L5 Physical and Chemical properties of Soil
1/23
Earn XP
Description and Tags
some definitions and keywords
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Physical properties of Soils
texture
Structure
Density
Porosity
Permeability
Colour
Temperature
Plasticity, Compressibility and Erodibility
Soil texture
Relative amount of sand, silt and clay
Soil fractions (soils separates → mineral part of soil)
Sand: gritty
Silt: floury when dry, silky when wet
Clay: Velvety
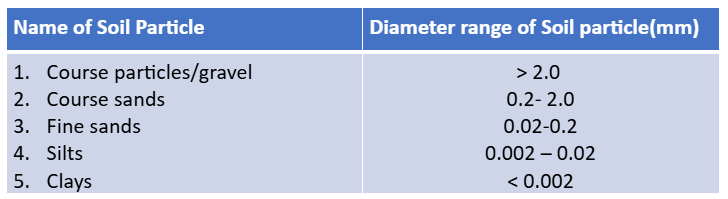
Equilateral triangle used bu USDA for textural classes of soil
Sand
Particles seen by unaided eye
Less important role in physiochemical activities
Sand increases size of pores between soil particles facilitating movement of air and water.
Silt
Coarse shows little physiochemical activity but finer grades play important role in some chemical processes
Silty soil has larger exposed surface area than sandy soil.
Contains sufficient quantities of nutrients, both organic and inorganic
Soils rich in silt possess high water holding capacity
Clays
Plasticity and smoothness when wet and harness when dry. Smallest size and colloidal nature when clay particles are exposed to extremely large surface area.
Take very active part in physiochemical reactions in soil
Have fine pores, poor drainage and aeration.
Acts as store house for water and nutrients.
Soil structure
Arrangement of soil particles into aggregates (peds)
Granular
Blocky (angular and sub angular
Platy
Columnar and prismatic
Massive (non structure)
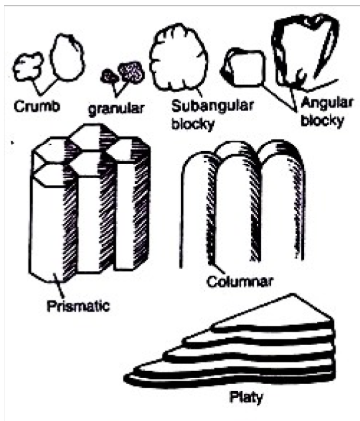
Soil structure associated with its functions
Fertility
Biodiversity
Rootability
Carbon sequestration
Nutrient cycling
Water cycling
Degraded soil structure is a decrease in soil services and increase in hazards.
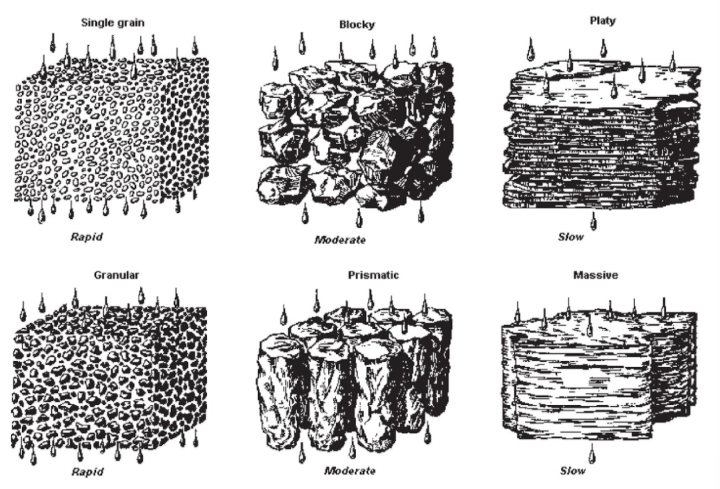
structure and texture together regulate porosity, density, compactness, retention and movement of water and air in soil
Effect of soil structure on water permeability
Particle density
A measure of the mass per unit volume of soil expressed with regard to volume of solids only Ranges from 1 – 3 mg m-3
Bulk density
A measure of the mass per unit volume of the entire soil sample (usually undisturbed) – a measure of the solids + pore spaces
Soil bulk density is influenced by both texture (sands are more dense) and structure
Bulk density is indicative of the pore space in a soil and ease with which roots may penetrate
Soil density and Pore space
Loose, well-aggregated, porous soils and those rich in organic matter have lower bulk density
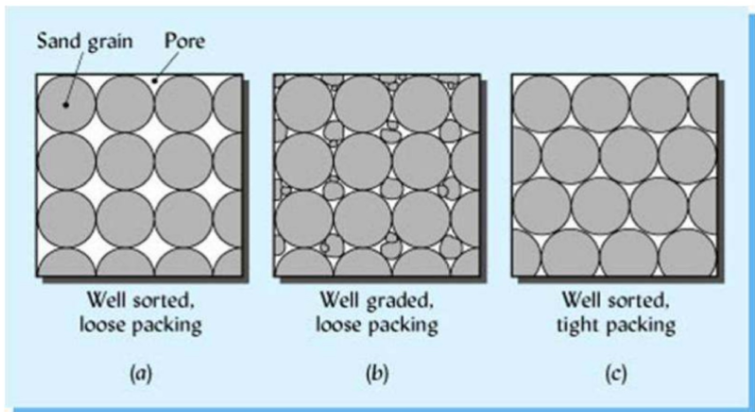
Soil colour
Inherited from the parental material (i.e., lithochromic) Or may be due to soil forming processes
Organic substances impart - black or dark greyish-black colour
Iron compounds a - brown, red and yellow colours of soils Iron oxides in combination with organic substances - brown colour which is most common soil colour
Silica, lime - light white and grey tinges to the soil.
Chemical properties of Soil
Inorganic matter composition
Organic matter composition
Cation Exchange Capacity of Soils
Buffering Capacity of Soils
Inorganic matter composition
Composed chiefly of aluminosilicates and oxides.
Through their surface electrochemical properties these minerals control the adsorption and transformation and behaviour of other chemical constituents.
Aluminosilicates are commonly called clay particles
Clay particles are colloidal in nature
Colloids
Thin, plate-like shape that reflect their layered chemical crystal structure
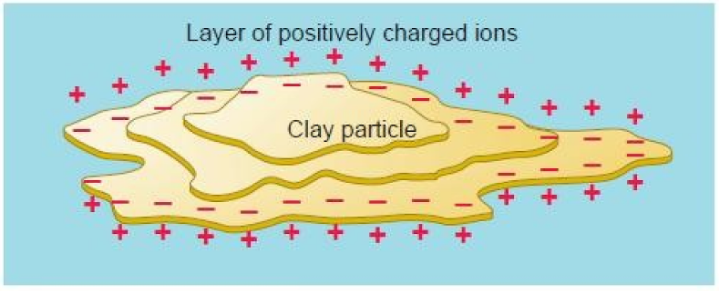
Colloid surfaces tend to be negatively charged because of their molecular structure - they attract and hold positively charged ions which often include nutrient bases such as calcium and magnesium
Organic matter
Organic matter - partially decomposed plant residues and humus
Humus - resistant product of decomposition and colloidal in nature
Humus contains the following organic molecules:
Amino acids – glycine, serine, alanine, glutamic acid
Proteins – purines, guanine, adenine
Aromatic molecules - Aromatic carboxylic acids
Aminosugars – glucosamine
Hexose sugars – glucose
The humus fraction also contains fats, oils, waxes, resins, tannin, lignin and some pigments.
Also contains colloidal structures that are resistant to decay.
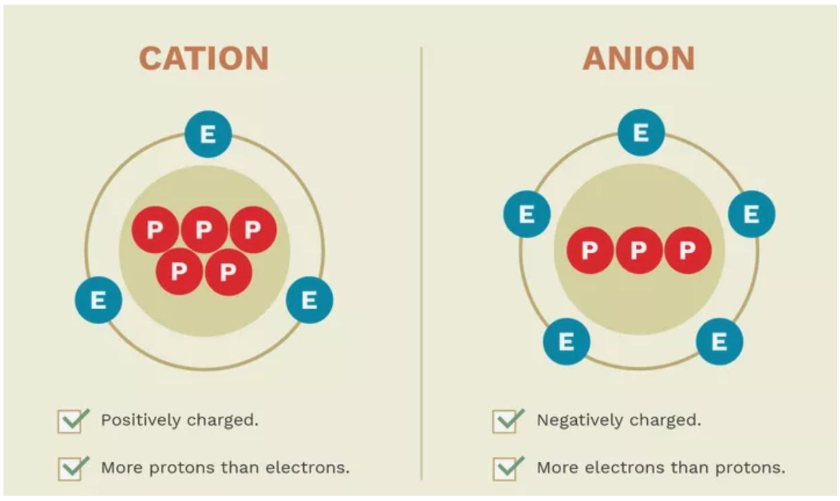
Ion
An ion is an atom with a positive or negative charge
Cation
A positively charged ion that has more protons than electrons, meaning it has lost one or more electrons
A +2 charge on an ion means it has lost two electrons, resulting in a net positive charge. This occurs when the atom transitions from a neutral state to a positively charged state, known as a cation.
Common +2 cations include magnesium (Mg²⁺) and calcium (Ca²⁺)
Anion
A negatively charged ion that has more electrons than protons, meaning it has gained one or more electrons
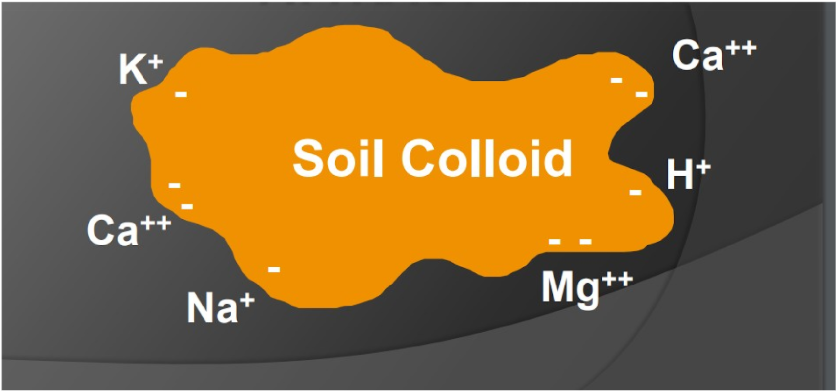
Cation Exchange Capacity (CEC)
Is the total capacity of a soil to hold exchangeable cations
Clay and organic matter have negative charges that can hold and release positively charged nutrients. (The cations are adsorbed onto the surface of the clay or humus)
That static charge keeps the nutrients from being washed away and holds them so they are available to plant roots and soil microorganisms.
Negatively charged colloids attract cations
Buffer capacity
The amount of acid or base a buffered solution can soak up before its pH will start to change significantly.
The buffer capacity of a soil is important in determining how its pH will change. Various minerals in soil help to buffer against changes in pH when an acid or base is added.
At high pH, calcium, magnesium and potassium oxides, together with carbonates, help to buffer pH changes
At acidic pH, aluminium oxides and iron hydroxides act as buffering agents; at intermediate pH levels, soil organic matter, mineral weathering and exchange reactions help to buffer the soil.
A higher buffer capacity means that the soil can absorb more acid and/or base without a significant change in pH.
In general, clay soils have higher buffer capacity than sandy soils, and a higher organic matter content tends to increase buffering capacity.
Bioavailablity
Available to Biological Organisms
May refer to ingestion or uptake of a given compound
May also refer to biological or physiological effects
By definition – chemical measurements remain proxy- must be linked to a biological organism