Gas Diffusion
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
gas diffusion
Gases move between respiratory compartments separated by membrane barriers via Passive Diffusion across the membrane
blood-tissue barrier
separates the systemic circulation from the tissue compartment
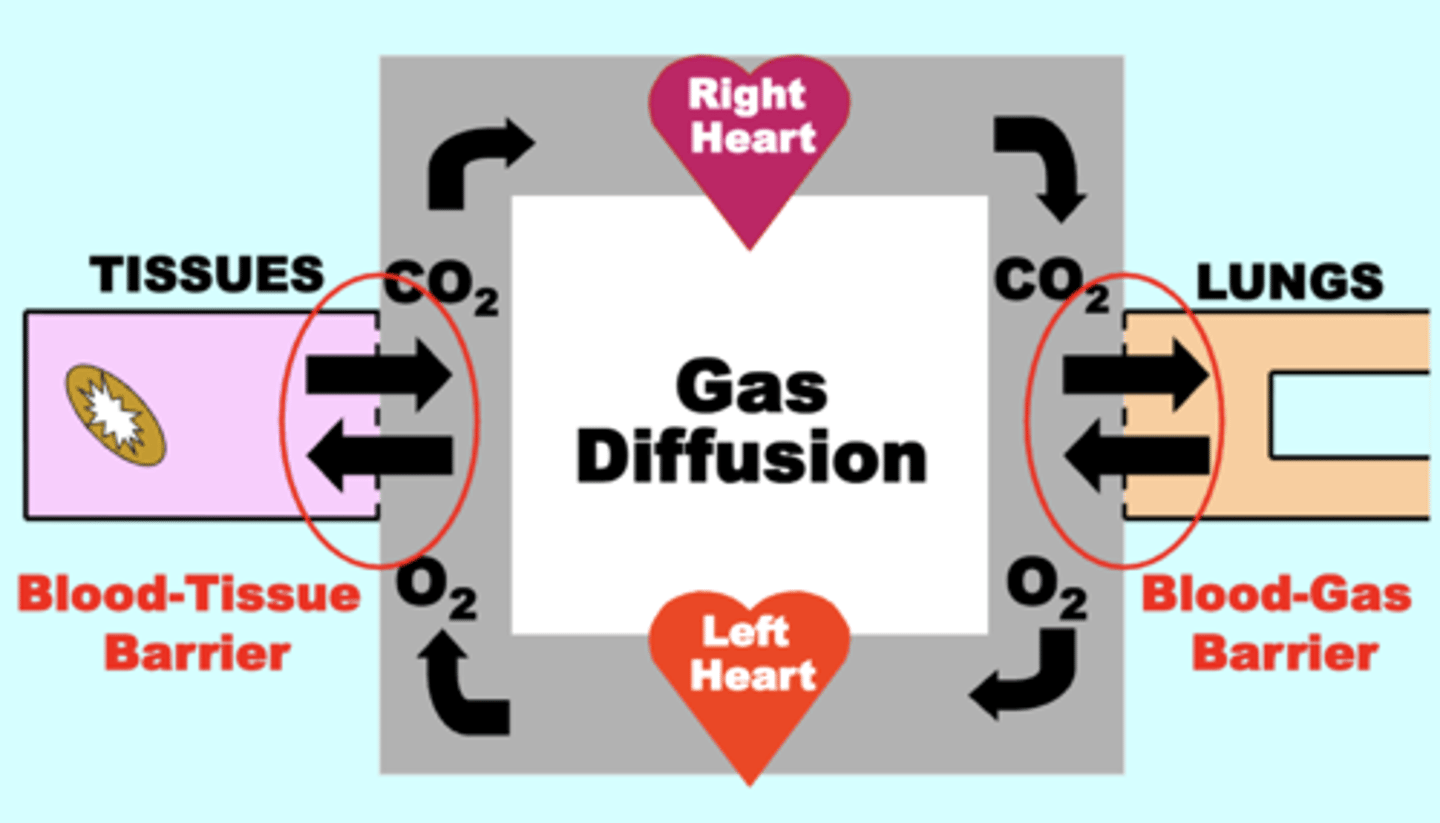
blood-gas barrier
separates the pulmonary circulation from alveolar gas (Lungs)
direction of gas diffusion is determined by?
the orientation and magnitude, respectively, of the Partial Pressure (Pgas) Gradient between compartments
direction of gas diffusion of O2 and CO2
O2: diffuses OUT of alveoli (PAO2 = 100 mm Hg) INTO venous blood (PvO2 = 40 mm Hg)
CO2: diffuses OUT of venous blood (PvCO2 = 46 mm Hg) INTO alveoli (PACO2 = 40 mm Hg)
Rate & Volume of gas diffusion across the barrier is influenced by?
the magnitude of the Pgas across the barrier, but also by other factors in terms of Fick’s Law parameters
Passive Diffusion of gases across is dependent upon?
the development and maintenance of Steady-State Gas Pressure Gradients between compartments
- direction & volume of flow of a particular gas is determined by the steady-state gas pressure difference between compartments
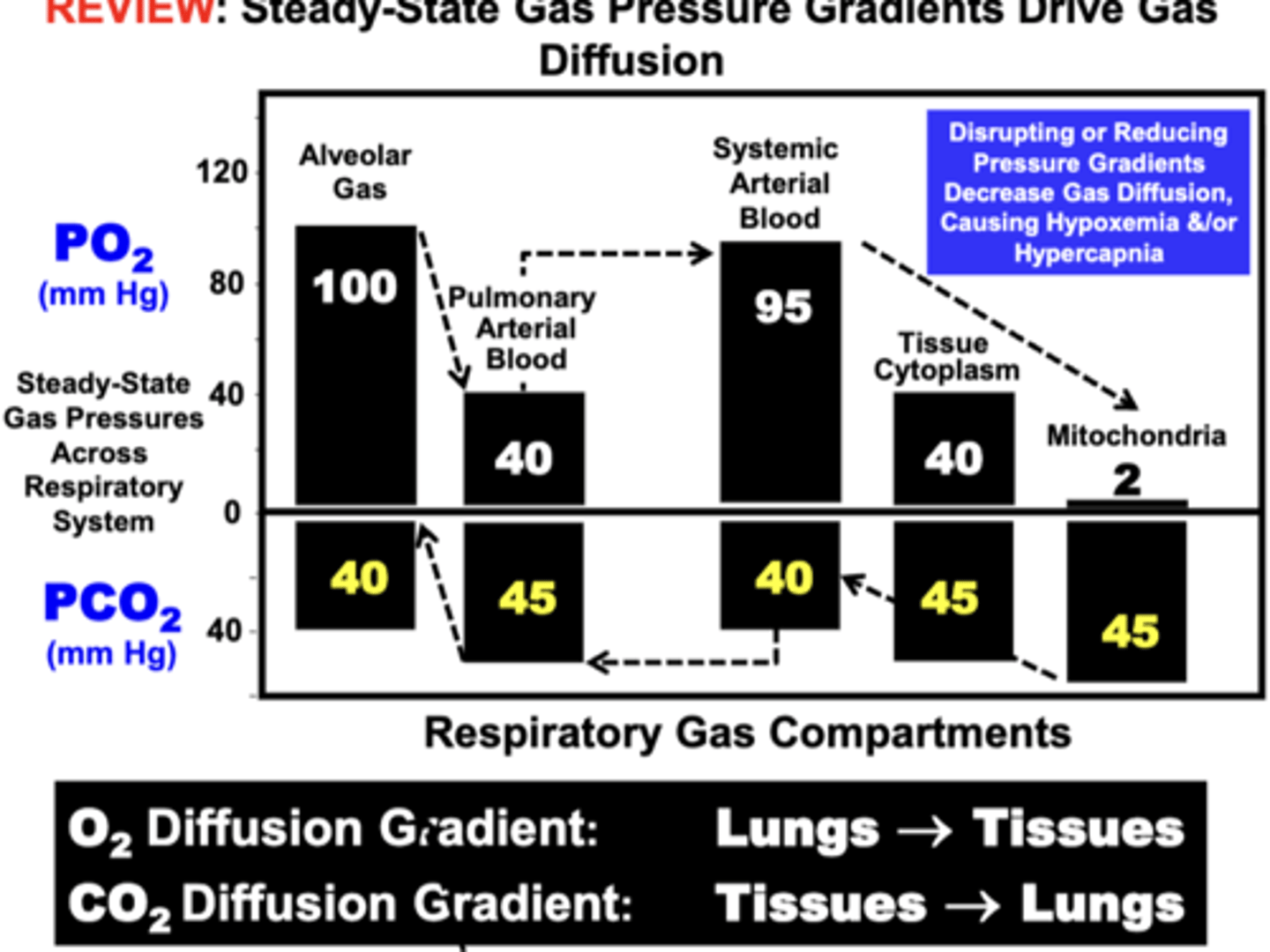
Direction of net O2 flow
from the Lungs toward → the Tissues
alveolar gas/lungs → (blood gas barrier) → pulmonary arterial blood → systemic arterial blood → (blood tissue barrier) → tissues → mitochondria
Direction of net CO2 flow
Tissues toward (→) the Lungs
disruption or reducing of steady state pressure gradients can result in?
a decrease of gas diffusion & gas exchange, potentially resulting in Hypoxemia &/or Hypercapnia
Decreasing Steady-State Gas Pressure Gradients between respiratory compartments decreases?
gas diffusion volume and rate between compartments
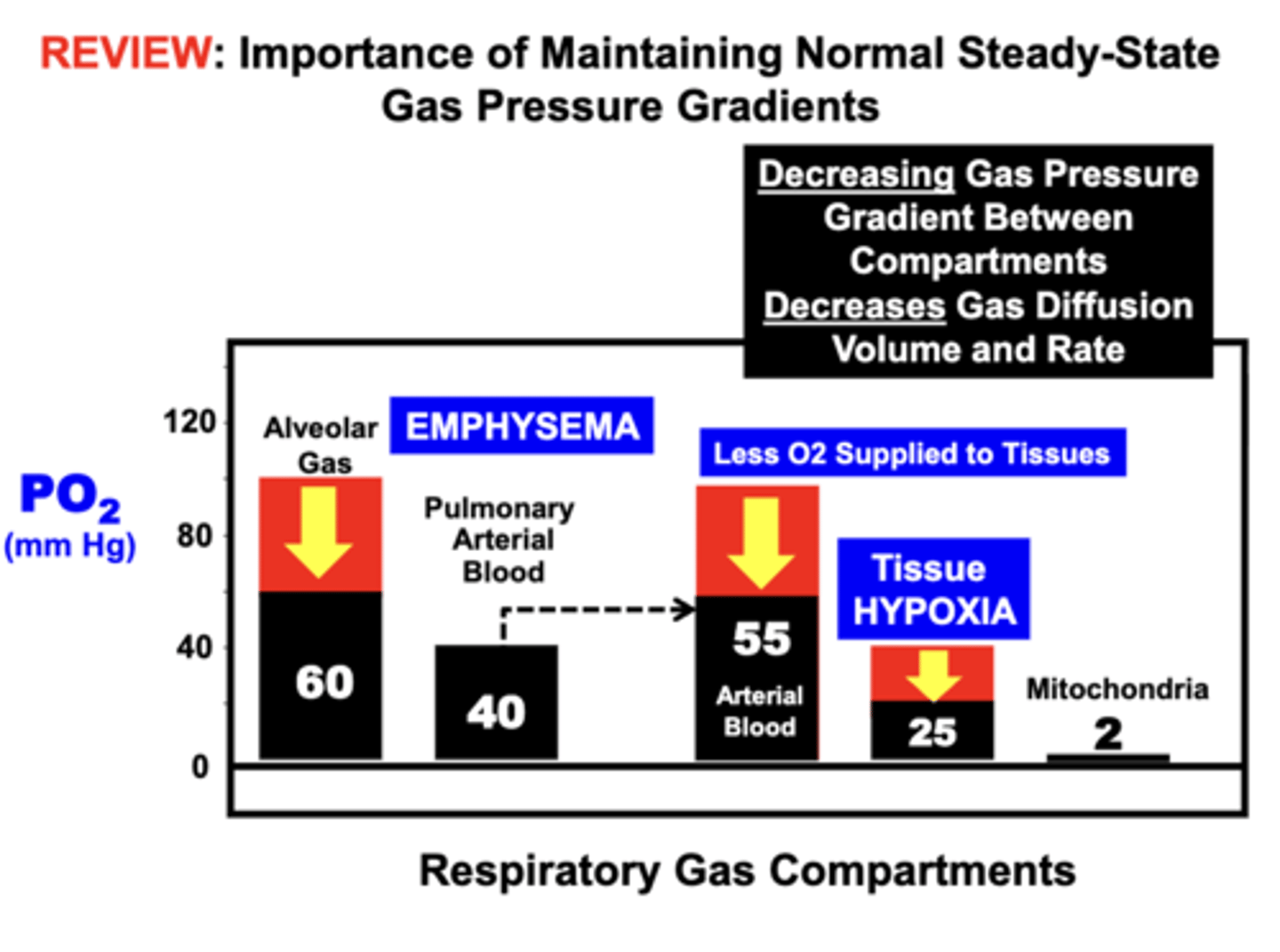
Emphysema
pulmonary disorder that ultimately causes Hypoxia by disrupting (decreasing) the gas pressure gradient between the lung compartment (Alveolar Gas) and pulmonary blood compartment (Pulmonary Arterial Blood)
- O2 is replaced in the blood, which decreases the O2 supply to the tissues leading to hypoxemia and tissue hypoxia
Increasing Steady-State Gas Pressure Gradients between respiratory compartments increases?
gas diffusion between compartments, which can restore normal O2 supply to tissues
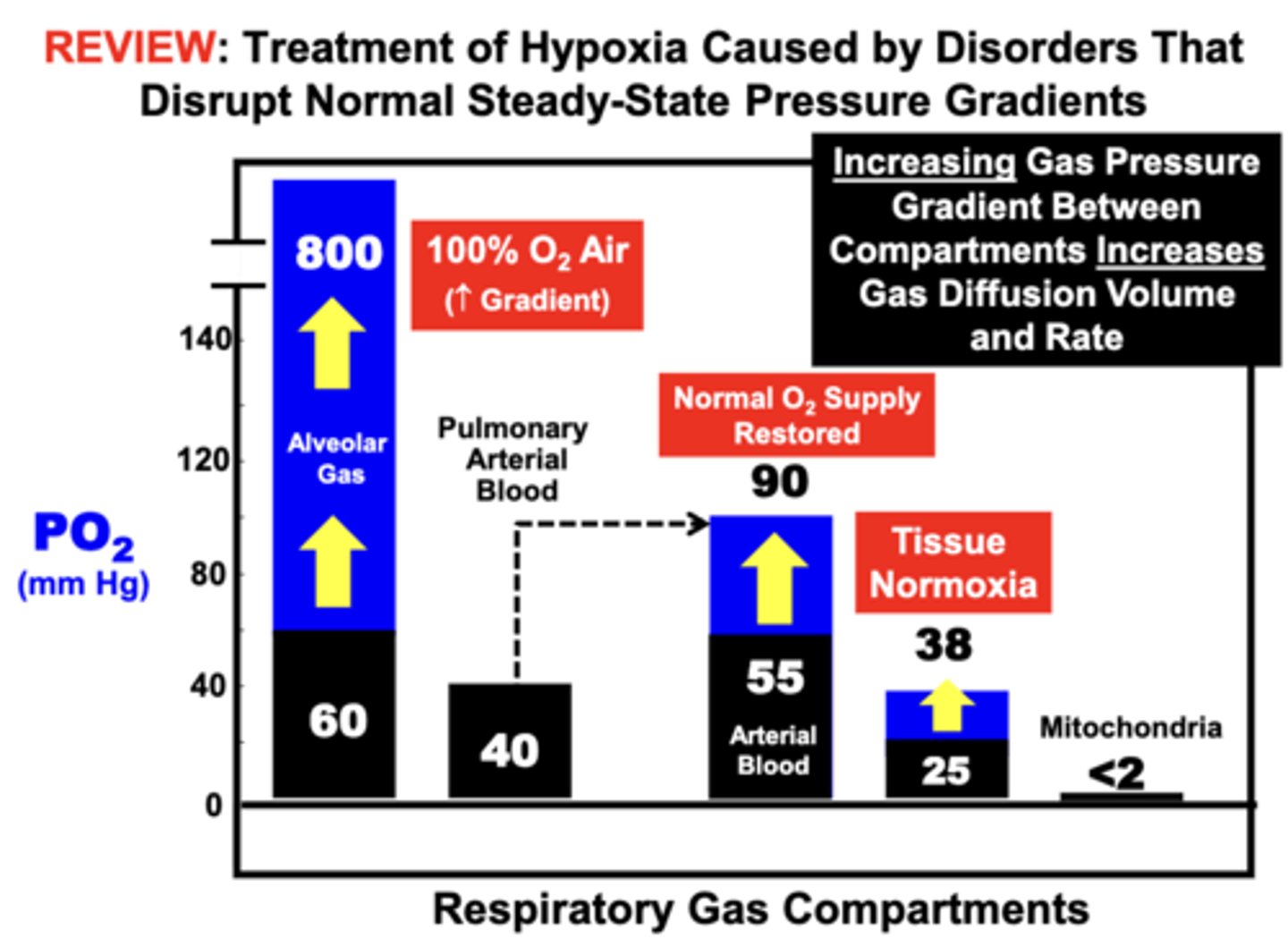
clinical treatment of Emphysema
with high FO2 air (100 O2 Air) is an effective treatment for Hypoxia
- increasing the gas pressure gradient between the lung compartment (Alveolar Gas...800 mmHg) and pulmonary blood compartment (Pulmonary Arterial Blood...40 mmHg) restores Arterial Blood PaO2 to near a normal level (90mmHg) such that O2 Supply is Restored to the tissues (Tissue Normoxia...38mmg)
differences in gas solubility influence the?
ability of gases to diffuse across membrane barriers
- higher solubility gases diffuse more easily across membrane barriers than lower solubility gases
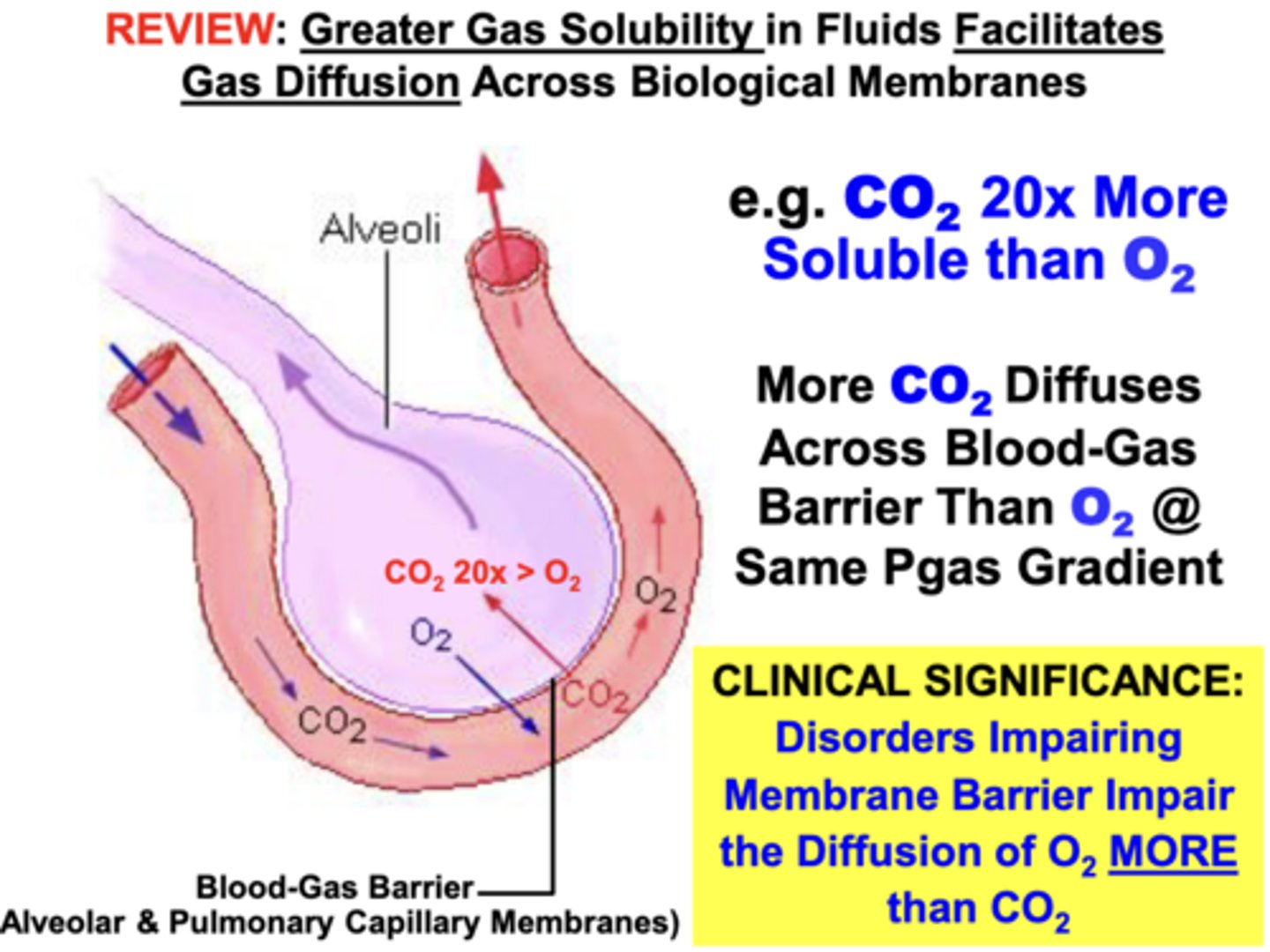
solubility properties of O2 and CO2
CO2 is 20x More Soluble than O2
- about 20x more CO2 than O2 can diffuse across the blood-gas barrier at the same partial pressure gradient if only solubility properties are considered
clinical relevance of the differing solubility properties of O2 and CO2
disorders damaging the integrity of the membrane barrier of lungs impair diffusion of O2 more than CO2
- ex. Inflammatory Fibrosis, which causes thickening of the blood-gas barrier
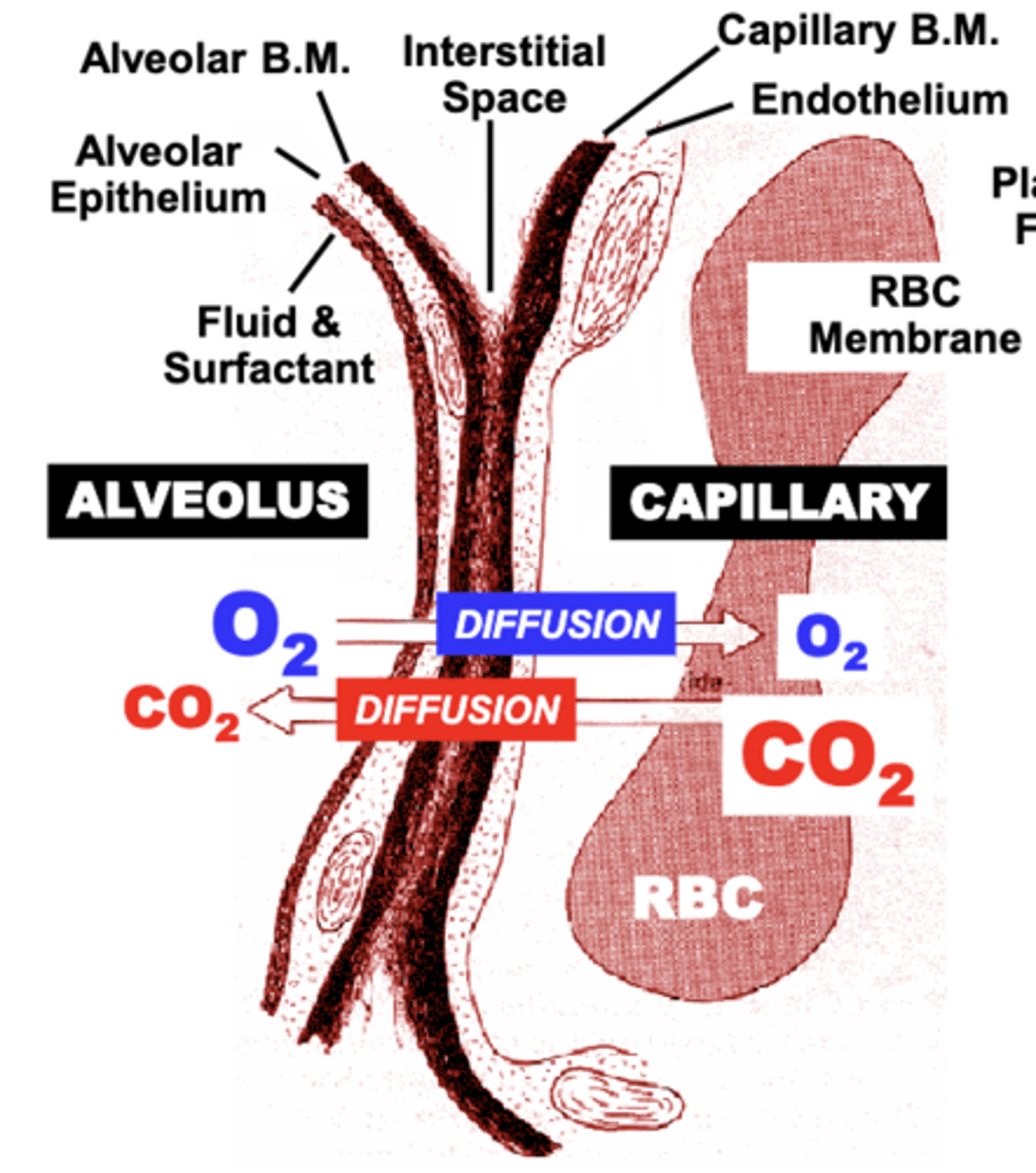
direction of diffusion of O2 and CO2 between the Alveolus, pulmonary capillary and RBC
Diffusion of O2 and CO2 between the Alveolus, pulmonary Capillary and RBC occurs in opposite directions
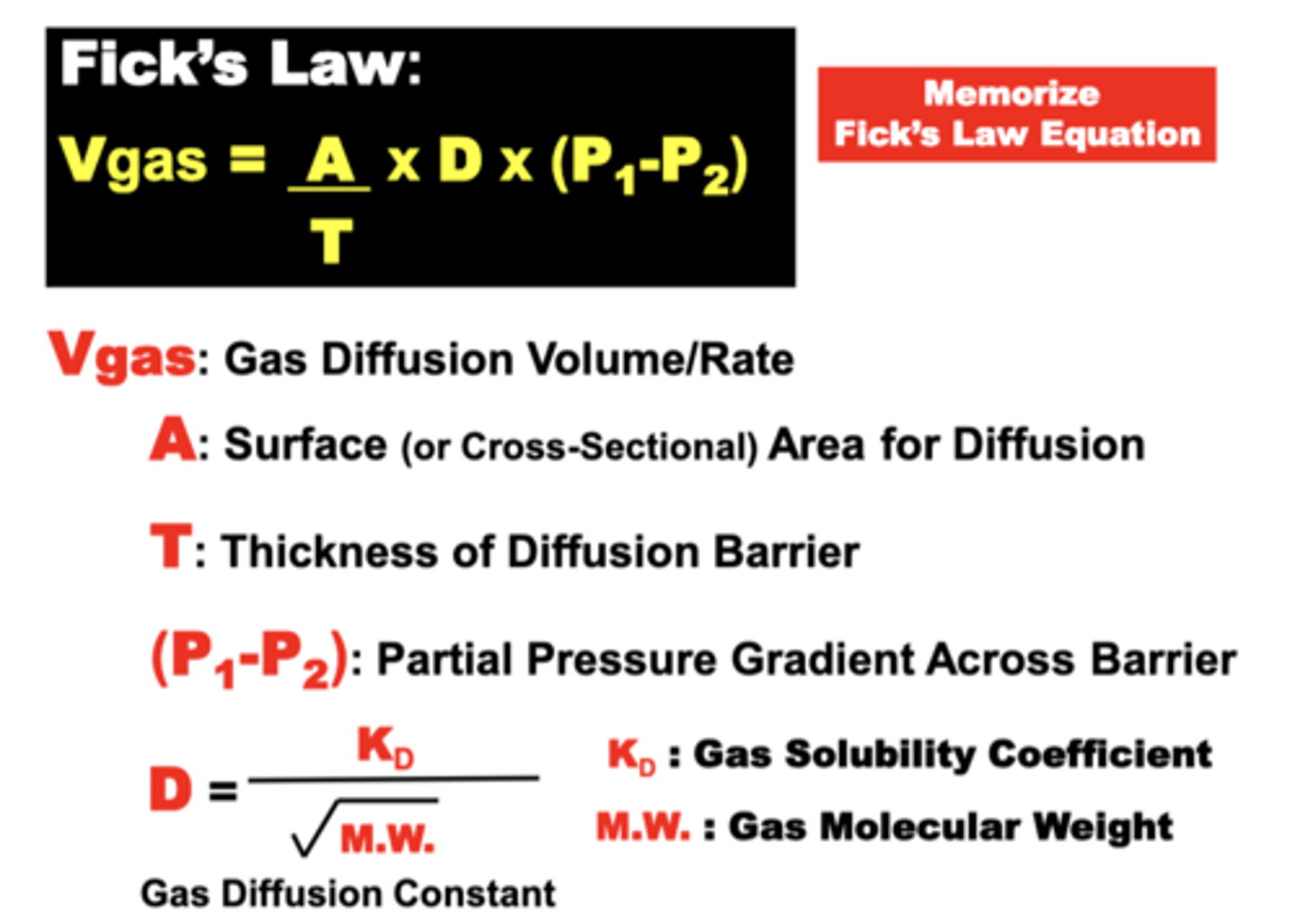
Fick's Law of Diffusion
Vgas = A/T x D x (P1-P2)
**only equation that must be memorized**
what is expressed by Fick's
expresses the variables that determine the volume of gas capable of diffusing across the blood-gas barrier or collectively across the whole lung over time (down a gas partial pressure gradient)
Gas Diffusion Volume (Vgas) transferred across a membrane is proportional to:
- the barrier Surface Area (A)
- Gas Partial Pressure Difference (P1 - P2) across the barrier
- a gas Diffusion Constant (D) related to specific Gas Properties
Vgas is inversely proportional to:
- the Diffusion Barrier Thickness (T)
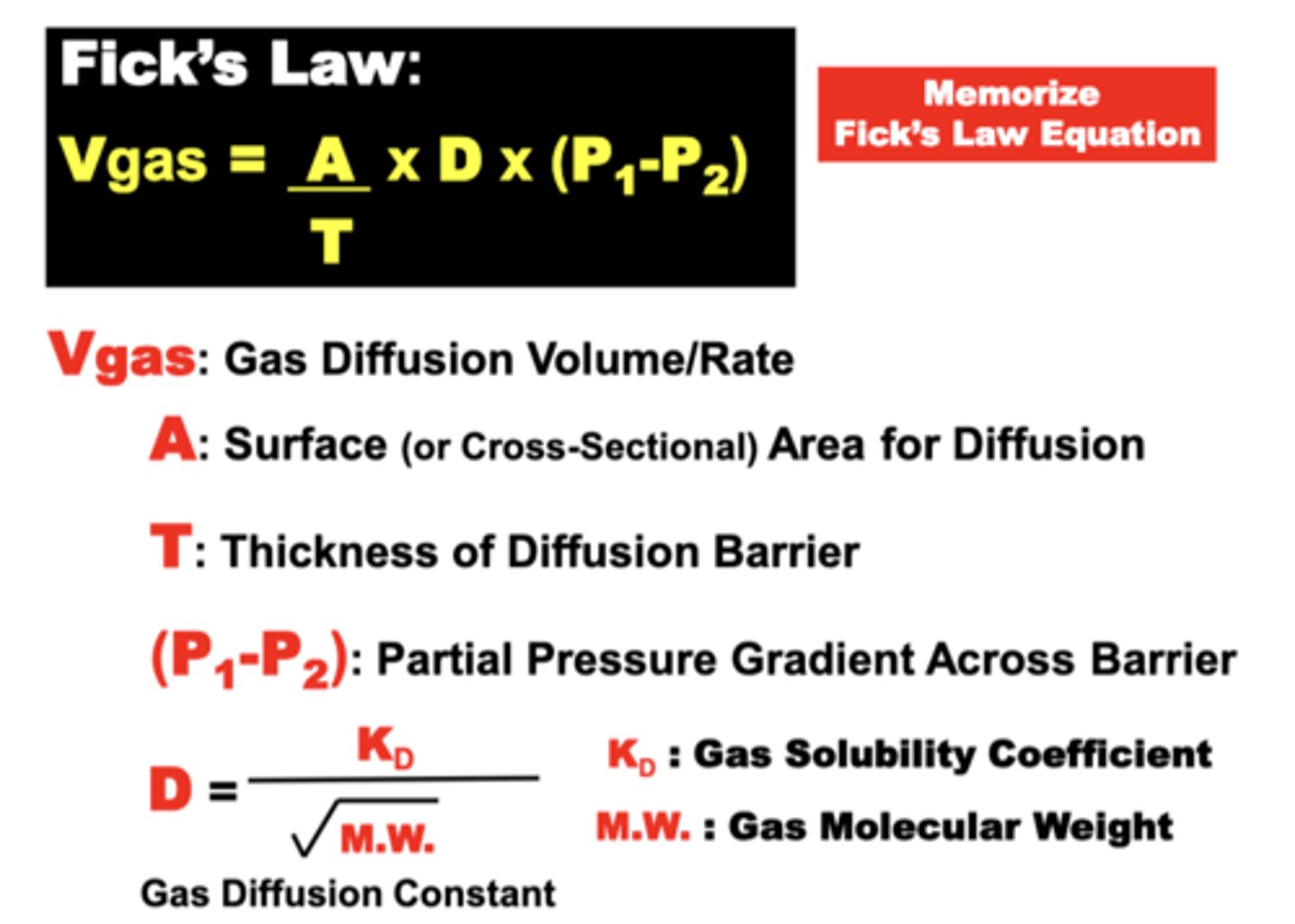
relationship of the diffusion constant
- proportional to gas’ Solubility
- inversely proportional to the sq. root of its Molecular Weight (M.W.)
= small, high solubility gases diffuse better than large and low solubility gases
basic physiological units of gas exchange
alveolus and pulmonary capillary
in terms of Fick's Law parameters, what is a nearly ideal surface for gas diffusion? why?
blood-gas barrier
1. Large Surface Area (A) of alveolar walls & capillaries (70-80 m2)
- results mainly from the numerous Alveoli (>300 million) surrounded by a dense capillary network
2. Short Diffusion Distance (T) facilitates gas movement
- only 0.3-0.6 μm separates alveolar gas from pulmonary blood in most regions of the lungs
- compare to 20-50 μm from capillary to cell in other peripheral tissues
3. Steep Partial Pressure Gradients (P1-P2) of O2 & CO2
- provide directionality and a strong driving force for high diffusion volume
4. Physical Properties (D) of the major respiratory gases (CO2 and O2)
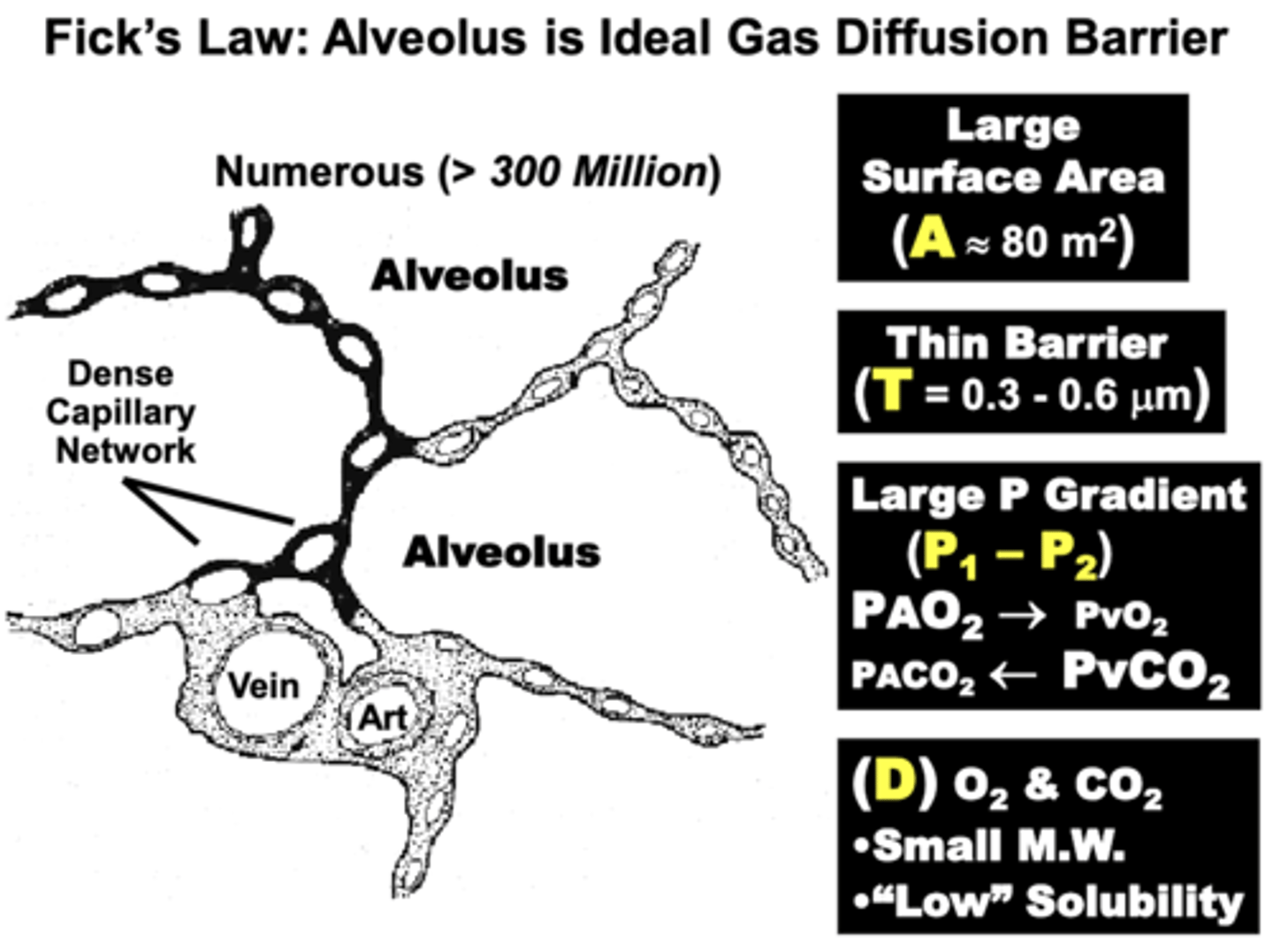
why are CO2 and O2 nearly ideal for efficient gas diffusion?
Their Physical Properties (D):
- CO2 & O2 are of small molecular weight, which favors gas diffusion
- Although the relative Low Solubility of both gases limits dissolved gas concentration, this trait allows Rapid Gas Equilibration across the membrane
= physiologically advantageous because it is estimated that a given RBC spends only 0.75 seconds exposed to alveolar gases at rest and may travel across in as little as 0.25 seconds during vigorous exercise
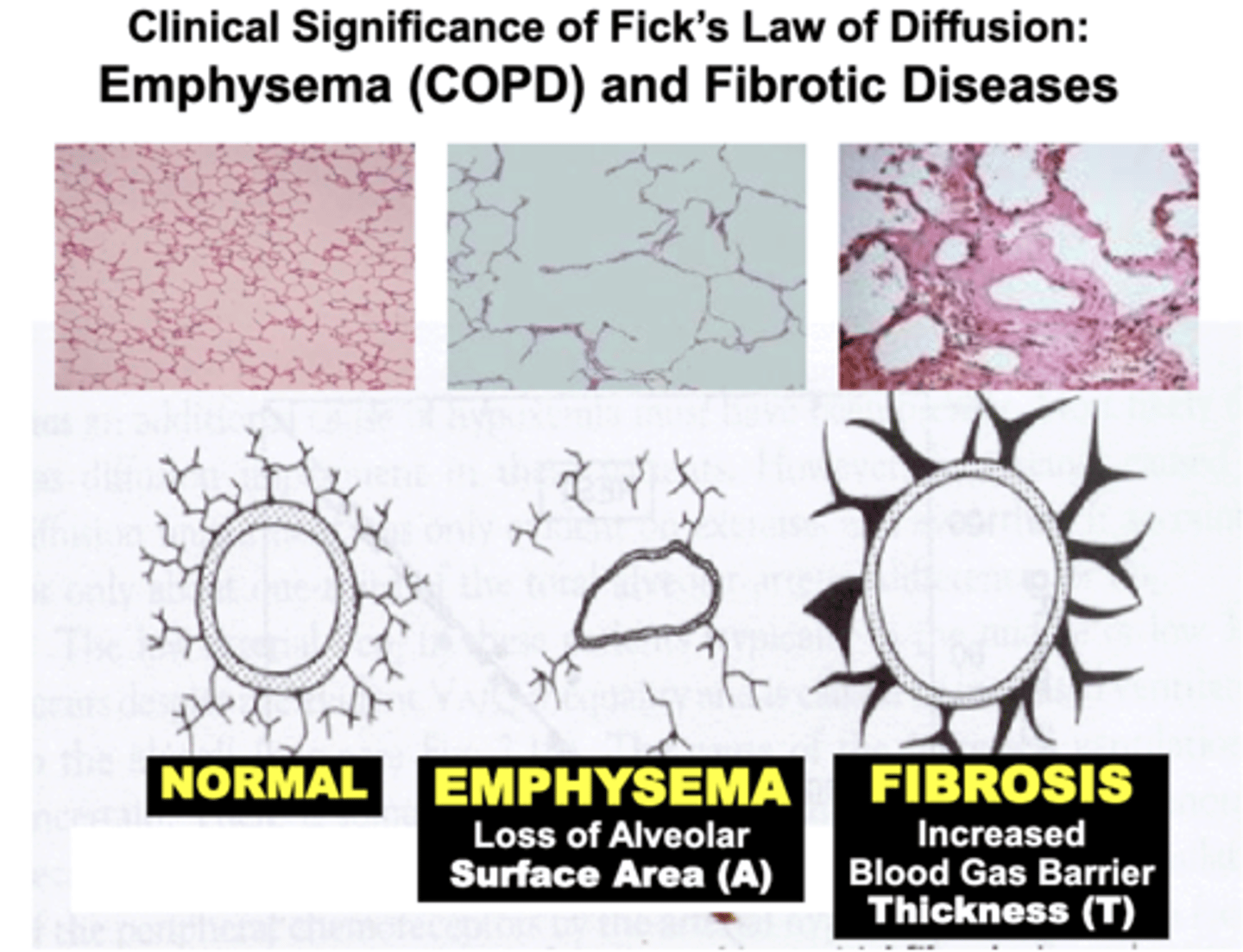
Clinical Significance of Fick's Law of Diffusion: Emphysema (COPD)
Pulmonary disorders such as COPD (e.g. Emphysema) result in a significant Loss of Alveolar Surface Area compared to Normal lungs
- results from destruction of the lung’s histological elastic (elastin & collagen) components (e.g. smoking)
= severely decrease gas diffusion of O2 and CO2 across the blood gas barrier
- pathophysiology is based on impairment of objective variables expressed by Fick’s Law of Diffusion
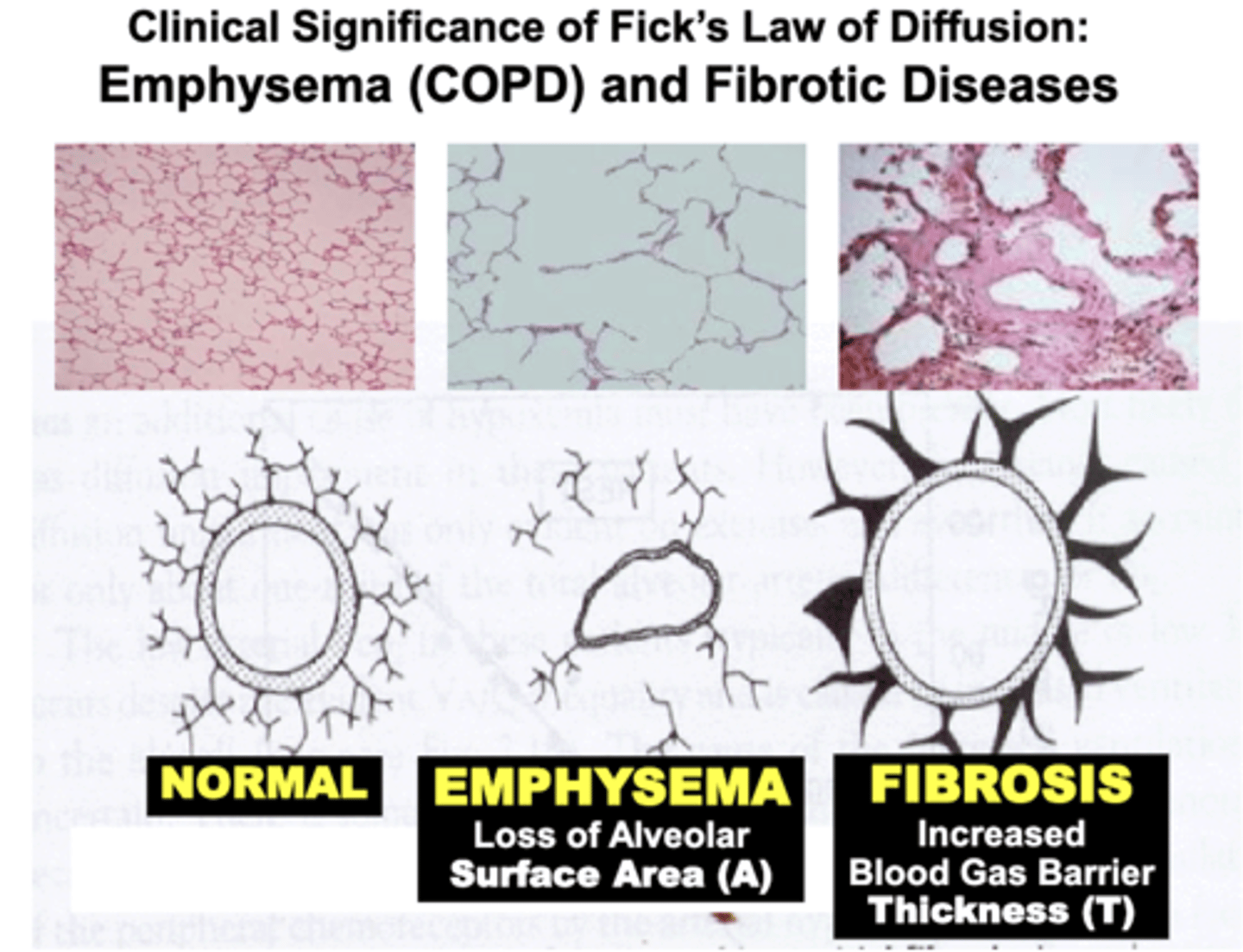
Clinical Significance of Fick's Law of Diffusion: Fibrotic Diseases
Patients with conditions termed Restrictive Pulmonary Disease (e.g. Lung Fibrosis) develop Increased Thickness of the Blood Gas Barrier compared to Normal lungs
- due to excessive formation of the lung’s histological elastic components (e.g. chronic infection and inflammation, toxins, etc.)
= severely decrease gas diffusion of O2 and CO2 across the blood gas barrier
- pathophysiology is based on impairment of objective variables expressed by Fick’s Law of Diffusion
Fick's Law: Treatment of Diffusion Disorders
Fick's Law expresses the basis for treatment of diffusion disorders
- impaired gas diffusion variables such as surface area (A) and barrier thickness (T) that result in decreased gas diffusion can be compensated for by increasing the partial pressure gradient (P1-P2) across the barrier
ex. a patient with hypoxemia caused by emphysema can be effectively treated by raising the FO2 of the air they breathe (e.g. 100% O2)
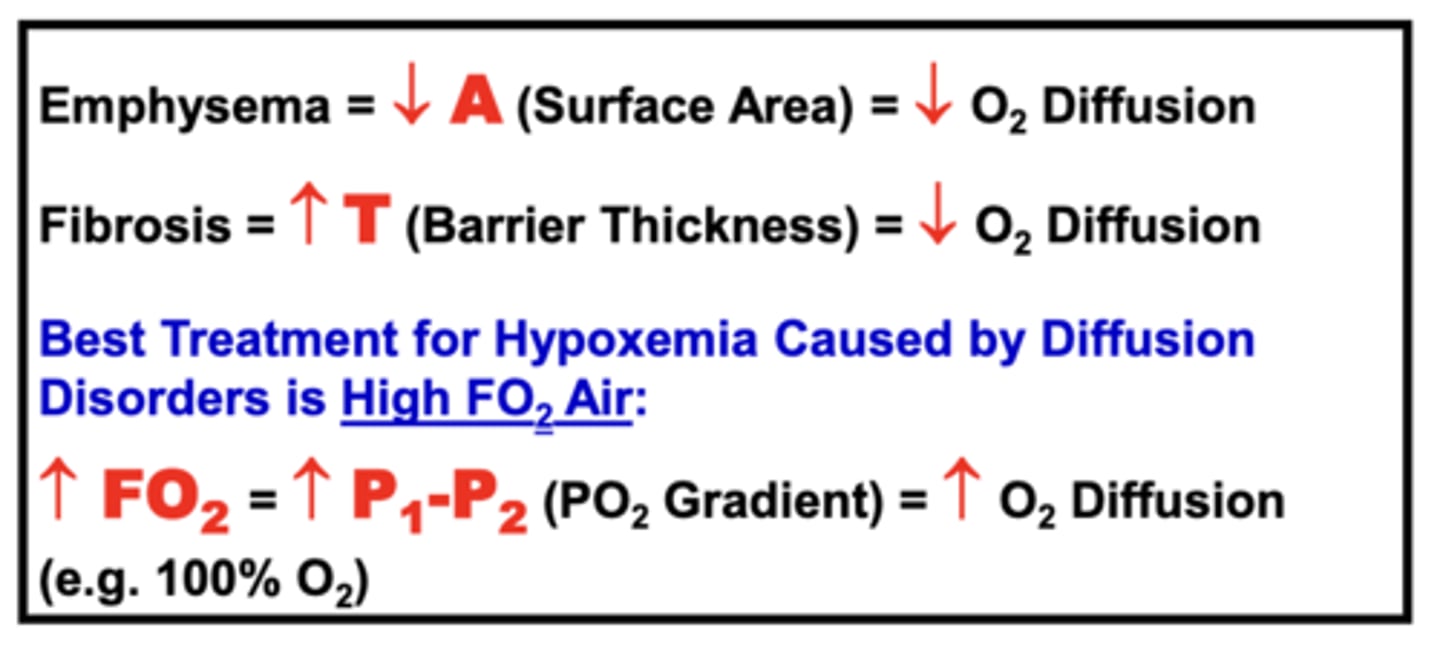
Best Treatment for Hypoxemia Caused by Diffusion Disorders
administration of High FO2 Air (e.g. 100% O2)
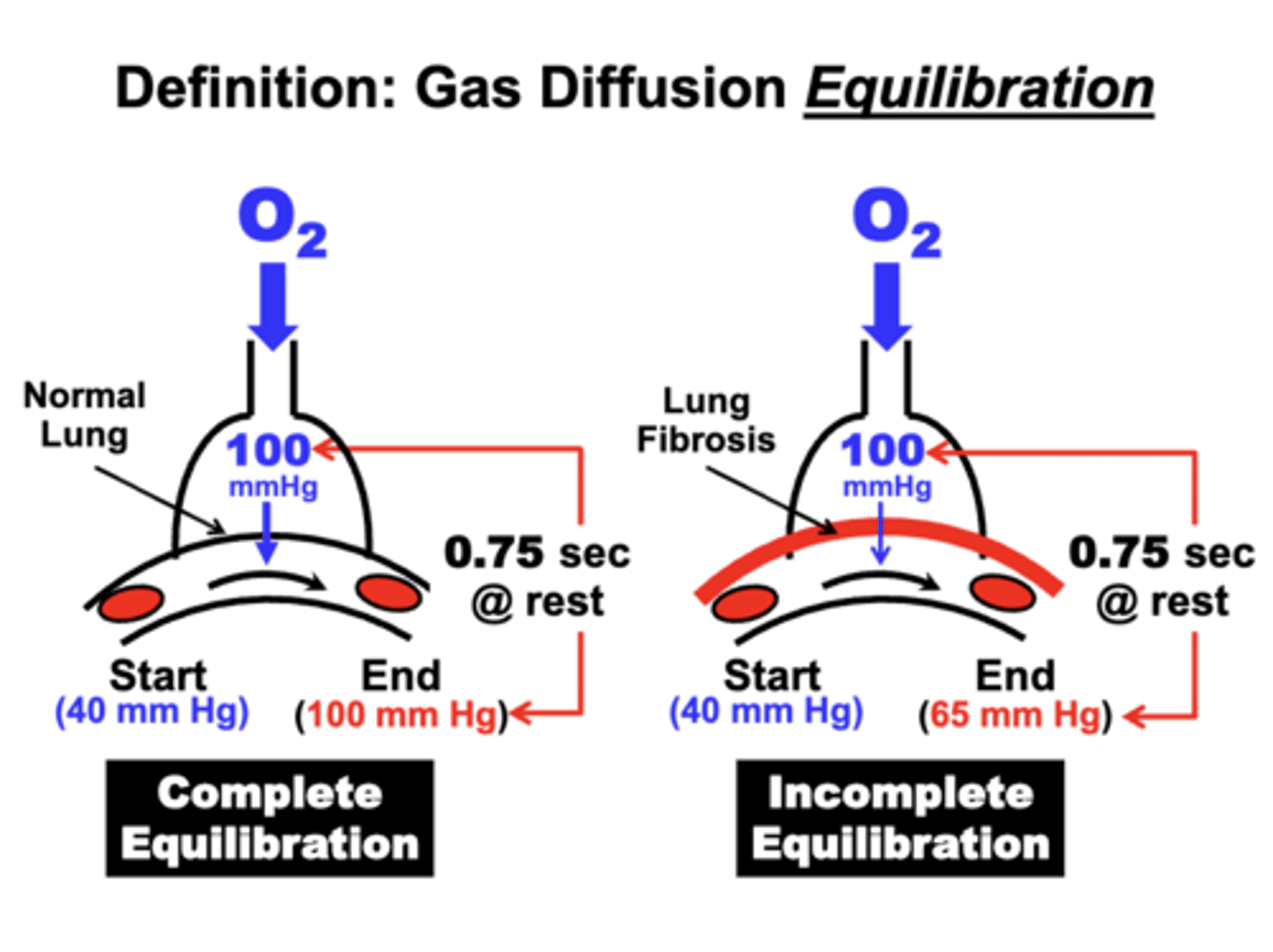
complete equilibration
occurs when blood PO2 is the same value as in the alveoli
o at this point, net O2 diffusion could stop and PO2 are restored
ex. Gas diffusion across the Blood-Gas Barrier
- PO2 value in pulmonary arterial blood (Systemic Venous Blood) returning to the lungs is 40 mm Hg
- Alveolar PAO2 is 100 mm Hg
- O2 will diffuse across the blood-gas barrier from the alveolar compartment into the blood
- Gas diffusion will continue until the blood PO2 increases to the same value (100 mm Hg) as in the alveoli
what is limited in equilibration?
there is a limited amount of time allowed for equilibration due to dynamics of pulmonary blood flow:
- At rest, only 0.75 sec. is allowed.
- During exercise, this may decrease to 0.25 sec
incomplete equilibration
occurs when blood PO2 fails to reach steady state value in alveoli during the available equilibration time
- due to decrease surface area or wall thickening
ex. lung fibrosis
- PO2 value in pulmonary arterial blood returning to the lungs is 40 mm Hg
- alveolar PAO2 is 100 mm Hg
- blood PO2 is only able to increase to 65 mm Hg
- fails to reach steady state value in alveoli during the available equilibration time
= eventually leads to hypoxemia and potentially hypercapnia
O2 Diffusion Time Course
normal individuals:
blood PO2 at rest equilibrates rapidly (0.25 sec) and completely (100 mm Hg) with the alveolar PAO2 well within the available perfusion period (about 0.75 sec at rest)
pulmonary disease :
development of respiratory disorders that impair alveolar membrane diffusing properties may prevent the blood from completely equilibrating with alveolar gases
- result in Incomplete O2 Equilibration Between Alveoli and Blood
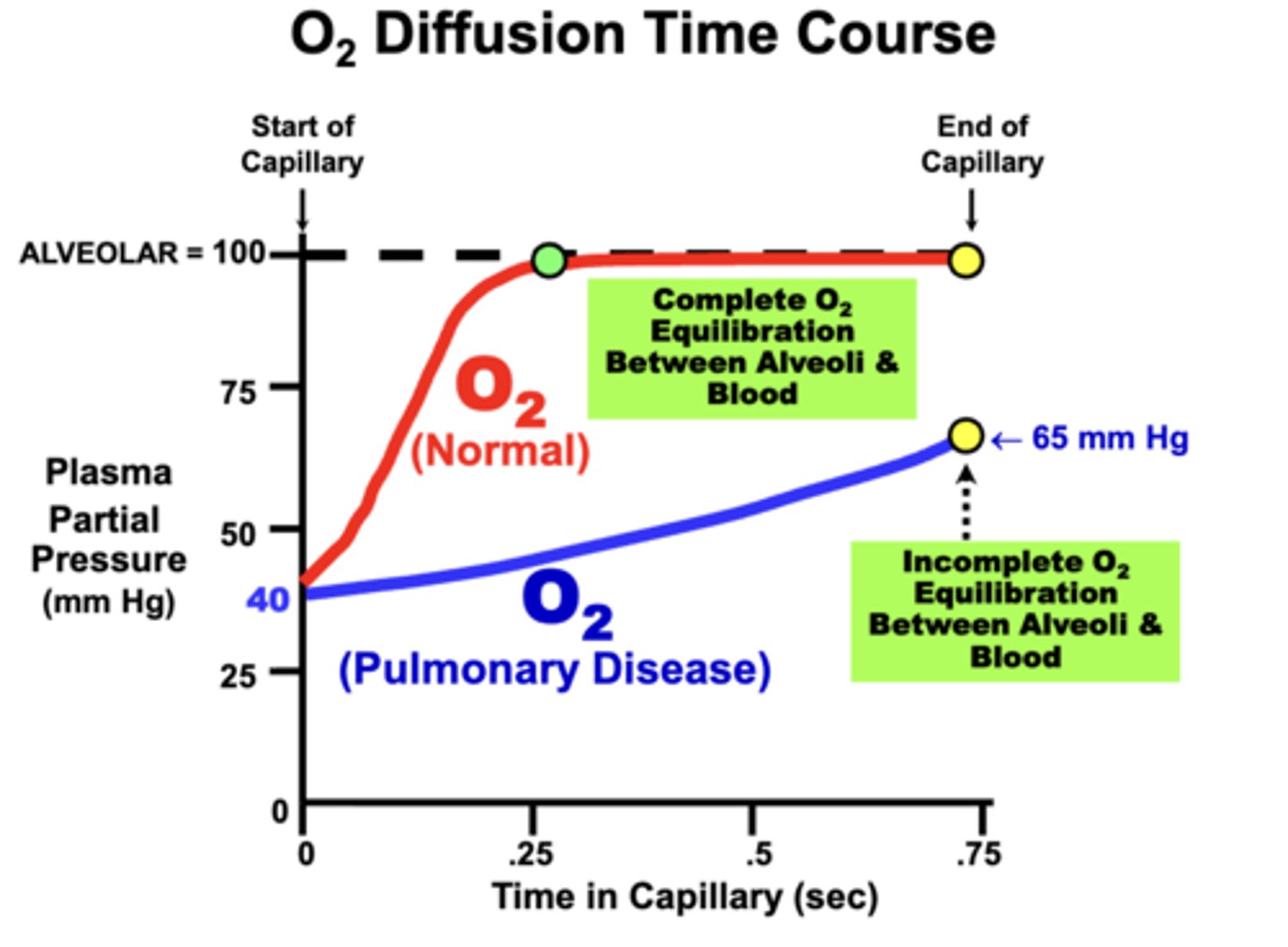
in terms of Fick's Law parameters, what can cause incomplete O2 equilibration to occur?
loss of diffusion surface area (e.g.emphysema) or alveolar wall thickening (e.g. fibrosis or inflammation)
- Clinically, this decreases arterial PaO2 and possibly Hb-O2 saturation
- Ultimately, O2 delivery to tissues may be compromised, depending on the severity of the condition
the ability of each gas to Equilibrate across the blood-gas barrier during an allotted time depends on what factors?
1. Diffusing Properties
2. Time Allowed for Equilibration
3. Gas Carrying Capacity of Blood
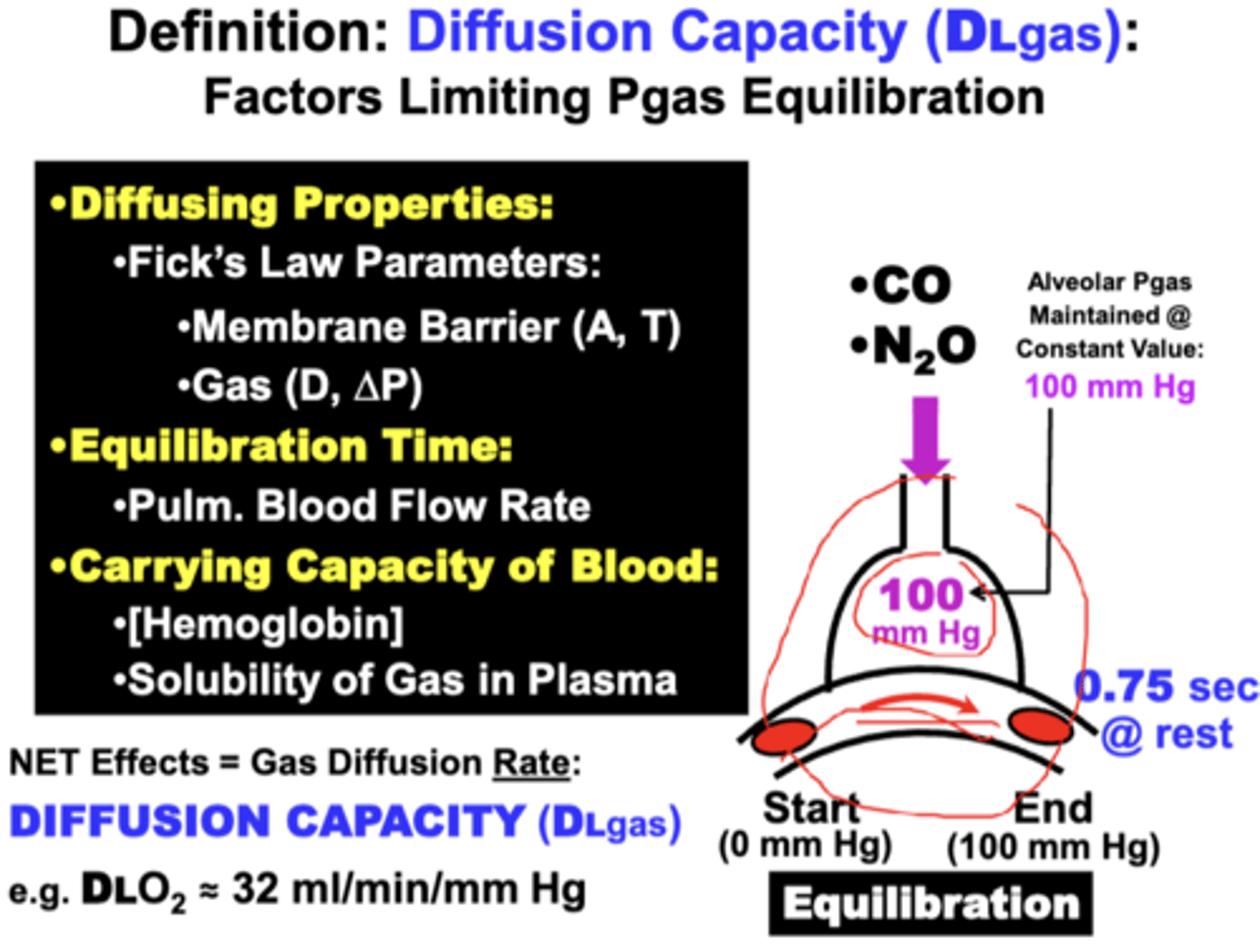
Diffusing Properties
fick's law parameters
of the membrane:
- surface area (A)
- membrane thickness (T)
of the gas:
- solubility coefficient (D)
- gas pressure gradient (ΔP)
equilibration time
normal time allowed for gas equilibration between the blood and lungs
- short (0.75 sec. @ rest)
Determined primarily by Pulmonary Blood Flow Rate.
ex. equilibration time can be reduced by increasing cardiac output during exercise.
gas carrying capacity of blood
for a particular gas:
- refers mainly to the Hemoglobin Capacity & Gas Solubility in blood
net effect of the equilibration factors
results in a rate of diffusion across the barrier termed the Diffusion Capacity (DLgas: e.g. DLO2), which is quantitatively expressed in units of ml/min/mm Hg
in terms of limited gas transfer, individual gases are classified how?
as Diffusion-Limited or Perfusion-Limited
- according to the predominant variable that limits the ability of the Pgas to equilibrate across a membrane barrier
limited gas transfer does not include?
Equilibration Time
- which is constant for all gases
- is, therefore, NOT a distinguishing parameter in the assay
Diffusion Limited
The rate at which gas transported is limited by the diffusion rate of gas across the membrane barrier
Perfusion Limited
The rate at which gas transported is limited by carrying capacity
CO gas diffusion is which type of limitation?
CO is classified as a Diffusion-Limited gas
- volume of CO capable of moving into the blood is limited by how rapidly CO can diffuse across the membrane barrier (i.e. diffusion rate)
- total volume of CO able to move into the blood from the lungs over the given time period is NOT limited by the capacity of blood to carry CO
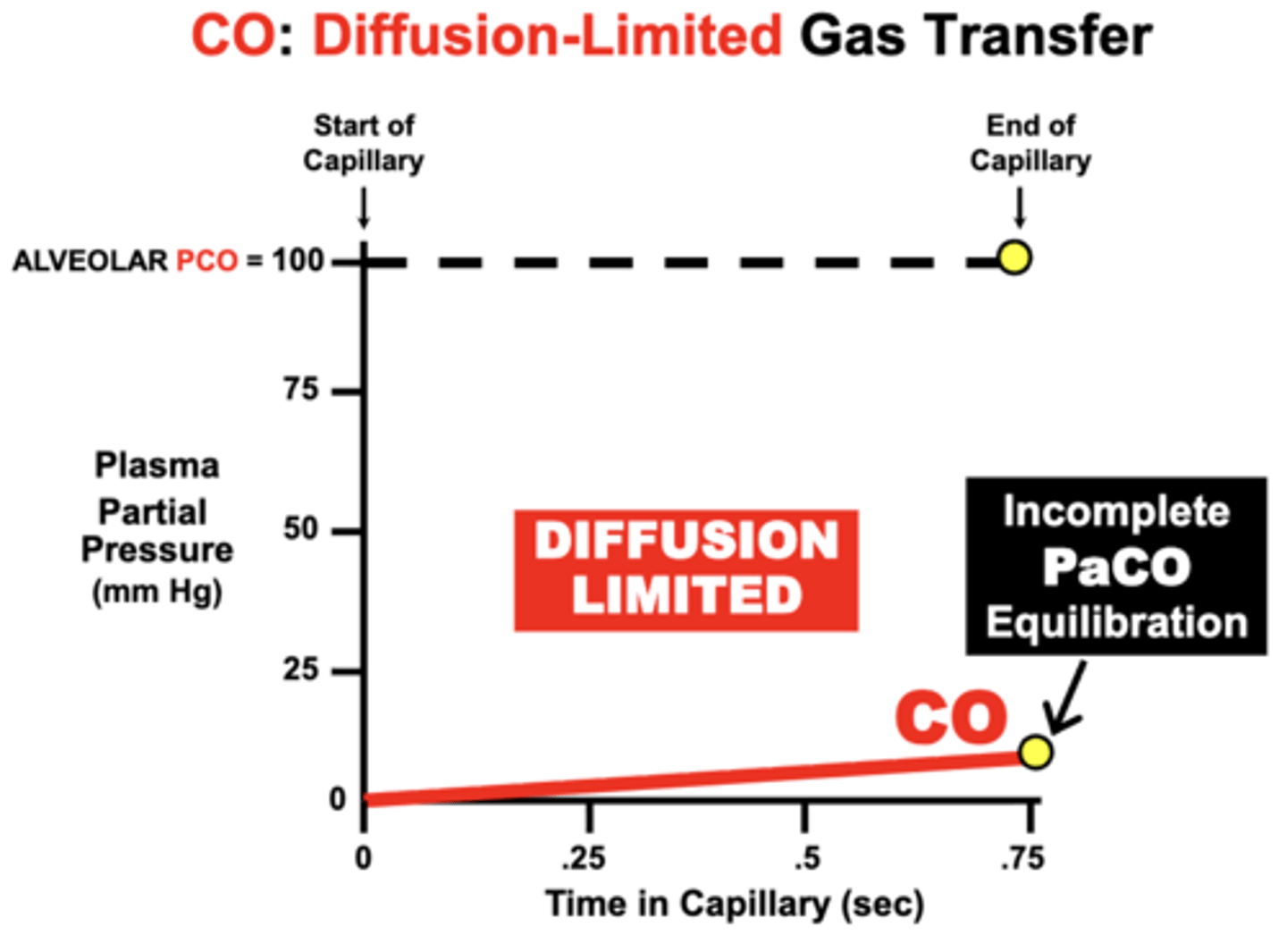
CO: Diffusion-Limited Gas Transfer
CO is maintained at a steady-state PCO of 100 mmHg within alveoli
- initial PCO (Start of Capillary) of the blood is 0 mm Hg and then rises as CO diffuses from the alveoli into blood
- PCO increases slowly by the time the blood reaches the End of Capillary
- indicates Incomplete PaCO Equilibration with the PACO during the 0.75 sec period of blood perfusion across the lungs
reason:
- while a small amount of CO becomes dissolved in plasma, the vast proportion of CO entering blood actually binds to Hb
- causes PCO to characteristically remain very LOW over the perfusion period
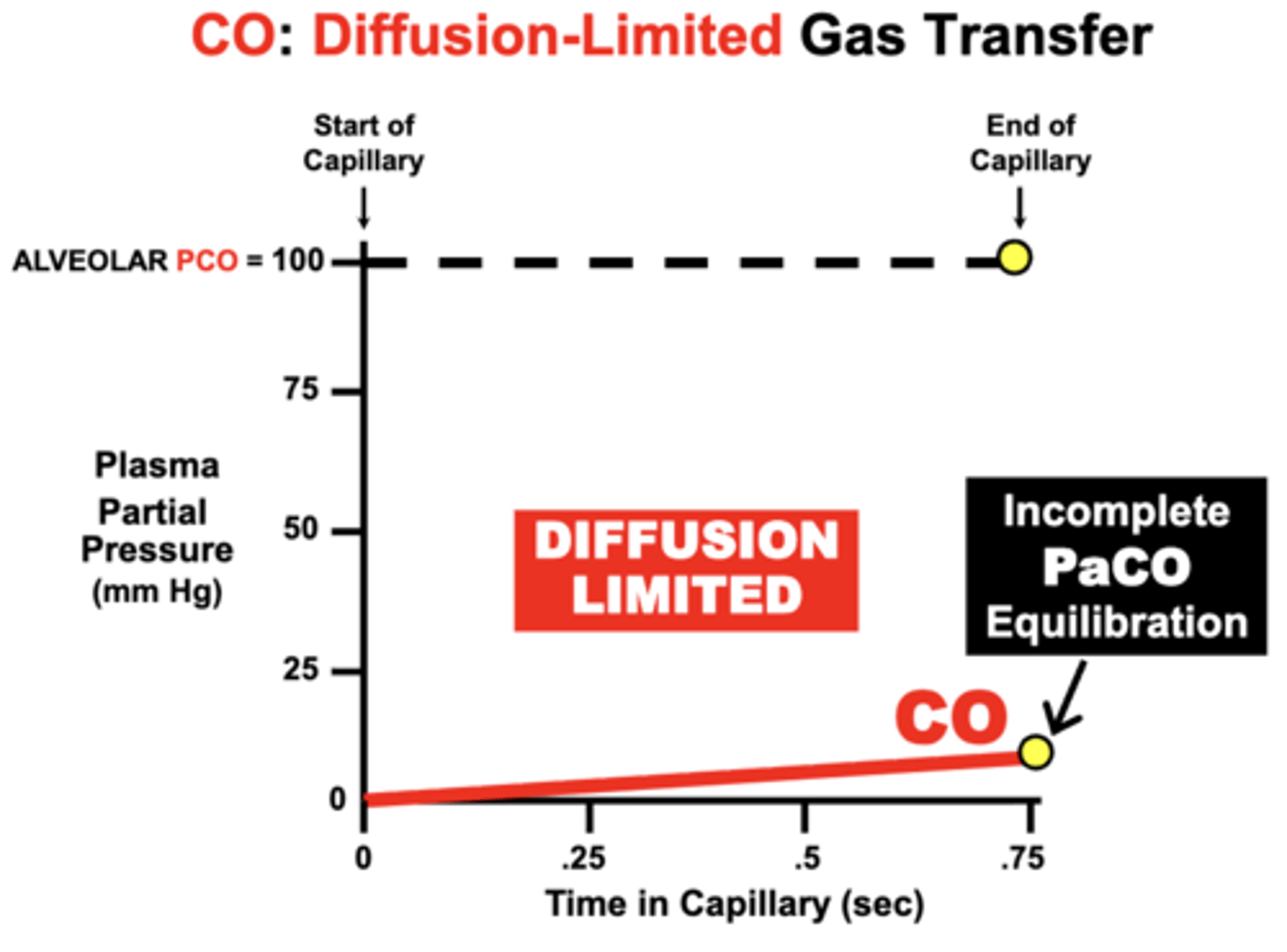
Clinical relevance of CO's equilibration
Because CO equilibration is almost exclusively determined by the lung’s membrane diffusion characteristics, CO is sometimes used diagnostically to determine the relative loss of normal lung Diffusion Capacity in patients with lung diseases impairing gas diffusion
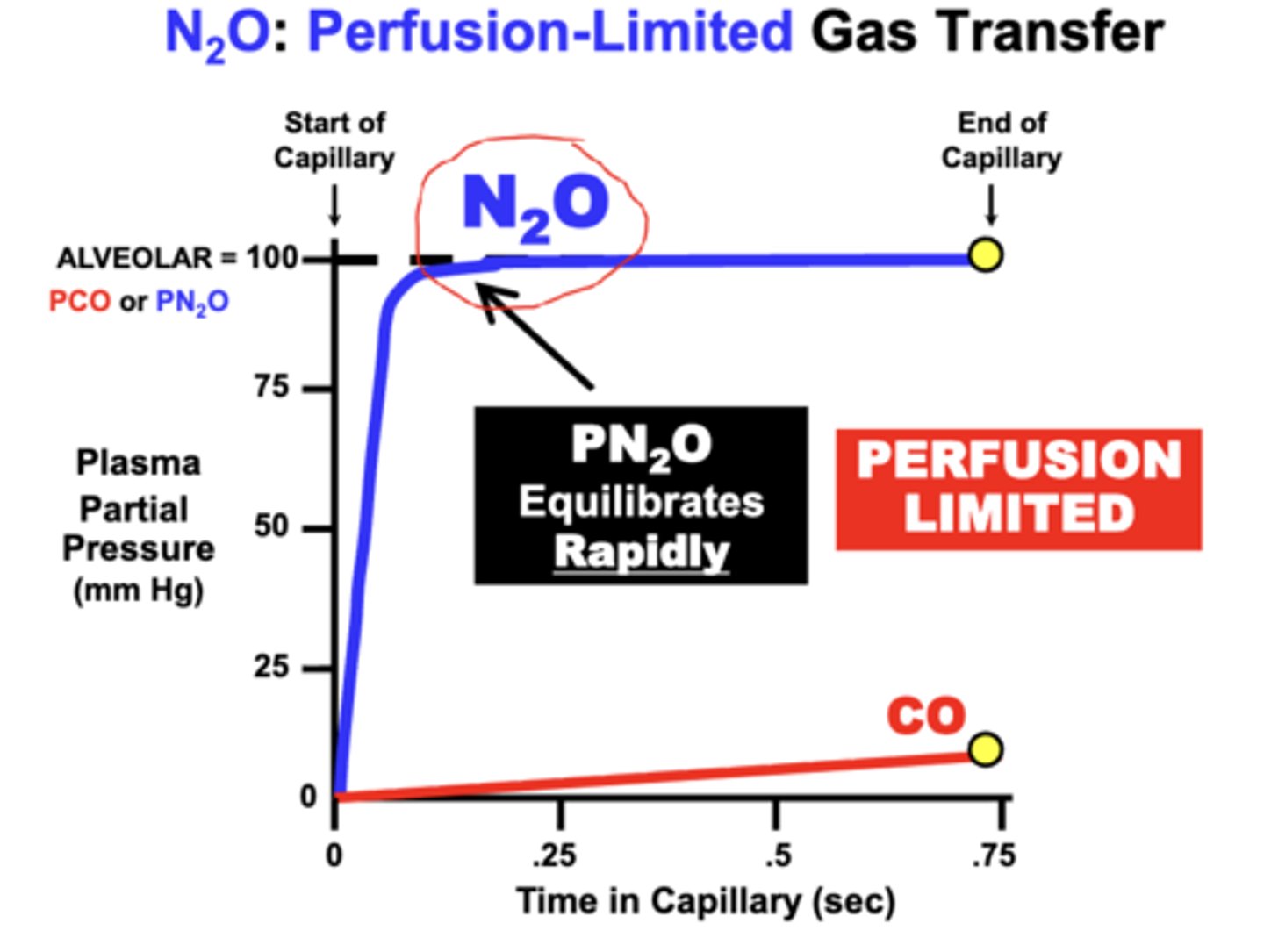
N2O gas diffusion is which type of limitation?
Nitrous Oxide (N2O) is classified as a Perfusion-Limited gas
N2O: Perfusion-Limited Gas Transfer
- there are no specific carriers in blood (e.g. Hb) for a gas such as Nitrous Oxide (N2O)
- so the total volume of N2O that diffuses into the blood can only become Dissolved in plasma
equilibration:
- PN2O of the blood Rapidly Equilibrates with the alveolar PAN2O during the perfusion period, because it is not a particularly soluble gas in plasma either
= maximum volume of N2O capable of diffusing into the blood over the given time period is primarily limited by the N2O carrying capacity of the blood
= Perfusion-Limited
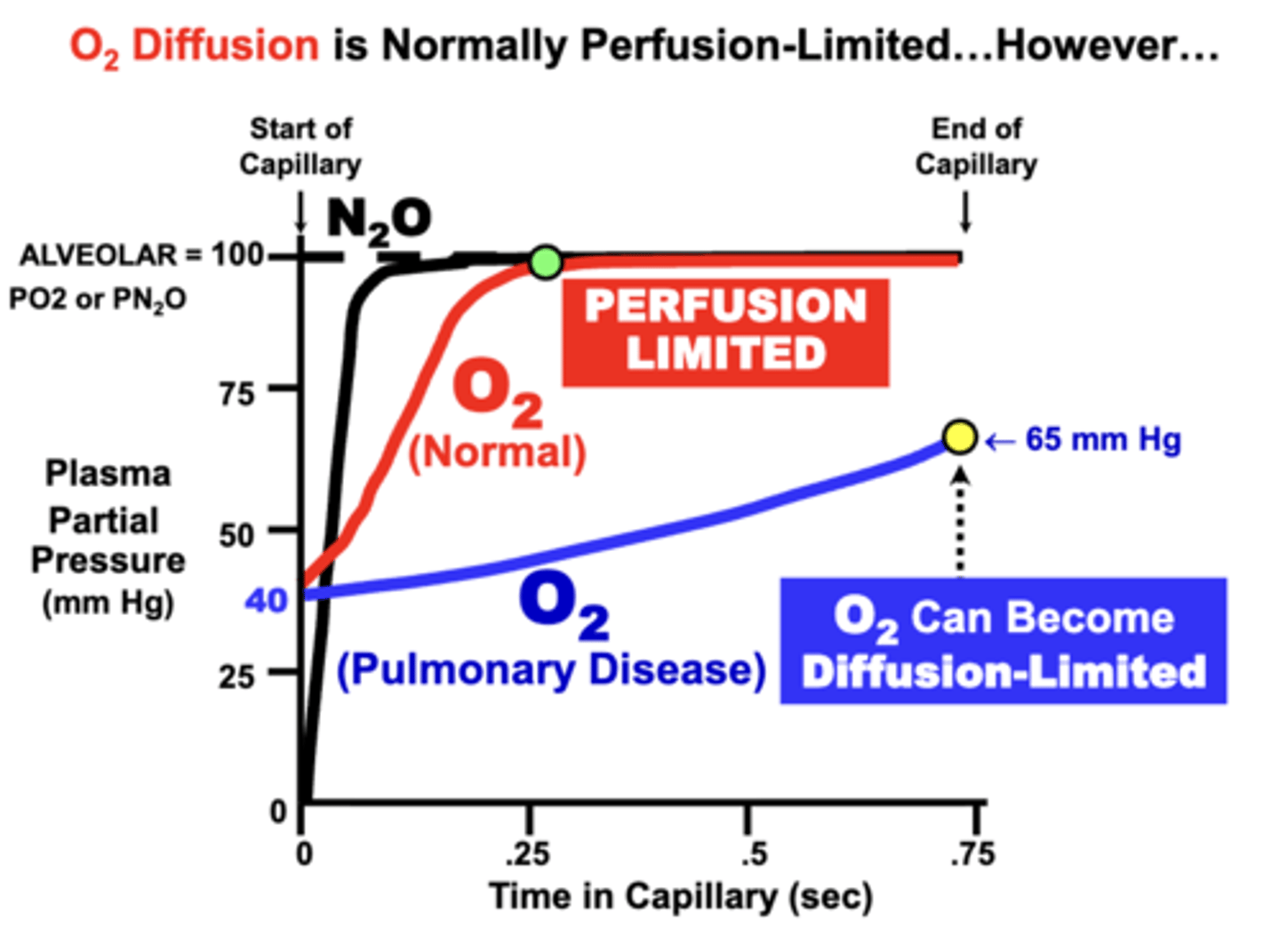
O2 equilibration classification
normally perfusion limited:
- blood PO2 of normal individuals at rest equilibrates rapidly (0.25sec) with alveolar PAO2 during the perfusion period
- plasma PO2 typically rises more rapidly than CO due to its lower affinity for Hb
O2 equilibration is NOT normally limited by the diffusion properties of the alveolar membrane and is classified as perfusion limited
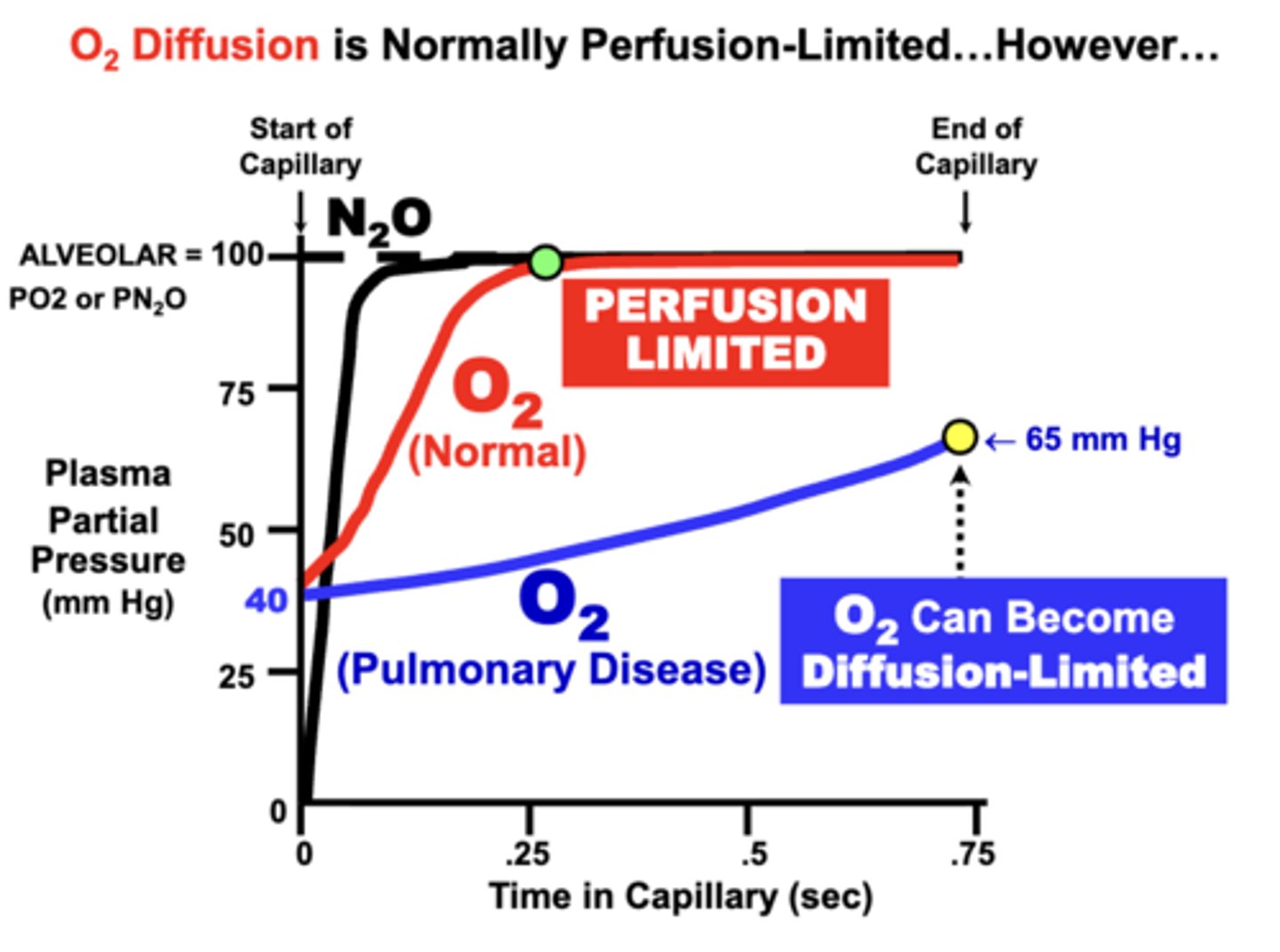
O2 as Diffusion Limited equilibration
development of respiratory disorders that impair alveolar membrane diffusing properties may prevent the blood from completely equilibrating with alveolar gases
- O2 transfer can become Diffusion-Limited
cause:
- in terms of Fick’s Law parameters, loss of diffusion surface area (e.g. emphysema) or alveolar wall thickening (e.g. fibrosis or inflammation)
- Clinically, this decreases arterial PaO2 and possibly Hb-O2 saturation
- Ultimately, O2 delivery to tissues may be reduced, depending on the severity of the condition
Reserve Diffusion Capacity of the Lungs
Large Reserve Diffusion Capacity:
- because the blood normally equilibrates quite rapidly (0.25 sec) at rest relative to the average amount of time allowed for equilibration (0.75 sec)
insures that blood becomes fully equilibrated with the alveolar compartment even during conditions that:
1) decrease equilibration time (e.g. exercise)
2) impair the diffusion properties of the lungs (e.g. reduced surface area, increased membrane thickness)
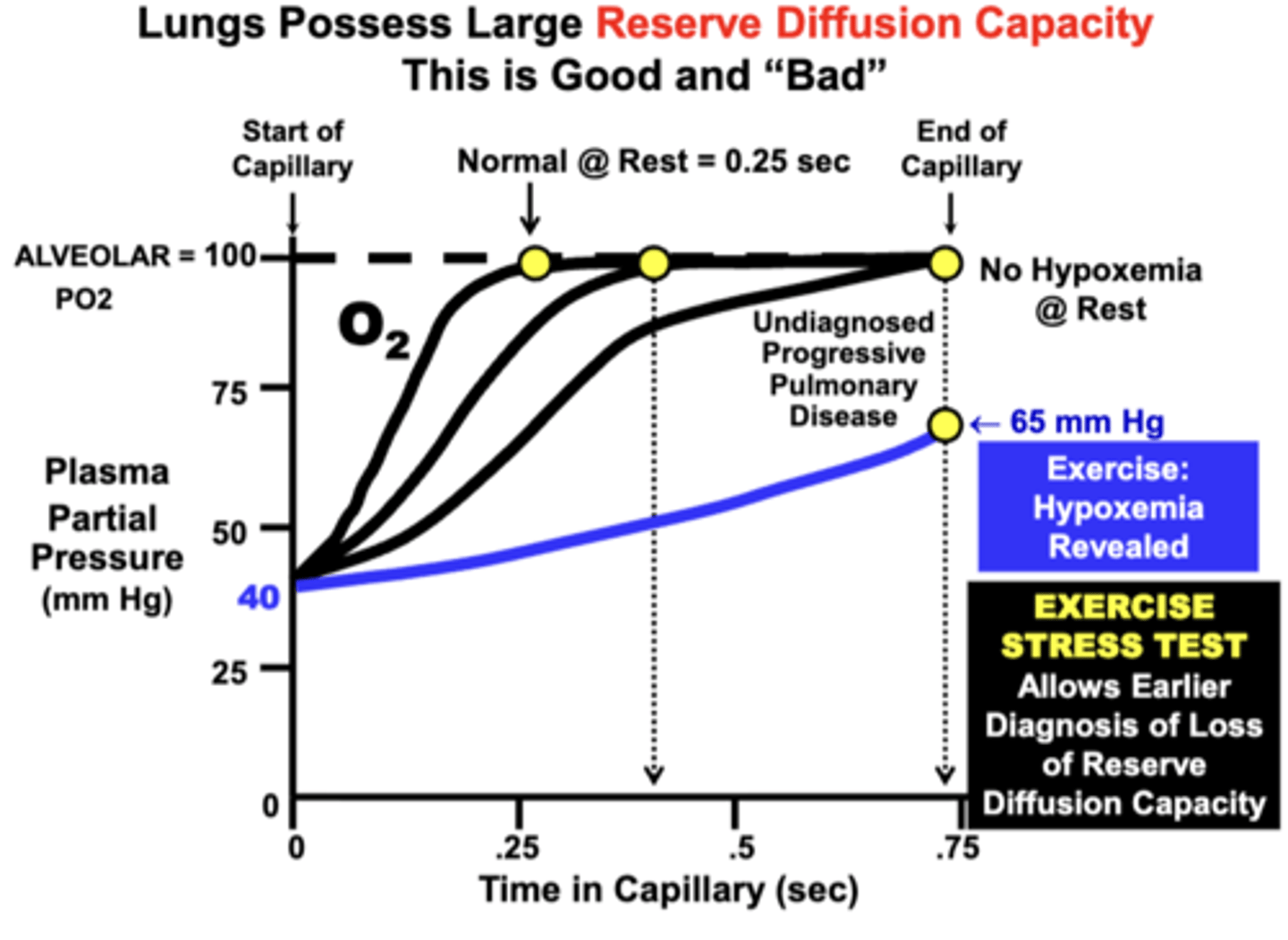
important clinical principle due to the larger reserve diffusion capacity of the lungs
a patient may possess a significant degree of pulmonary impairment, but have only mild or no symptoms at rest
Patient with Progressively Worsening Pulmonary Disease:
- at rest: is still able to fully re-saturate hemoglobin even though equilibration time is significantly increased over time (No Hypoxemia Evident @ Rest)
- during exercise: hypoxemia becomes evident (Hypoxemia Revealed During Exercise)
= Based on this effect, this is one reason why an Exercise Stress Test is administered diagnostically to patients suspected of developing a lung diffusion or other cardiopulmonary disorder: Earlier Diagnosis
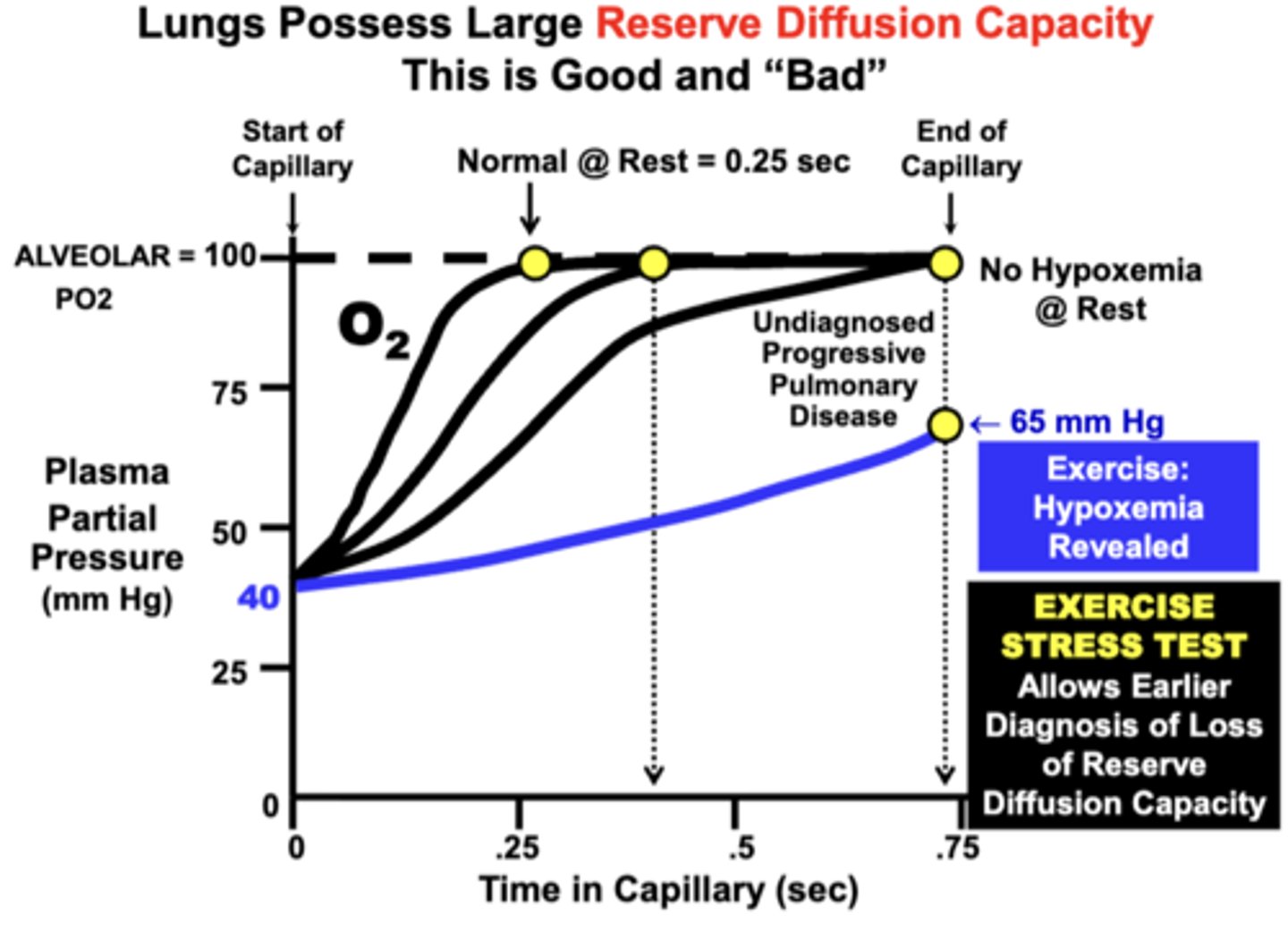
physiological basis of the best treatment for hypoxemia
Most Effective Treatment of Hypoxemia Due to a Diffusion Impairment is to have the patient Breathe Higher FO2 Air
- apparent from Fick’s Law of Diffusion, which indicates that increasing the partial pressure gradient (P1-P2) across the diffusion barrier, proportionately increases the rate of gas diffusion (Vgas)
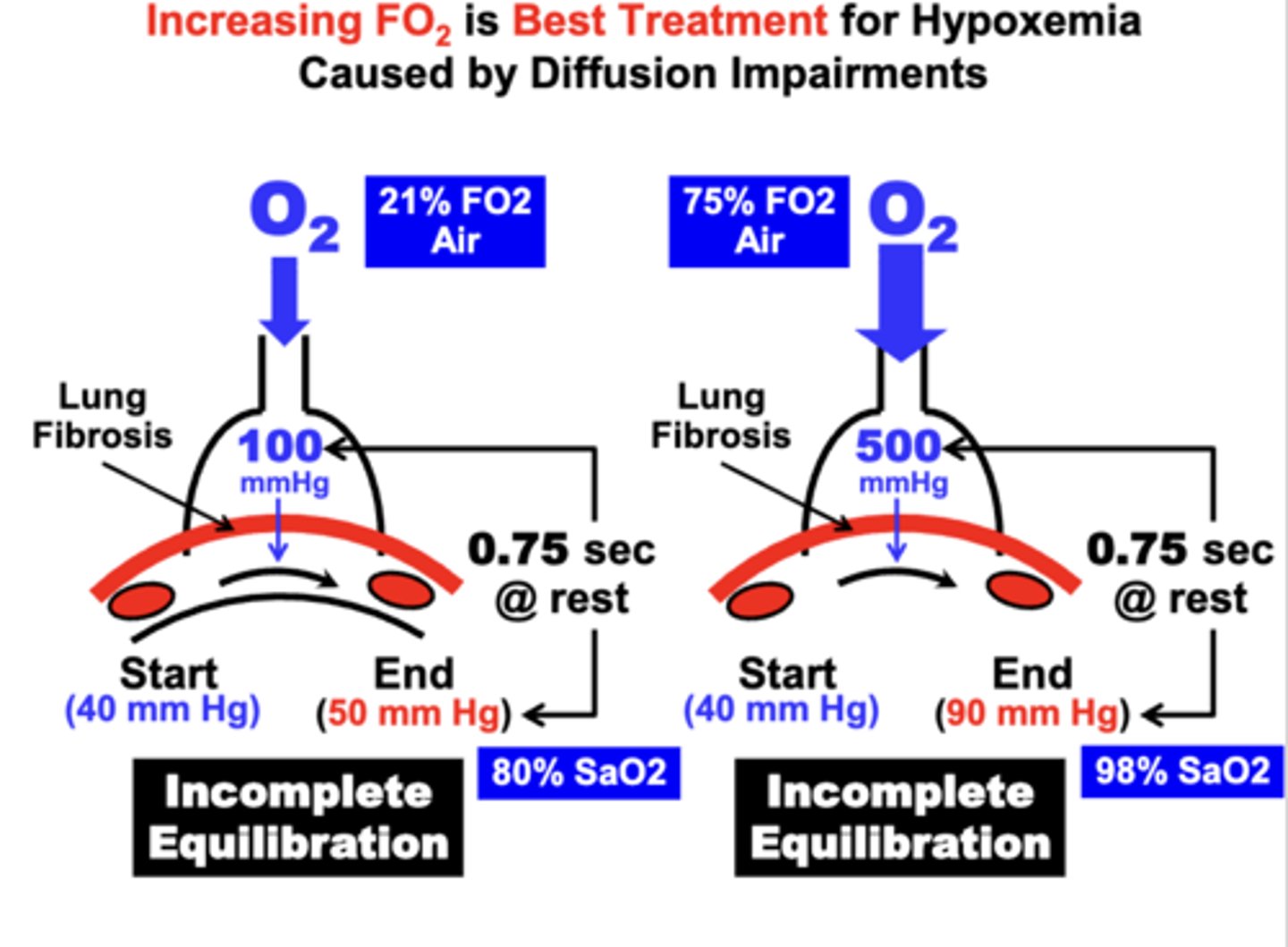
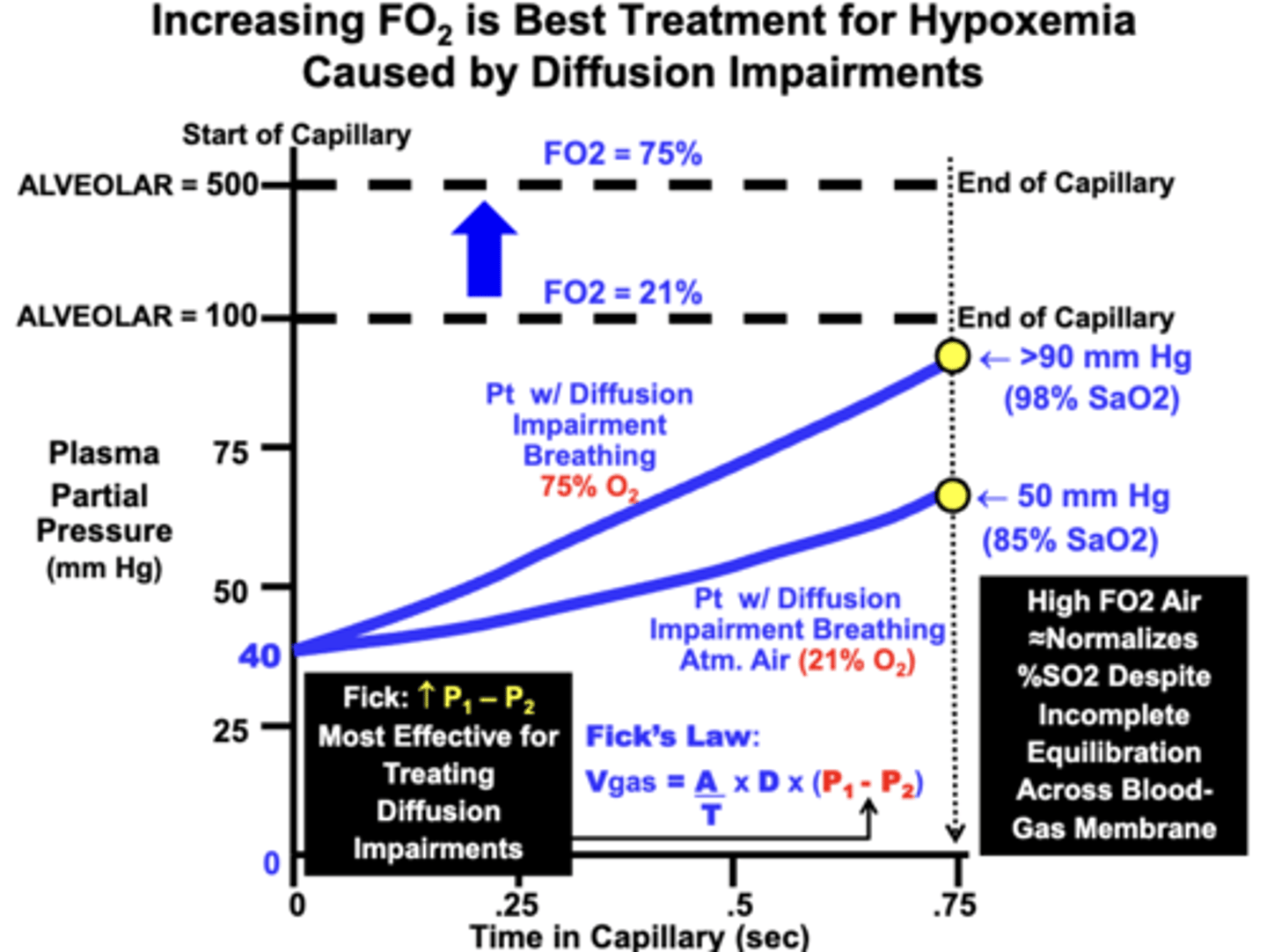
outcome of treating hypoxemia with high FO2 air
By increasing the alveolar PO2 from 100 mm Hg to 500 mm Hg by breathing 75% O2
= the amount of gas allowed to diffuse from the alveolar into the blood is increased between 7-8-fold
- results in a higher blood PO2 during the perfusion time period compared to the patient breathing atmospheric air
- importantly, a significantly higher Hb-O2 saturation is achieved (98% SaO2) compared to the lower FO2 (<90%)
- the equilibration “gap” between the alveolar and blood PO2 is actually increased by breathing the higher FO2 air
- the more important clinical outcome is that the PO2 is raised to near 100 mm Hg, which is the value necessary to achieve nearly 100% O2
- optimizes adequate O2 delivery to the tissues, which is the primary objective of treatment
- in this case, full equilibration is the less important goal.
CO2 vs O2 equilibration
- CO2 Diffuses Across Blood-Gas Barrier in the Opposite Direction from O2
- CO2 transport is more complicated than for O2 because it is carried as multiple biochemical forms
- CO2 transfer across the blood-gas barrier depends on uncertain rates of biochemical equilibration reactions
CO2 equilibration limitation
CO2 Diffusion is Most Likely Perfusion-Limited
- based on the relative low plasma solubility of CO2 which allows rapid equilibration with alveoli
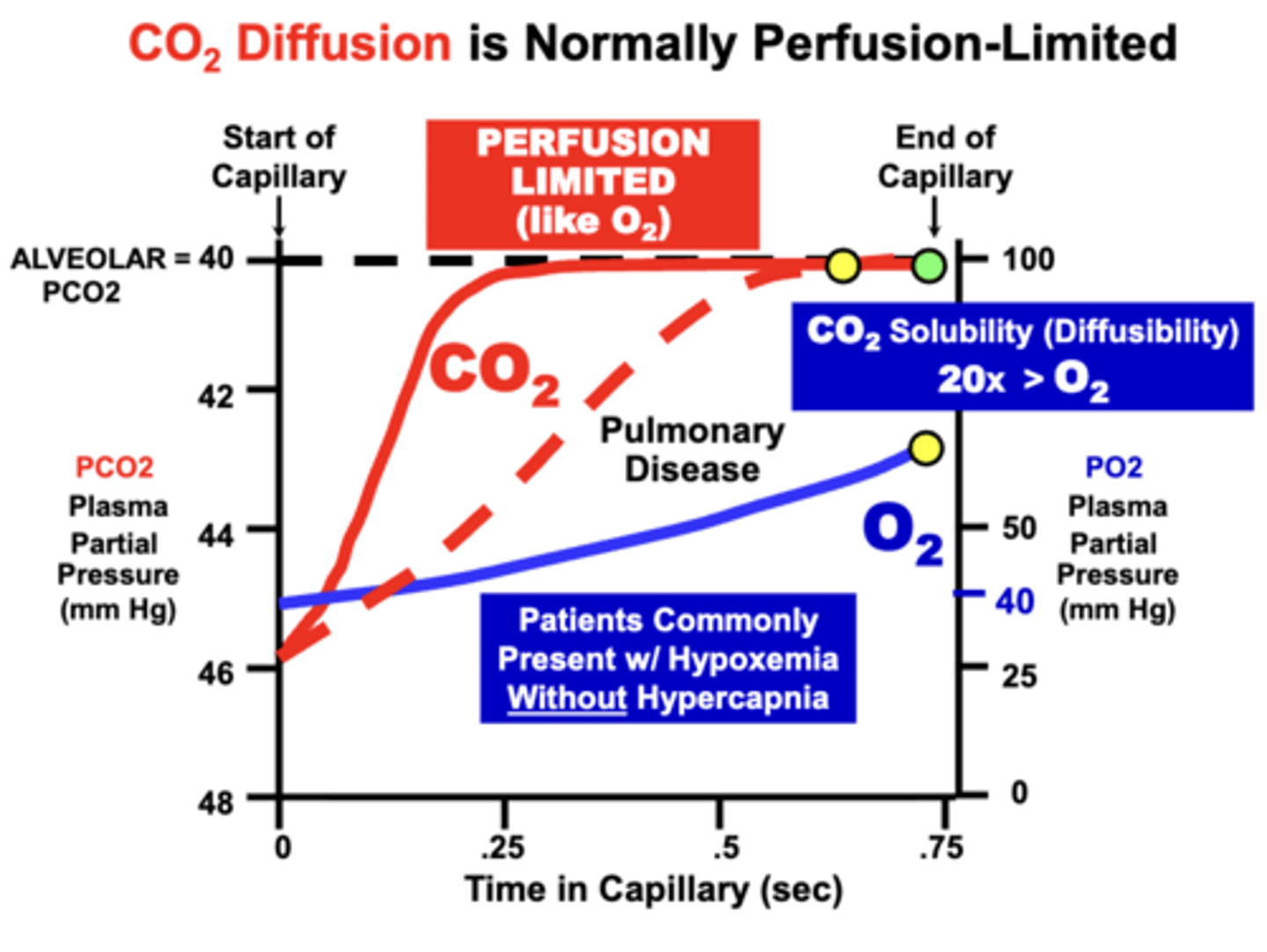
Like O2, CO2 can become?
Diffusion-Limited
- although more severe membrane diffusion impairments are generally required to prevent equilibration
- due to the higher (20x) relative solubility of CO2 compared to O2, which allows continued CO2 equilibration despite a severely diminished membrane diffusion capacity
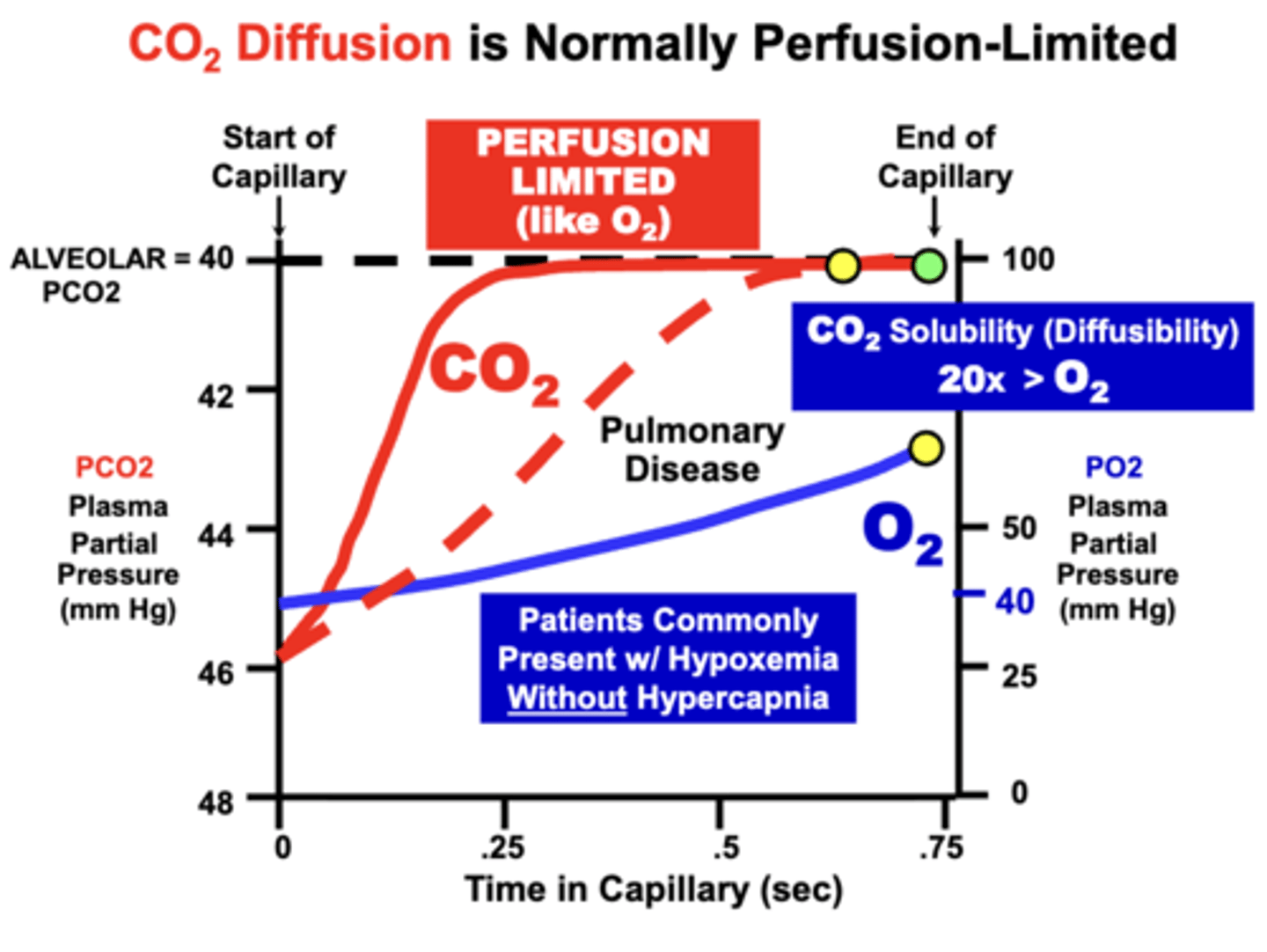
CO2 is often maintained during what conditions?
CO2 equilibration is often maintained during conditions that severely impair O2 diffusion.
- that is, patients will commonly develop Hypoxemia, but without Hypercapnia.
Diffusion Impairment Principles
1. Diffusion Impairments ALWAYS Decrease PaO2
2. Due to the greater diffusion capacity of CO2, PaCO2 may remain Normal or Increase depending on the severity of the impairment
3. Most Effective Single Treatment for Diffusion Impaired Hypoxemia is Hyperoxia
- hyperoxia normalizes hypoxemia by increasing the O2 diffusion gradient according to Fick's