phenolics
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
97 Terms
do phenolics have a nutrional value
no
where are phenolics mainly present
mainly present in raw materials from plant origin
what do phenolics do in plants
Phenolics serve diverse biological functions in plants, such as protecting the plant against insects and UV-light, and attracting pollinators
give the definition for phenolic compound
A molecule that contains at least one aromatic ring with one or more hydroxyl-groups
give examples of phenolic rich foods
Coffee
Tea
Cocoa
Berries
Grapes
Wine and juices
Herbs and spices (u would need to eat a lot tho)-(dried oregano highest)
why is the term phenolic compounds or phenolics preffered over polyphenols
1. The prefix "poly" suggests multiple hydroxyl groups. However, many common phenolics found in nature, such as p-coumaric acid and p-hydroxybenzoic acid, are monophenols, possessing only one hydroxyl-group on an aromatic ring.
2. The term is inconsistent: some scientists restrict "polyphenols" only to the polymeric forms, while others use it broadly for monomeric, oligomeric, and polymeric forms.
• Using "phenolic compounds" or "phenolics" helps to avoid this ambiguity.
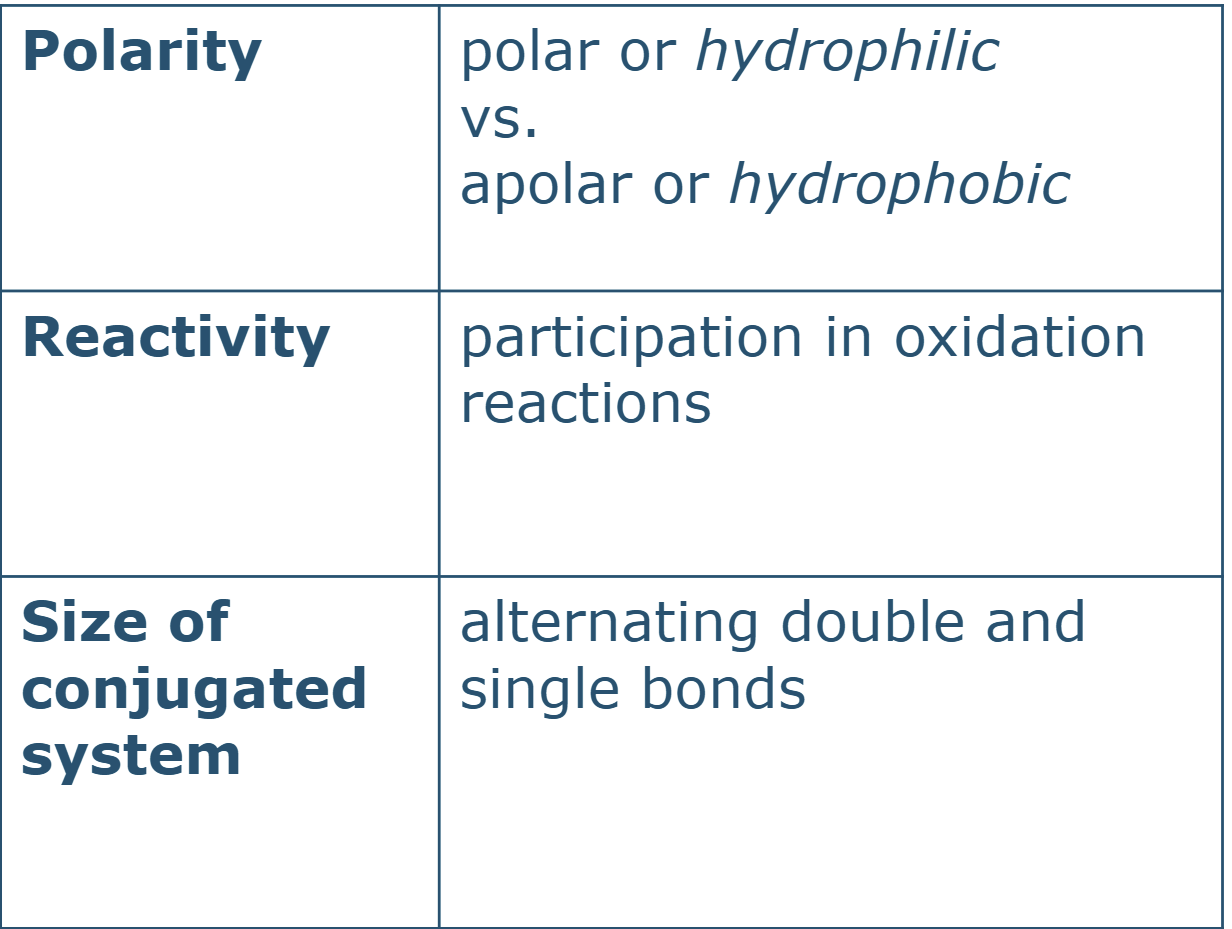
what are the 3 structural variations in phenolics
polarity
reactivity
size of conjugated system
what kind of polarity do phenolics usually have
Phenolics are typically medium polar
→ limited water solubility
what does a large conjugated system mean
Large conjugated system: aborb visible light and leads to observed colour
how many double bonds needed to see colour
Rule of thumb:
Conjugated system < 8 bonds = no colour
>8 =colour
what are the 6 structural features typically present in phenolics
hydroxylation
methylation
glycosylation
carboxylation
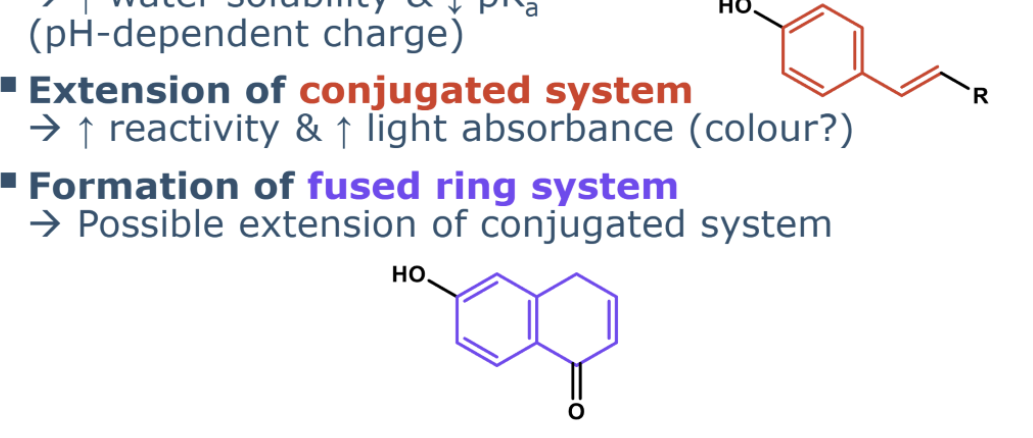
extension of conjugated system
formation fused ring system
describe hydroxylation
adding OH group
increase in polarity
increases reactivity (higher susceptibility to oxidation/higher antioxidant capacity)
higher water solubility
higher oxidation
higher antioxidant act
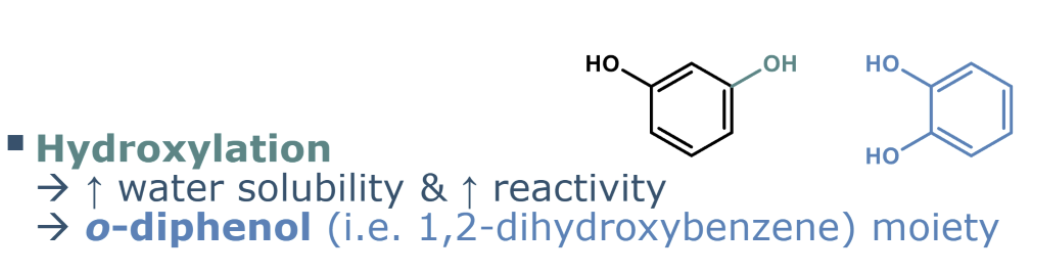
what is o-diphenol susceptible to
The o-diphenol structure (1,2-dihydroxybenzene) is especially susceptible to oxidation.
O- ortho position , 2 oh groups next to eachother on aromatic ring
describe methylation
adding CH3 to the OH
reduces the polarity
reduces reactivity
reduces water solubility
redcues oxidation
reduces antioxidant act

describe glycosylation
adding a saccharide
increases polarity…. increases water solubilty
reduces reactivity….reduces oxidation, reuces antiocidant act

describe carboxylation
Adding COOH
increases polarity.., increases water solubility
lowers pka….lowers food ph
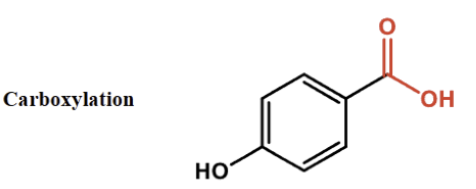
describe extension of conjugugated system
(e.g., via alkenes or fused rings)
Increases light absorbance. I
increases reactivity due to more possibilities for resonance structures.
increased oxidation
increased antioxidant act
Leads to color if the conjugated system becomes long enough (typically ≥ eight conjugated double bonds). Important for antioxidant activity.
what do phenolic compounds occur as in nature
glycosides
Phenolics as glycosides in plants
→ better water-soluble and less reactive
This combination of increased solubility and reduced reactivity makes glycosides easier for the plant to store
what are the structures of the six main classes of monomeric phenolicsc
1. Simple phenolics
2. Hydroxybenzoic acids
3. Hydroxycinnamic acids
4. Hydroxycinnamyl alcohols
5. Stilbenoids
6.flavanoids
hydroxycinnamic acids and hydroxycinnamyl alcohols serve as
nature’s building blocks.
Esterification-hydroxycinnamoyl esters
Amidation- phenolamides
Oxidative coupling- form di-, oligo-, and polymeric phenolics, such as lignins and lignans
Reduction- forming hydroxycinnamyl alcohols)

monomeric phenolics can be converted to di-, oligo-, and polymeric phenolic compounds via
oxidative coupling
This process is often initiated by the enzyme polyphenoloxidase (PPO), which converts phenolics to highly reactive o-quinones.
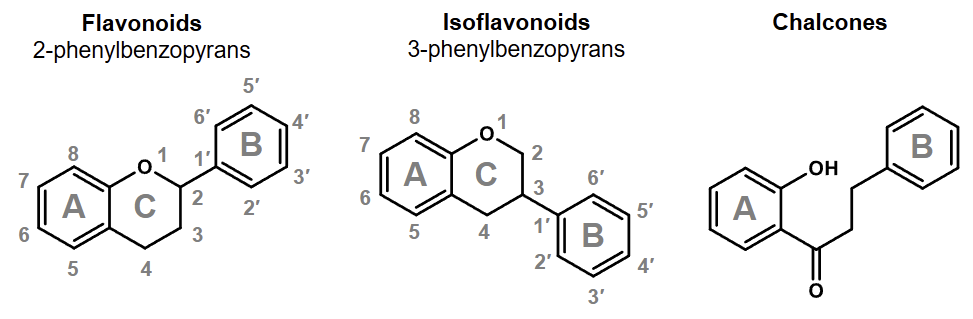
what does the term flavonoids refer to
isoflavnoids (2nd )
flavanoids (3 rd)
may include chalcones
give 4 examples of main classes of di-, oligo-, and polymeric phenolic compounds
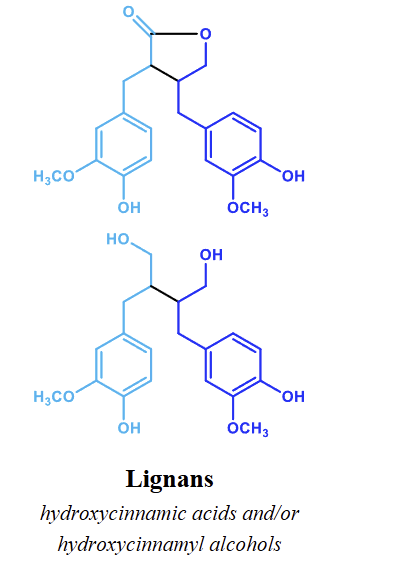
● Lignans
● Lignins
● Condensed tannins
● Hydrolysable tannins
how are lignans made
Hydroxycinnamic acids and/or hydroxycinnamyl alcohols.
how is lignin made
Primarily hydroxycinnamyl alcohols (monolignols)
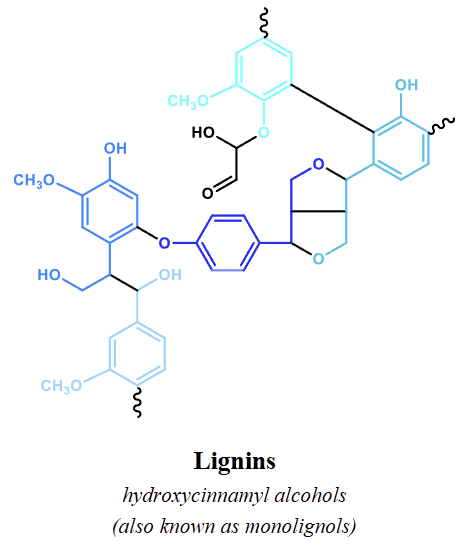
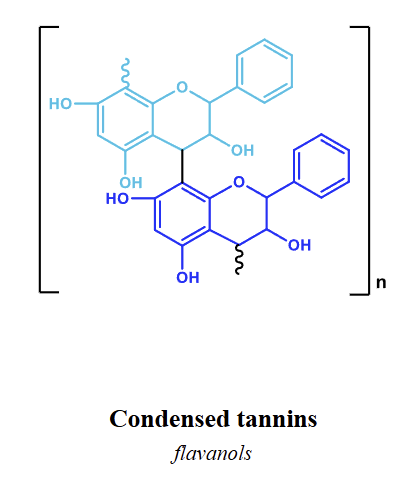
how are condensed tannins made
Flavanols
how are hydrolysabe tannins made
Gallic acid or derivatives (Glycosidic core) + a central monosaccharide
Explain how condensed tannins and hydrolysable tannins can influence food properties
Tannis: Oligo- and polymeric phenolic compounds that strongly interact with proteins
The interaction of tannins with proteins is not restricted to animal skin proteins: All types of proteins, including food proteins, can interact with tannins. This interaction can influence food properties in various ways, including:
1. The formation of insoluble particles, which leads to turbidity of products (see §4.8);
2. The inhibition of enzymatic reactions by the inactivation of enzymes;
3. As a result of (2), diminished digestibility of vegetable raw materials, due to inhibition of digestive
enzymes;
4. Astringency, a dry, puckering mouthfeel
where does phenolic compounds antioxidant activity originate from
Their antioxidant activity originates from their ability to act as reducing agents (e.g. by scavenging radicals) and as metal chelators (i.e. they can bind metals, preventing those metals form acting as pro-oxidants).
consumption of antioxidants has beneficial effects for?
consumption of antioxidants has beneficial effects for human health, for instance, by lowering the risk for cardiovascular disease and cancer
what are the 2 mechanims phenolics exibit antioxidant activity
Reducing oxidised compounds
→ e.g. by radical scavenging
Chelating metals
= binding metal ions
Phenolic compounds are able to act as antioxidants due to their main structural feature
hydroxyl-group(s)
attached to an aromatic ring
describe radical scavenging
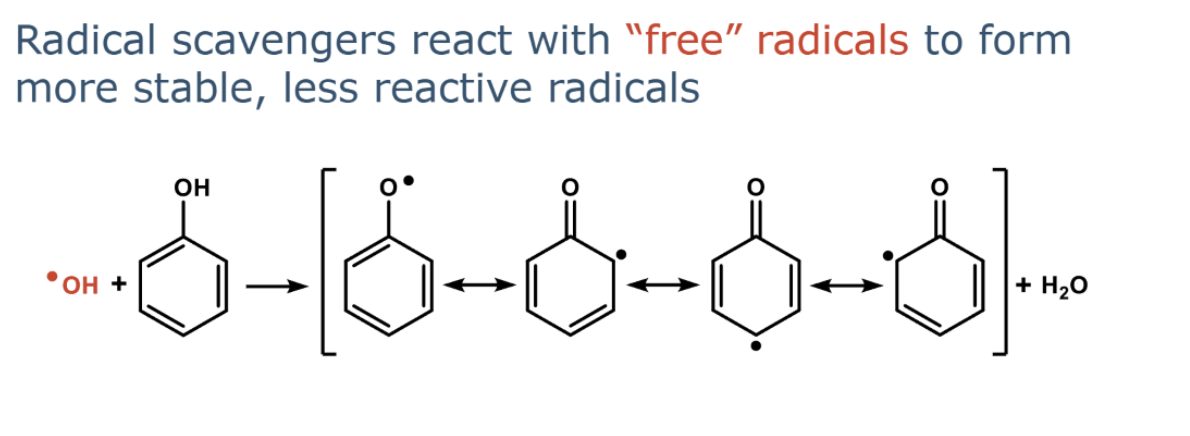
are resonance stabilised radicals more or less reactive
Resonance stabilised radicals are much less reactive than non-stabilised radicals
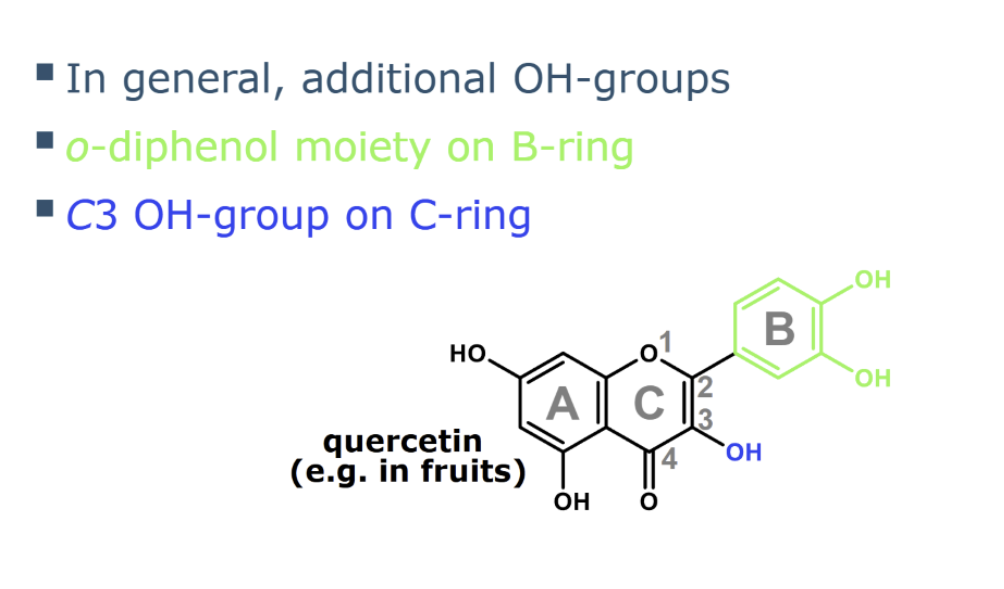
what are desirable structural features for radical scavenging
is chelation direct or indirect antioxidant acitivty
inidrect
are bound metal ions less or more likely to participate in redox reactions
Bound metal ions are less likely to participate in redox reactions, thus metal
chelation effectively leads to a more stable food product
what is a strong metal binding site for flavanoids
o-diphenol moiety on the B-ring of flavonoids is one of the most important structural features, as it
is a strong metal binding site
what are desirable structural features for metal chelation
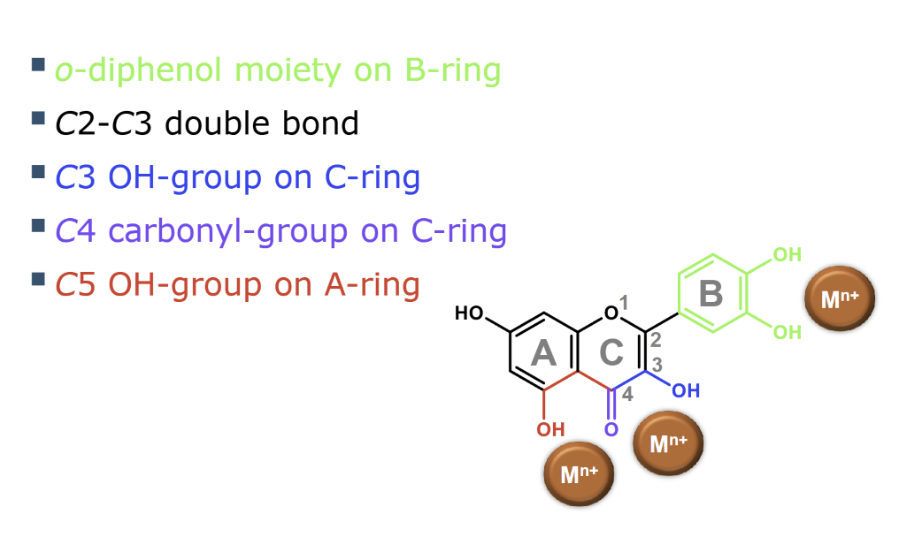
what enzyme catalyses oxidation of phenolic compounds
PPO
Polyphenoloxidases
▪ Phenolics as substrates
▪ Oxygen as electron acceptor
▪ Two copper ions in active site
why does oxidation only take place in damged cells
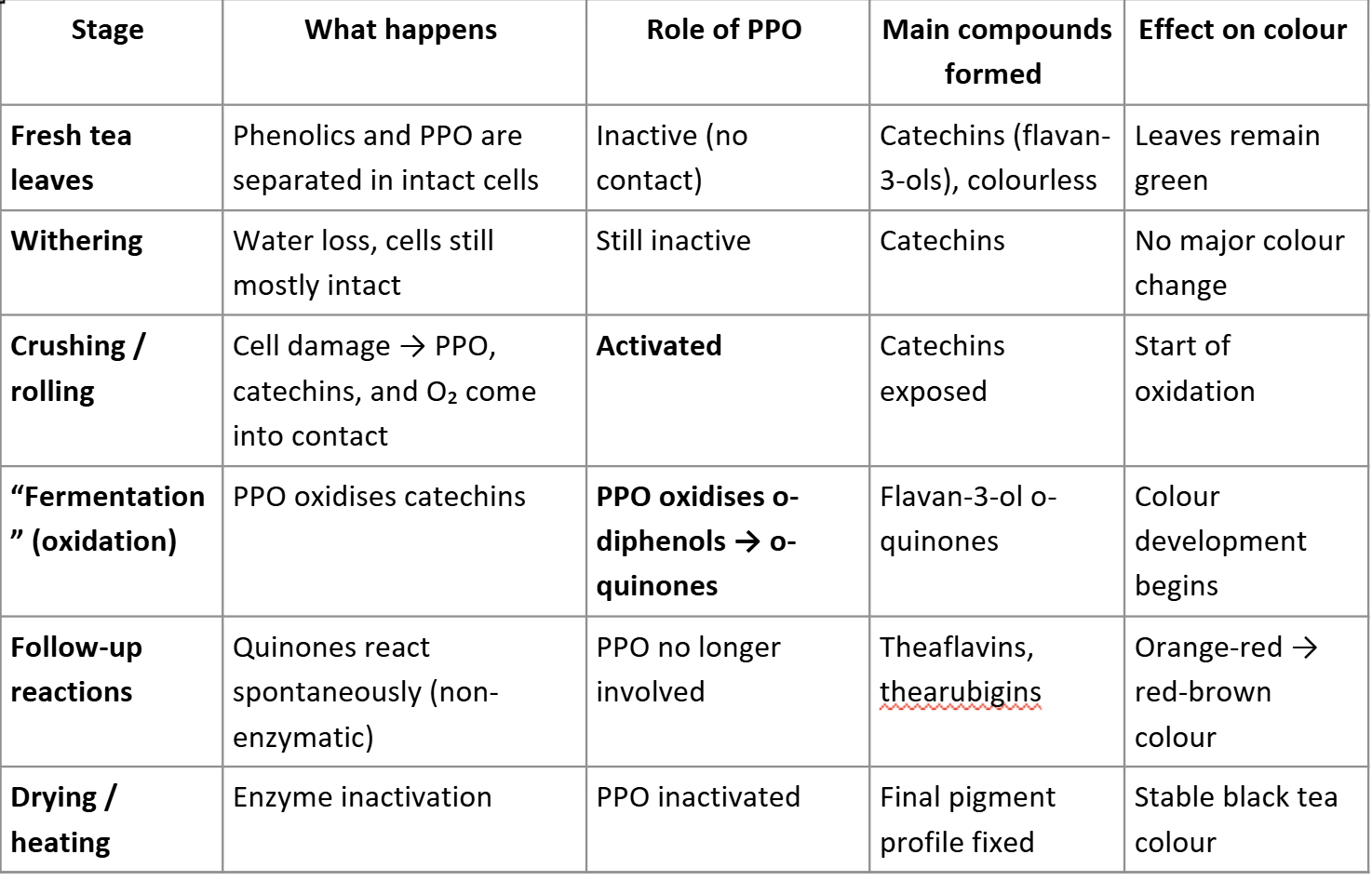
In intact plant tissue, enzymatic oxidation by PPO does not take place because phenolic compounds and PPO are not in contact with each other, they are present in different compartments in the plant cells.
If the plant tissue is
damaged during harvesting or processing (e.g. cutting), enzymatic oxidation can take place because PPO, phenolics, and oxygen (from air) all come into contact with each other.
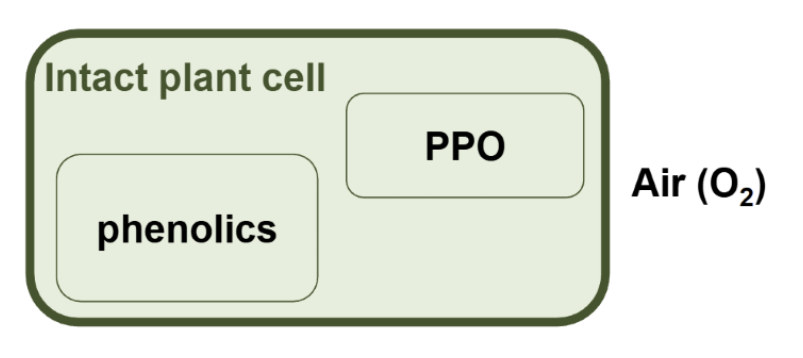
what does PPO do
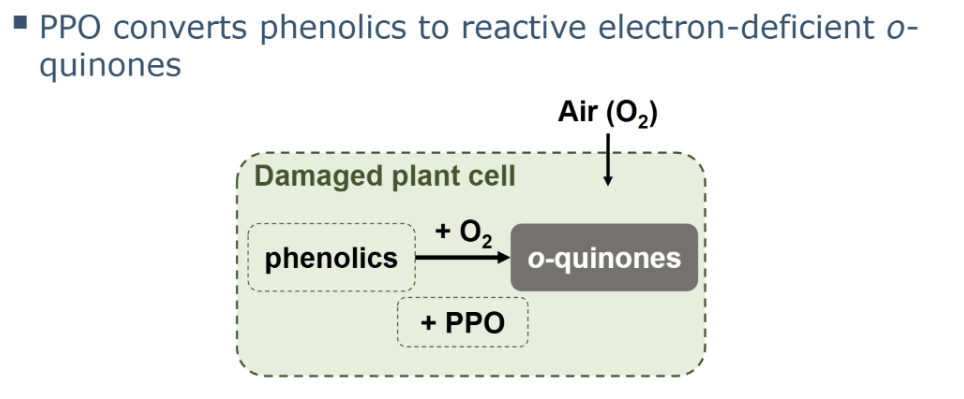
o-diphenol moieties importance in oxidation.
Why important for oxidation: o-diphenols are excellent PPO substrates and are easily oxidized to o-quinones
in which step is PPO involved in
It is important to remember that only the first step (i.e. the initial enzymatic reaction) is in fact an enzymatic
reaction that is catalysed by PPO. The formed o-quinones are highly reactive and will quickly react in spontaneous
follow-up reactions, without further involvement of PPO
what kind of follow up reactions are there for o-diphenol
1.Quinones react with phenolics: Formation of phenolic dimers and phenolic oligomers= orange brown pigments ,Interaction with proteins. formation of phenolic polymers= insoluble brown pigments
quinones react with other food molecules: changes in flavour ,colour and appearance
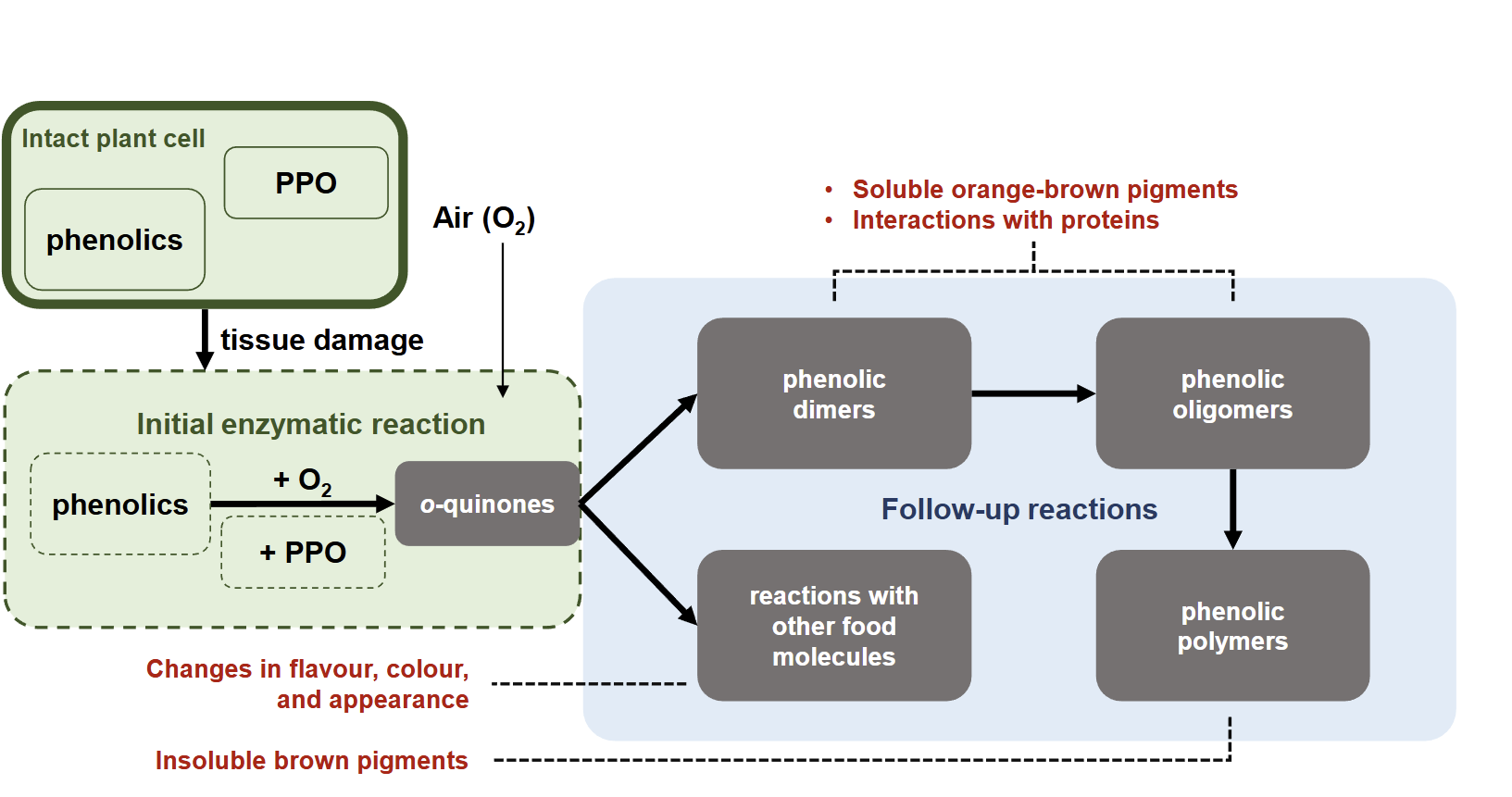
give an example of the effect of o-quinone in food
Enzymatic browning
what is a quinone
A quinone is a class of organic compounds derived from aromatic rings (like benzene) with two carbonyl (C=O) groups in a conjugated cyclic structure
role of cresolase and catecholase activity in oxidation by PPOs
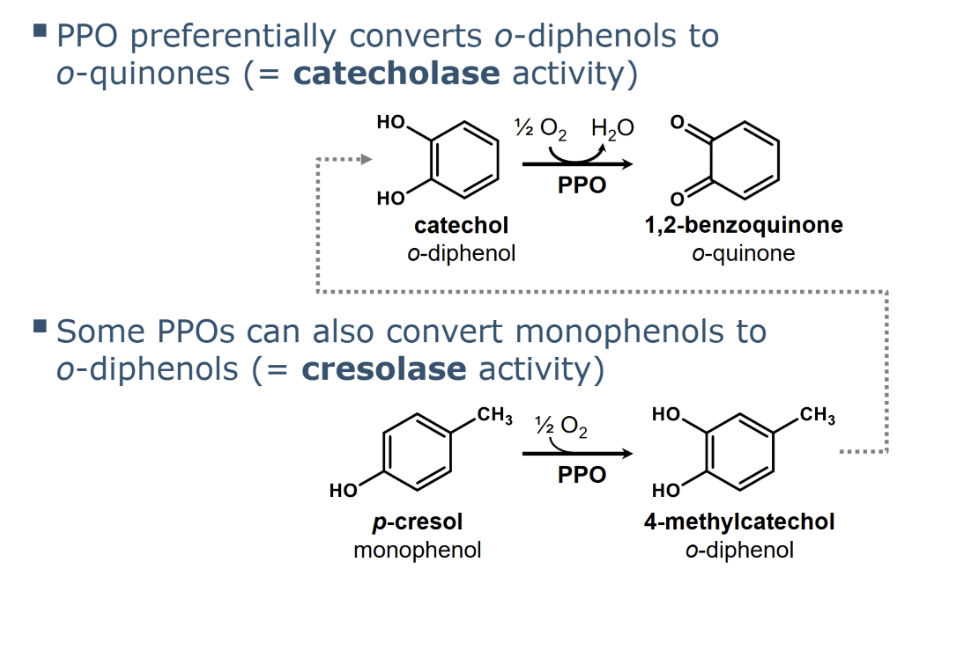
cresolase activity
Some PPOs can convert monophenols to o-diphenols
via hydroxylation. In this reaction, the PPO utilises ½ mole of O2 to perform addition of a hydroxyl-group to the
aromatic ring at the position ortho to the first hydroxyl-group
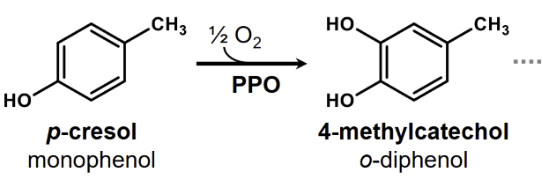
do all PPOs have cresolase activity
However, not all PPOs have cresolase activity, some only possess
catecholase activity, which is discussed in the next section. PPOs that don’t have cresolase activity cannot
oxidise monophenols to o-quinones
do all PPO have catecheoase activity
All PPOs possess catecholase activity, which is the main reaction catalysed by PPOs
describe speed of catecholase and cresolase
Cresolase typicsally slow
while catecholase fast
monophenols brown much
more slowly than o-diphenols, because?
monophenols brown much
more slowly than o-diphenols, because hydroxylation is the rate limiting step
which factors influence the formation of o-quinones by PPO.
what are the 2 types of phenolic protein reactions
covalent and non covalent
describe covalent and non covalent protein phenolic formation
Covalent----> irreversable: formation of protein phenolic conjugates
Non covalent---> reversible: formation of protein phenolic complexes
Explain how covalent protein-phenolic conjugates are formed
reaction of electron-deficient o-quinones with nucleophilic
groups.
Typical nucleophilic groups that may be present in food molecules like proteins and peptides are amine
(–NH2) or thiol (–SH) groups. Amine and thiol groups are found in proteins and peptides as parts of the side
chains of amino acid residues (e.g. cysteine has a thiol group, lysine has an amine group).

are thiols are amines stringer nucleophiles
Thiols are much stronger nucleophiles than amines. Thus thiols are much more reactive towards o-quinones, so they react much faster
Upon further oxidation of the protein-bound o-diphenol
protein-bound o-quinones are formed. This can lead to reactions that form various types of cross-links between proteins
protein-bound o-quinone reacts with a non-oxidised phenolic, this can potentially lead to
browning of the protein via
similar reactions to those that occur in enzymatic browning of phenolics
Explain the interactions that lead to formation of non-covalent protein-phenolic complexes.
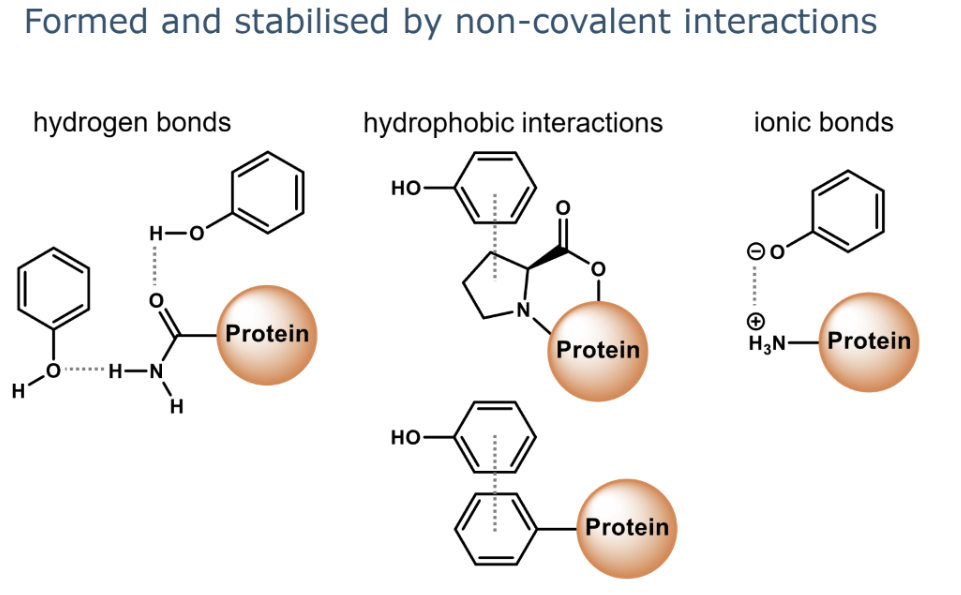
for protein phenolic complexes what factors does it depend on
1.protein structure and characteristics: type of side chains,he accessibility of reactive amino acid side chains
2.Phenolic compound structure and characteristics: including the hydrophobicity, solubility, andmolecular size.
3.The pH of the food product or ingredient determines the charge of the proteins and the phenolics.
do monomeric phenolic compounds form string protein phenol complexes
Monomeric phenolic compounds generally do not form strong protein-phenolic
complexes or stable cross-links between proteins.
do polymeric phenolic compounds that consist
of more than 10 to 15 monomeric subunits form strong phenolic protein complexes
polymeric phenolic compounds that consist
of more than 10 to 15 monomeric subunits can also not form stable complexes with proteins anymore, due to the fact that these molecules are poorly water-soluble, too large, and too rigid to approach proteins in solution
protein-phenolic complexation is maximal around
In general, protein-phenolic complexation is maximal around the iso-electric
point of the proteins.
for protein phenolic complexes what promotes ionic bonds vs hydrophobic
at neutral or alkaline pH carboxylic acid groups are dissociated and thus negatively
charged, enabling formation ionic bonds.
Phenolic compounds that are not charged are more hydrophobic, which promotes hydrophobic interactions.
phenolic/protein ratio influences aggregation and precipitation.
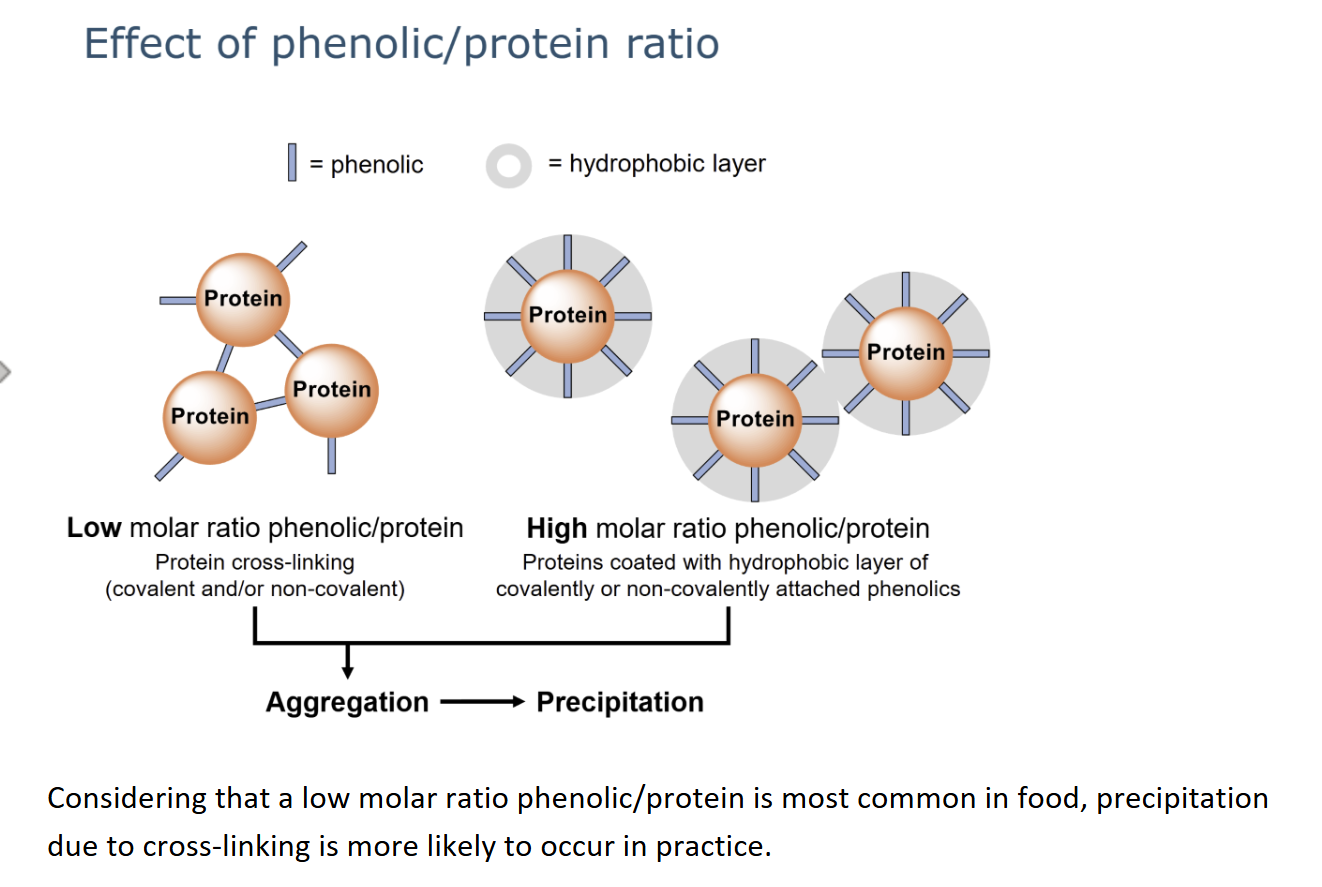
the methods that can be used to prevent or reduce protein-phenolic interactions
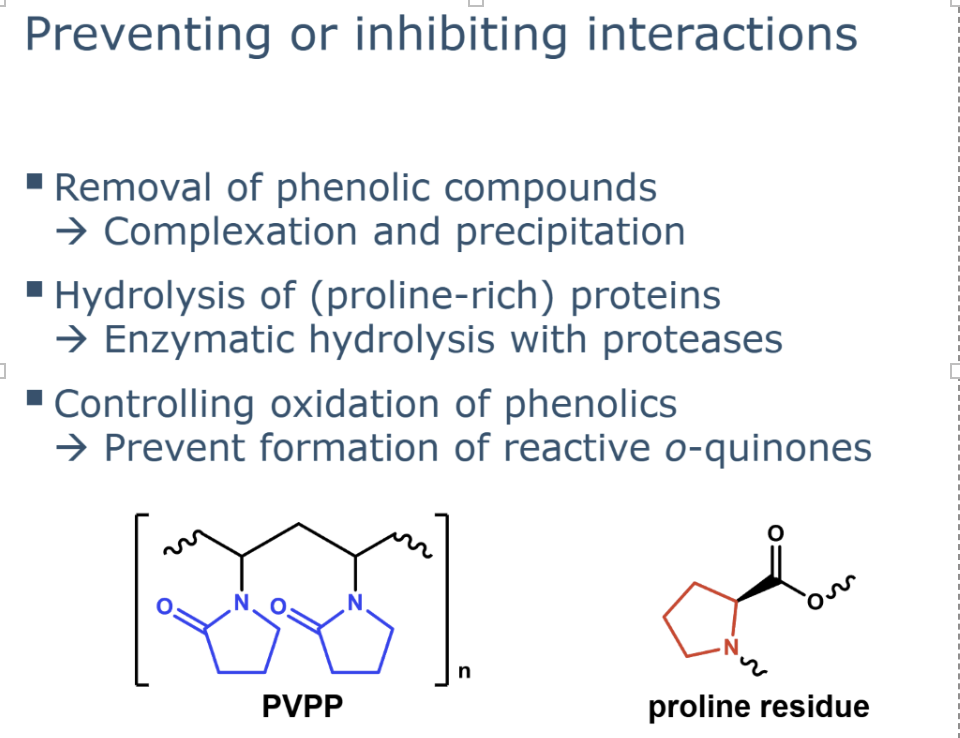
how can u remove phenolic compounds
Principle
Mainly di- and oligomeric phenolics form strong complexes with proteins.
Proline-rich proteins (e.g. gelatin) bind phenolics via hydrophobic interactions, caused by stacking of aromatic rings of phenolics with pyrrolidine rings of proline residues.
Practice
Gelatin is added to bind phenolics, causing aggregation and precipitation, which is then removed by filtration or sedimentation.
Alternatively, protein-analogues are used, most commonly polyvinylpolypyrrolidone (PVPP).
PVPP contains pyrrolidone side chains that resemble proline, giving it a high capacity to bind phenolics.
Result
Removal of oligomeric phenolics strongly reduces later protein–phenolic complex formation during processing, storage, or consumption.
describe hydrolysis of proline rich proteins
Principle
Enzymatic hydrolysis reduces protein molecular size and disrupts hydrophobic regions.
Practice
Proteases are used, especially those that cleave near proline residues.
These proteases are particularly effective for proline-rich proteins, while having limited effects on other proteins.
Result
Smaller proteins with disrupted hydrophobic regions have a lower tendency to interact with multiple phenolics, reducing cross-linking, aggregation, and precipitation.
describe Controlling oxidation of phenolics
Principle
Covalent protein–phenolic interactions mainly occur via o-quinones.
Preventing phenolic oxidation limits quinone formation.
Practice
Control PPO activity by:
Adding antioxidants
Using conditions that inhibit or inactivate PPO
Result
Reduced o-quinone formation leads to fewer follow-up reactions and thus fewer covalent protein–phenolic interactions.
Effect of phenolic compounds on appearance
formation of a haze, i.e. turbidity

What are the methods that can be used to control and inhibit enzymatic browning
1.eliminate oxygen
2.lowering pH
3.heat induced denaturation PPO
4.cooling
adding chelating agents
adding sulfite
adding thiol compounds
removal phenolic compounds
what is oxidative coupling
oxidative coupling is formation of a brown colour, therefore, the sequence of reactions initiated by PPO is also known as enzymatic browning
explain the mechanism of oxidative coupling
Route C. Coupled oxidation, the dimer is oxidised by an o-quinone. In this process this o-quinone is
reduced to an o-diphenol while simultaneously forming the dimer o-quinone. The dimer o-
quinone can then react with a non-oxidised phenolic
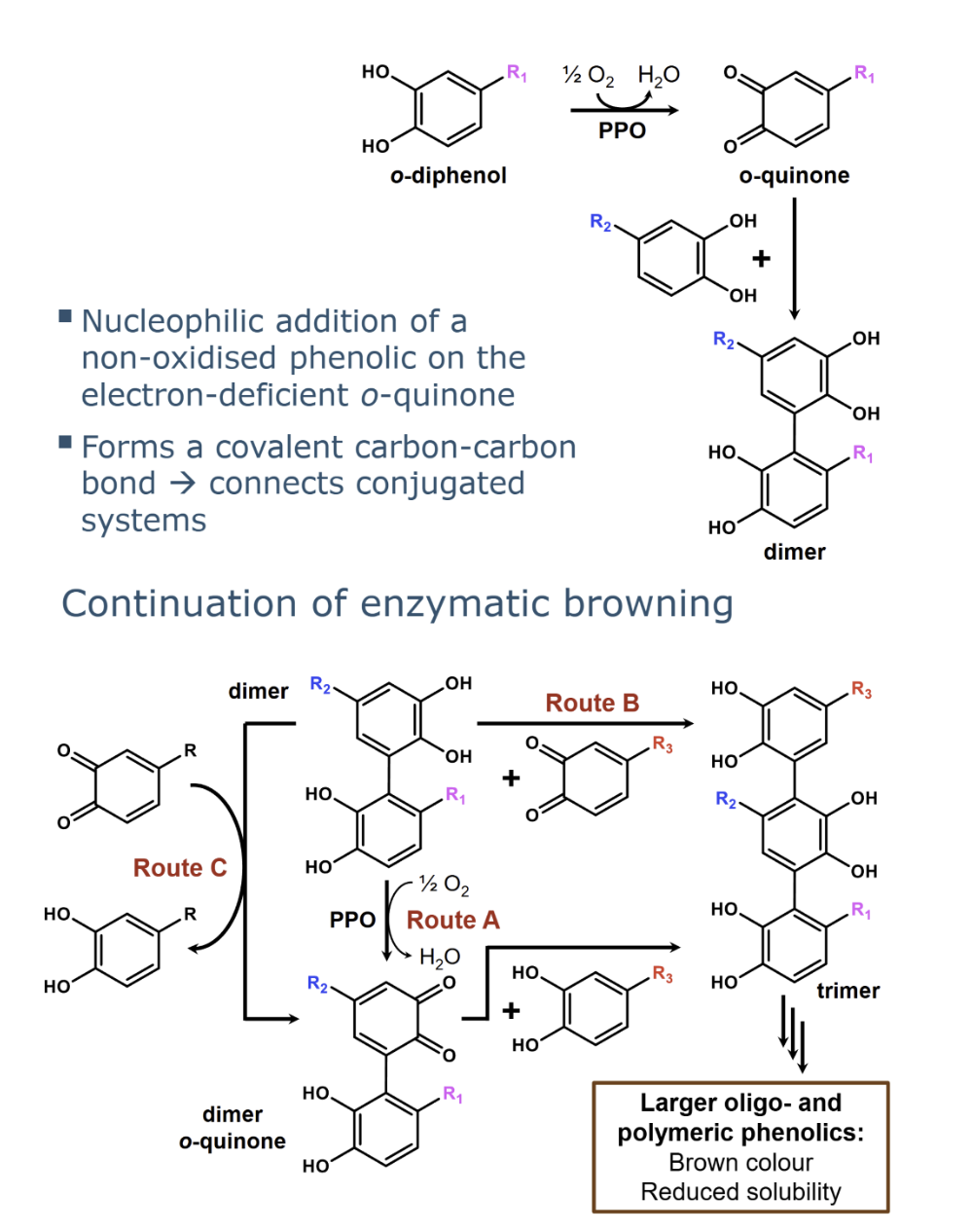
with increasing size of coupling products the water solubility….
With increasing size of the coupling products, their water solubility
decreases. Trimers and oligomers of 6-8 phenolic monomers as building blocks are brown pigments that are typically still water-soluble to some extent. Very large oligomers (> 6-8 monomeric subunits) and polymers are
typically not water-soluble at all, they are insoluble brown pigments.
anthocyanins and anthocyanidins, the presence of colour is a result of
anthocyanins and anthocyanidins, the presence of colour is a result of the conjugated system that connects
the A-ring and the B-ring, via the C-ring
8 double bonds

whats difference between anthocyanin or anthocyanidin
If R4 at C3 is a saccharide, then the structure is an anthocyanin (i.e. a glycoside), whereas if R4 is H, then it is an
anthocyanidin (i.e. an aglycon)
what colour is anthocyanina nd anthocyadinin responsible for
responsible for the red,
violet, or blue colour of popular fruits, like blue berries and raspberries, and some vegetables, like red cabbage
what colour is anthocyanina nd anthocyadinin for acidic , slightly acidic and alkaline
Acidic pH- positive charge = red
Slightly acid – neutral = violet
Alkaline- negative charge = deep blue
what colour is anthocyanina nd anthocyadinin if water is added.
Water addition breaks conjugation in the C-ring, causing loss of colour
is flavor and aroma volatile or non volatile
Flavour = taste (non-volatile) & aroma (volatile)
whats a bitter fruit example
Isoflavanoids known to be bitter
grapefruit, which contains bitter flavanone glycosides
what affects bitterness
the linkage position of the saccharide unit in flavanone glycosides affects their
bitterness
1→2 = bitter
1→6 = not bitte
what is astringency
Astringency: Caused by precipitation of proline-rich proteins
in saliva due to protein- phenolic interactions
where is astringency most relavant in
most relevant in food products such as wine and tea that are rich in
oligomeric phenolic compounds
astringency increases with?
Astringency increases with the strength of protein–phenolic binding
describe the astrigency mechansim
Phenolic compounds (especially tannins) interact with salivary proteins
These proteins are often proline-rich salivary proteins
Phenolics form strong non-covalent complexes with proteins
This can lead to:
Aggregation
Cross-linking
Precipitation of salivary proteins
Precipitation reduces saliva’s lubricating function
Result → dry and rough mouthfeel (astringency)
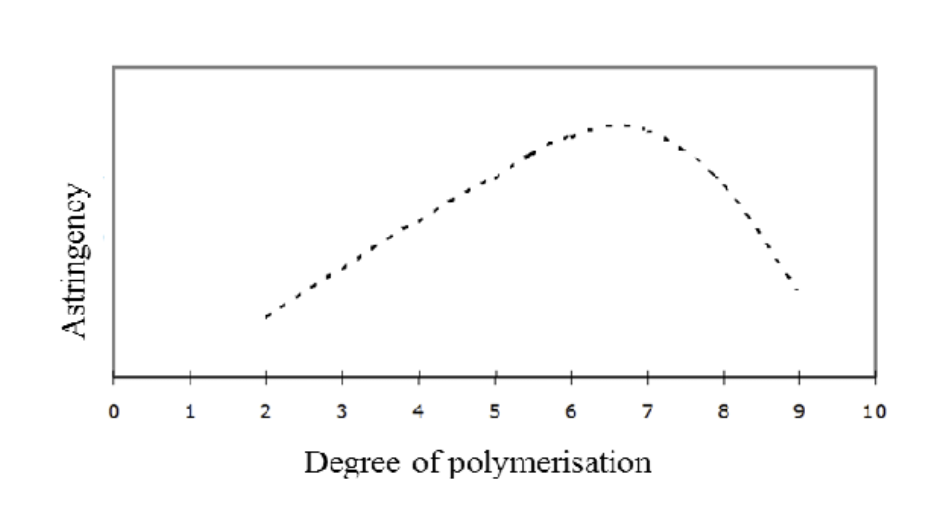
Influence of degree of polymerisation (DP) on astringency
Effect of DP on protein binding
Low DP (monomers, dimers)
Weak protein binding
Low astringency
Medium DP (oligomers, DP ≈ 5–7)
Strong protein binding
Efficient cross-linking of proteins
Maximum astringency
High DP (large polymers)
Reduced solubility or flexibility
Less effective interaction with proteins
Decreased astringency
Effect of phenolic compounds on aroma
phenolics can affect the aroma of foods by acting as aroma compounds
themselves or via o-quinones participating in Strecker degradation to form amino acid-
derived aldehyde aromas.
Why phenolics are challenging to analyse
Phenolics show high structural diversity
what can be used to detect phenolics
UV-Vis spectroscopy and the Folin-Ciocalteu assay can be used to detect
phenolics
describe UV–Vis spectrophotometry for phenolics
Principle
UV–Vis spectrophotometry measures absorption of light in the range ~200–700 nm
Phenolics always contain at least one aromatic ring
Aromatic rings absorb light in the UV region
Why it works for phenolics
Aromatic rings → UV absorption
Larger conjugated systems → absorption shifts toward the visible region
Therefore:
Simple phenolics → mainly UV absorption
Highly conjugated phenolics → may absorb visible light (colour)
Use
Direct detection of phenolics in solution
Measurement of absorbance at specific wavelengths
Basis for many colorimetric assays
describe Folin–Ciocalteu assay
Folin–Ciocalteu reagent contains two metals
Phenolic compounds:
Are oxidised
Reduce the metals in the reagent
Reduced metals form a bright blue colour
Colour intensity is proportional to phenolic concentration
📌 This makes it a colorimetric assay, measured by UV–Vis spectrophotometry
Quantification
A calibration curve is prepared with a known phenolic
Most commonly gallic acid
Results expressed as:
Gallic Acid Equivalents (GAE)
whats advantage and disadvantage of
Folin–Ciocalteu assay
Advantages
Simple
Fast
Low-cost
Does not require advanced equipment
Widely used in research and industry
Main disadvantage (VERY IMPORTANT FOR EXAM)
The assay is not specific for phenolics
The reagent reacts with any reducing compound, including:
Reducing sugars
Ascorbic acid
Therefore:
TPC values may be overestimated
Accuracy depends on sample composition
what is the main phenolic compound in tea
flavan-3-ols (catechins) are the main phenolic compounds in tea leaves and green
tea.
Explain the role of fermentation by PPO in the formation of colour in black tea production