Physiology Oct. 7th
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Does surface area scale linearly with mass?
No, surface area doesn’t scale linearly with mass as organisms increase in size
Ex. Rotifers are an example due to their high surface area relative to mass

Explain the Nature of Diffusion
Gas exchange occurs through diffusion from areas of high concentration to low concentration.
Efficiency of diffusion is time-dependent and correlates with distance.
Example: Diffusion across a cell membrane (10 nanometers thick) takes about 100 nanoseconds.
In larger distances, such as those in larger organisms (e.g., human nerve cells), reliance on diffusion alone becomes impractical.

Explain Gas Exchange in Plants
Getting CO2 out of the external medium (air) and into the leaf
Within the leaf CO2 needs to get into the chloroplast (Rubisco)
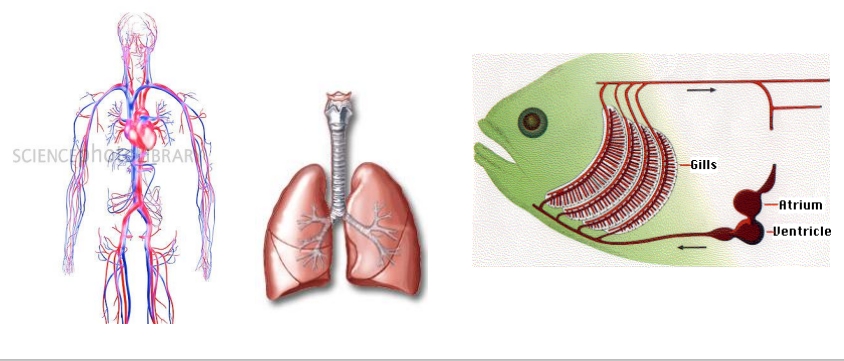
Gas Exchange in Animals
Getting O2 out of the external medium and into the cells
Often via the circulatory system in animals
Getting CO2 out of the cells and into the external medium
How much CO2 and O2 is found in the air?
Air (gases)
~21% O2
~0.04% CO2
Cold Seawater (in solution)
~0.8% O2
~93% of global CO2
What is the ideal gas law?
Describes how gases behave under various conditions, assuming gas molecules are perfect spheres in constant motion.
Involves variables like pressure (P), volume (V), and temperature (T).
Pressure and temperature are directly proportional (as one increases, so does the other).
Pressure and volume are inversely proportional (as volume decreases, pressure increases).
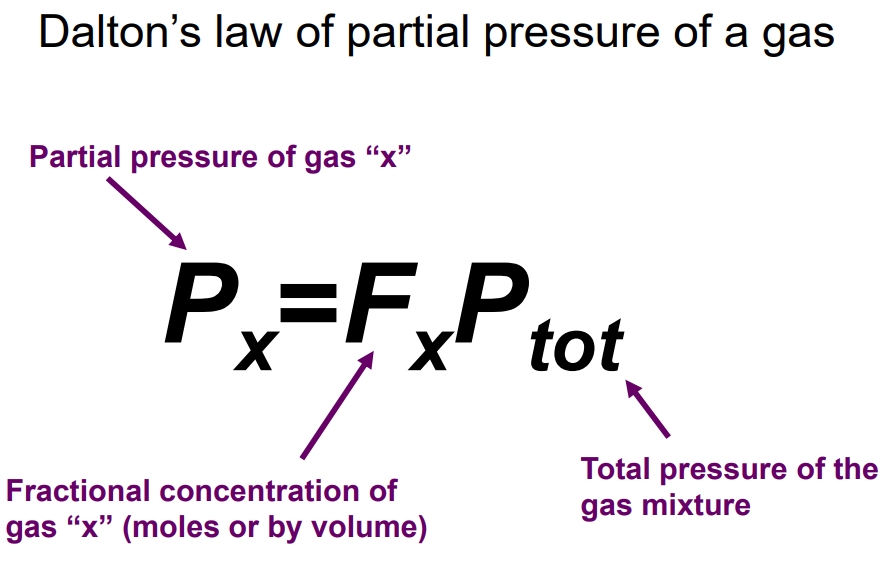
What is Daltons Law of Partial Pressures?
Total pressure exerted by a system of gases is the sum of the partial pressures of individual gases.
Increasing one partial pressure can increase the total pressure without affecting other partial pressures, as each gas contributes independently to the total pressure based on its concentration.
Example notations for atmospheric gases:
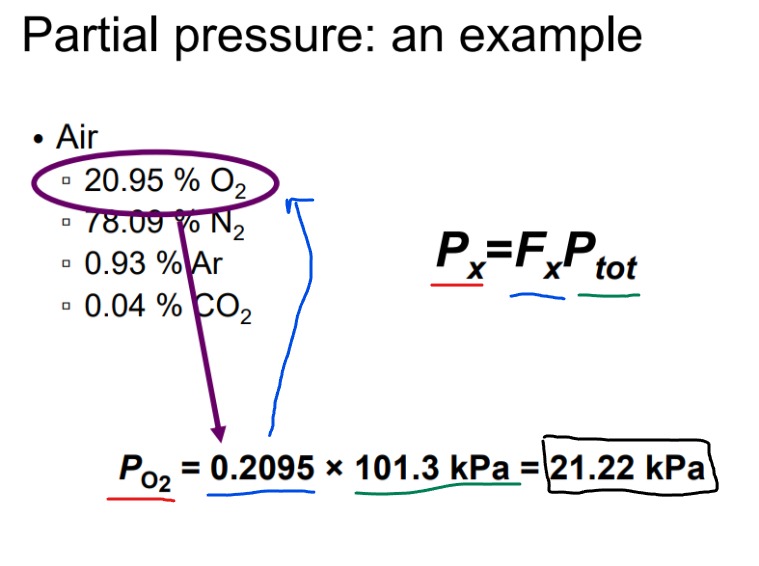
Atmospheric pressure: 101.3 kPa at sea level.
Ex. What’s the partial pressure of O2 if its 20.95% of air?
What is Henrys Law?
Describes the relationship between the partial pressure of a gas and its concentration in a liquid.
Solubility varies by gas (e.g. carbon dioxide is more soluble than oxygen)

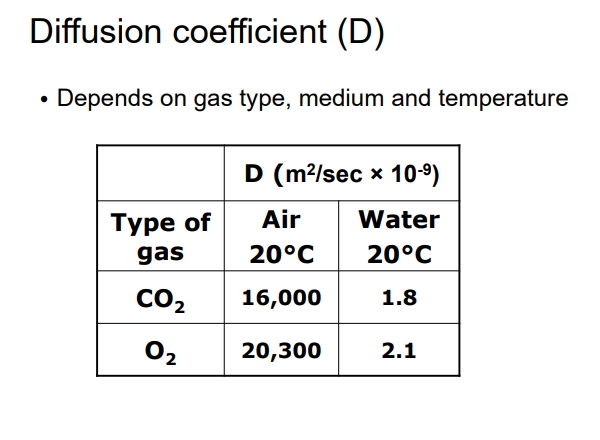
What is the diffusion coefficient?
Unique for each gas and can be influenced by the medium (air vs water).
Diffusion occurs faster in air compared to water (10,000 times faster).
What can effect the rate at which a gas diffuses?
The concentration of a gas directly affects its diffusion rate because diffusion depends on the concentration gradient
Larger the concentration difference = faster diffusion and vice vera
The area available for diffusion effects the the diffusion rate
wider the opening of a bottle (or the greater the surface area), the more gas molecules can move across, or out, at once

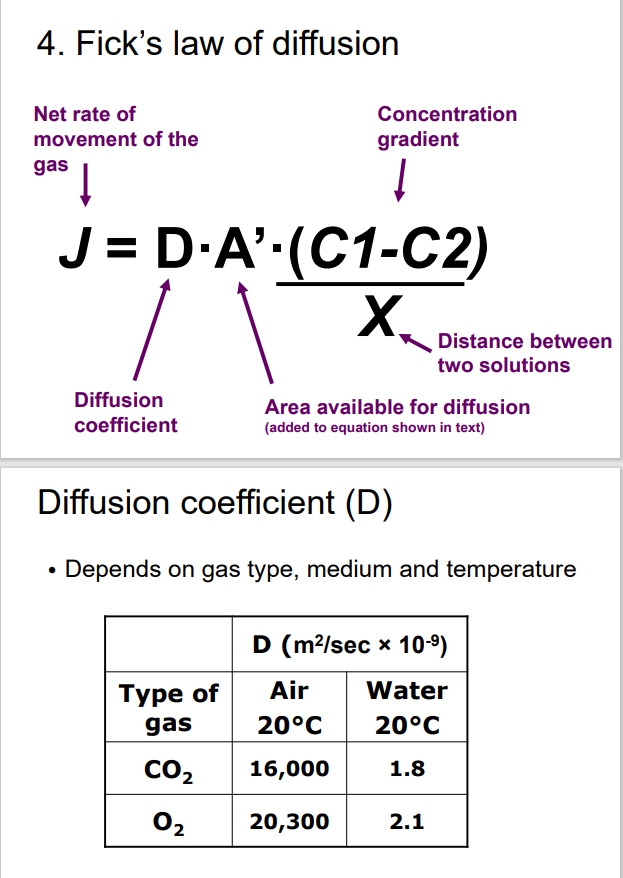
What is Fick’s Law of Diffusion?
Governs the net rate of movement of gases through diffusion.
Important to note that diffusion rates slow with increased distance.
What are the factors that affect gas solubility?
Temperature
Higher temperatures lead to decreased gas solubility.
Cold soda holds fizz better because CO₂ stays dissolved. Warm soda goes flat quickly — the CO₂ solubility drops as temperature rises (CO2 molecules move quicker when heated and won’t be able to stay in solution; want to get out into the air)
Salinity
Increased salinity reduces dissolved gas quantities.
Whats the equation once we put it all together?
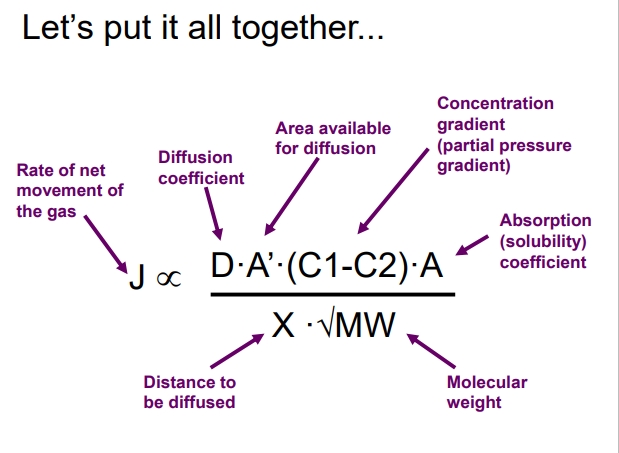
What variables of the previous equation can be modified?
Surface area, Concentration gradients, Distance over which diffusion occurs (A’, C1-C2, and X)
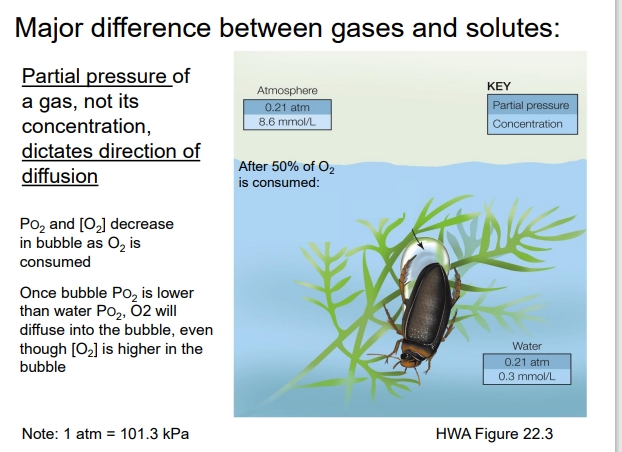
Whats the difference between gases and solutes?
For gases, its partial pressure, not concentration, that determines the direction of diffusion.
diffusion happens from areas of high partial pressure → low partial pressure, not necessarily from high concentration → low concentration.
For solutes (like salt or sugar in water), diffusion depends on concentration gradients — molecules move from high concentration to low concentration.
How does the beetle example explain this?
The beetle traps an air bubble under its body while underwater.
Inside the bubble: O₂ is used up as the beetle breathes, so PO₂ in the bubble drops.
The water around the bubble still has a higher PO₂, even though the O₂ concentration in water is lower than in the bubble (air).
Because gases diffuse according to partial pressure, O₂ from the water diffuses into the bubble, replenishing it.
(O₂ can move into the bubble even if the bubble still has more O₂ molecules (higher concentration)as long as its partial pressure is lower than the surrounding water’s)
Explain Convection vs.Diffusion
Diffusion
more passive of gas molecules from high to low concentration (or partial pressure)
its slow over long distances
works well for small organisms or across thin membranes (like alveoli in lungs)
Convection
bulk flow of air or fluid, carrying gases with it.
It’s much faster than diffusion because it physically moves large amounts of gas.
Driven by pressure differences, temperature gradients, or external forces (like stirring or wind).
Think of it as air circulation instead of random molecular movement.
Blood flow moving oxygen through your body.
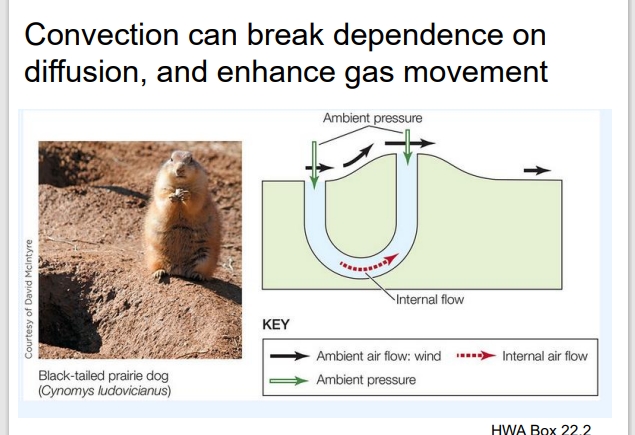
How does the Prairie Dog example help explain this?
The diagram of the prairie dog burrow shows how convection enhances gas exchange underground:
Wind moves over the two burrow openings, creating different pressures (low pressure at one end, high pressure at the other).
Upwind (left): Air hits directly, slows down, and “piles up” — this creates higher pressure.
Downwind (right): Air speeds up as it flows past, which lowers pressure.
Result: Air flows from left → right through the burrow (high → low pressure).
This pressure difference causes air to flow through the tunnels — that’s convection.
The flowing air continuously brings in fresh oxygen (O₂) and removes carbon dioxide (CO₂), which keeps the air in the burrow breathable.
If they relied only on diffusion, gas exchange would be much slower, and oxygen might not reach deeper parts of the burrow.
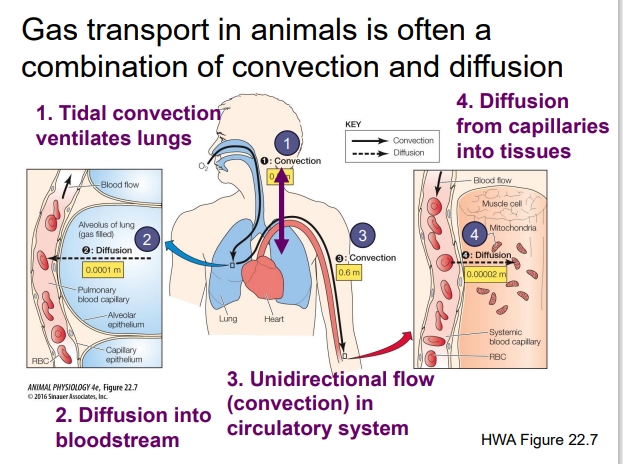
Explain gas transport in animals
Is often a combination of convection and diffusion
Tidal Convection Ventilates the Lungs:
Convection = bulk movement of air (breathing in and out).
Air enters the lungs by tidal ventilation — meaning air flows in during inhalation and out during exhalation.
This movement brings fresh O₂ to the alveoli and removes CO₂ from the body.
Think of this as the “delivery system” that refreshes the air inside the lungs.
Diffusion into the Bloodstream:
Once air reaches the alveoli (tiny air sacs), gas exchange happens by diffusion.
O₂ diffuses from high partial pressure in alveoli → low partial pressure in blood.
CO₂ diffuses the opposite way (from blood → alveoli).
This step is microscopic — gases move only a few micrometers across the alveolar and capillary membranes.
Unidirectional Flow (Convection) in the Circulatory System:
Once gases are in the blood, convection takes over again.
The heart pumps blood through vessels — a bulk flow that carries O₂-rich blood to tissues and brings CO₂-rich blood back to the lungs.
This is convection on a larger scale, like a delivery route.
Diffusion from Capillaries into Tissues:
When oxygenated blood reaches body tissues, O₂ diffuses from blood to cells where it’s used for metabolism.
CO₂ diffuses from cells to blood to be carried back to the lungs.
Again, this happens over short distances, driven by partial pressure gradients.