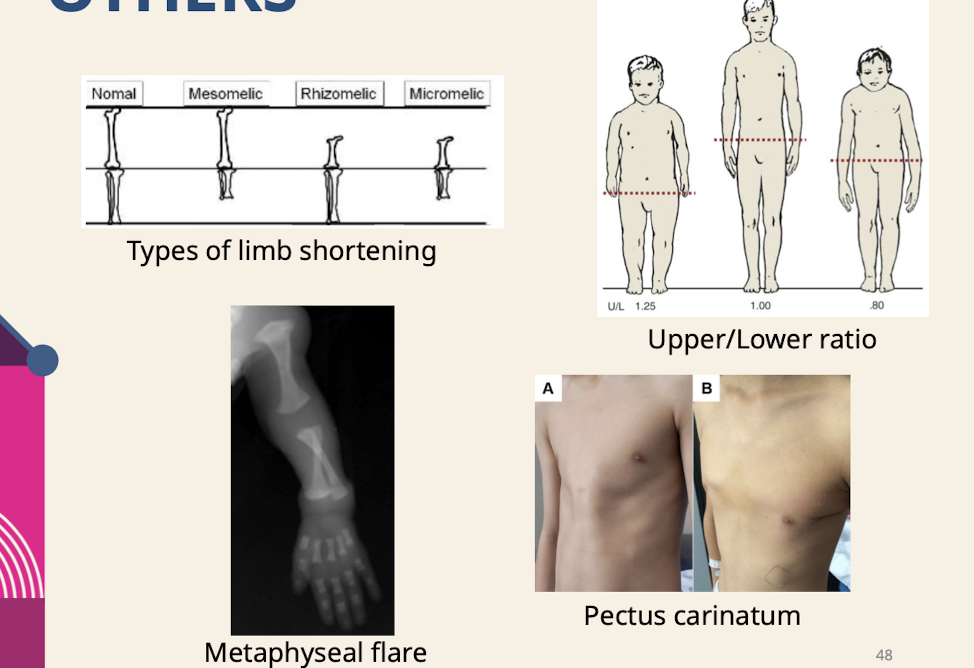
What is the combination of anomalies that may be suggestive of a genetic etiology?
The constellation of both major and minor anomalies.
Why is it important to see pictures of family members when performing dysmorphology?
Noting a minor anomaly that is present in other family members may suggest it is a usual feature of that family.
What detailed history is included in the prenatal and birth history during a genetics assessment?
Teratogen exposure; prenatal screening/testing; gestational age at delivery; method of delivery.
What details are included in the neonatal history during a genetics assessment?
Newborn screening results; neonatal complications.
What details are included in the developmental history during a genetics assessment?
History of developmental delay or regressions.
A patient presents with ptosis; strabismus; hypertelorism (far apart orbits); and low set ears. What is the suspected diagnosis (Case 1)
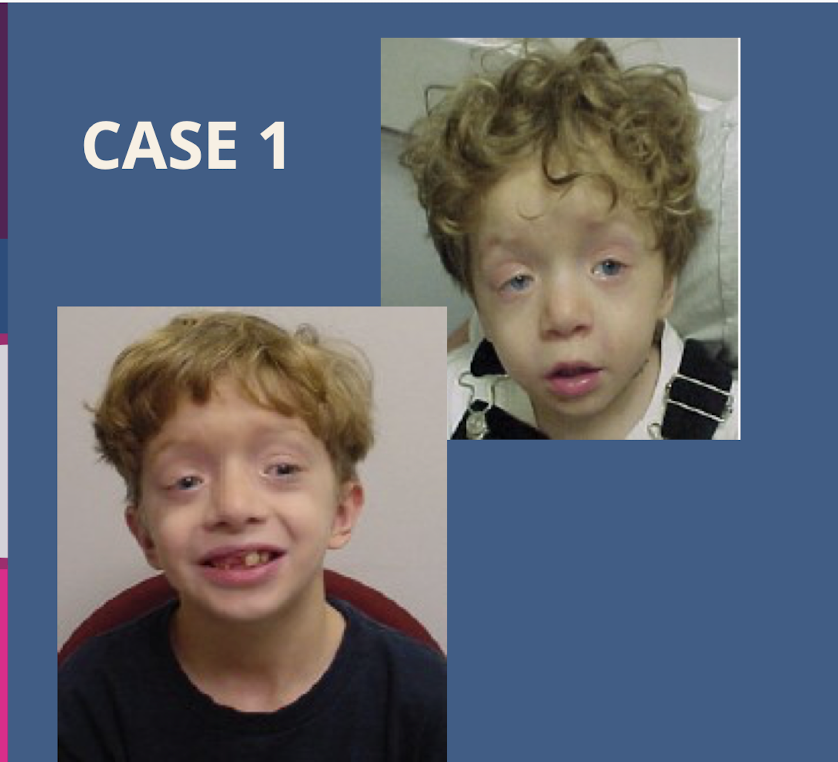
Noonan syndrome.
A patient presents with hooded eyelids; bulbous nose; midface hypoplasia; micrognathia; and overfolded ears. What is the suspected diagnosis (Case 2)

22q11.2 deletion syndrome.
A patient presents with low-set ears; frontal bossing; triangular facies; midface hypoplasia; and blue sclera. What is the suspected diagnosis (Case 3)
Osteogenesis imperfecta (Type III).
Associated with broken bones, really weak bones.
A patient presents with a thin upper lip; smooth philtrum; midface hypoplasia; short palpebral fissures; and low-set nasal bridge. What is the suspected diagnosis?

Fetal alcohol syndrome.
In a pedigree analysis where only males are affected; all through carrier females; and there is no male-to-male transmission; what is the pattern of inheritance?
X-linked.
In a pedigree analysis showing a vertical pattern; equal sex distribution; male-to-male transmission; and every affected child having an affected parent; what is the pattern of inheritance?
Autosomal dominant.
In a pedigree analysis showing vertical transmission and equal sex distribution; but no affected individuals in the first generation and two affected in the second generation; what complicating factor should be suspected?
Autosomal dominant with germline mosaicism.
Germline (gonadal) mosaicism: Parent’s somatic cells are normal, but some gametes carry the mutation → multiple affected children despite unaffected parent.
If a pedigree analysis shows nearly all affected individuals are male and all affected males are related through carrier females; what is the pattern of inheritance?
X-linked.
What is the cornerstone of genetic disease?
Mendelian inheritance is the cornerstone of genetic disease; but there are exceptions.
In the context of genetic disease; what pattern of inheritance might exceptions sometimes lean toward being "the rule"?
Particularly multifactorial inheritance.
Regarding dysmorphology; what should clinicians focus on rather than finding one or two minor anomalies?
It is less about finding one or two dysmorphisms and more about seeing the forest for the trees.