Quantum Model of the Atom test
1/21
Earn XP
Description and Tags
I didn't add ANY of the electron configuration questions because I feel you'd learn better by writing them out ദ്ദി˙-˙ )✧
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
How does the particle nature of light explain the photoelectric effect
Light must behave as a particle, as packets of energy must be moving a high enough frequencies in order to knock electron loose; this is what makes the metal emit electrons.
Order the following frequencies of electromagnetic radiation in order of energy: Microwaves, infrared, ultraviolet, gamma
Energy and frequency are directly proportional, so these are in order. (directly proportional means as one variable increases, the other variable also increases)
What is c?
The speed of light; 3.0E8 m/s
What is a quantum of energy?
the minimal energy that can be lost or gained by an atom.
What is light’s quantum?
Light’s quantum is the photon
Explain why the Hydrogen emission line spectrum only had specific frequencies
Electrons emit a photon when they go from an excited state to a grounded state. They only emit along certain frequencies because electrons orbit in discrete energy levels .
What is the Bohr model of a hydrogen atom?
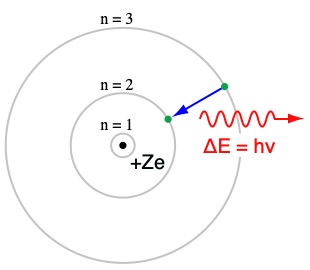
What are some flaws with the Bohr model?
It isn’t entirely accurate and only works with hydrogen and doesn’t account for the chemical behavior of atoms
What are each of the quantum numbers and what do they mean?
n= main energy level and can only be in integers (the energy level and distance of electron from nucleus
L is the shape of the orbital
m is orientation of the orbital around nucleus
Spin is +½ or -½
What is Heisenberg’s Uncertainty Principle
We can’t calculate both the velocity and position of an electron
What did the Schrödinger Wave Equation explain?
The probability that an electron would be in a given place, which is the orbital
Define the Aufbau Principle
Electrons will occupy the lowest energy level that will receive them
Define Pauli’s Exclusion Principle
No two electrons can have the same 4 quantum numbers
Define Hund’s Rule
Each orbital of equal energy will have one electron before it is occupied by a second electron
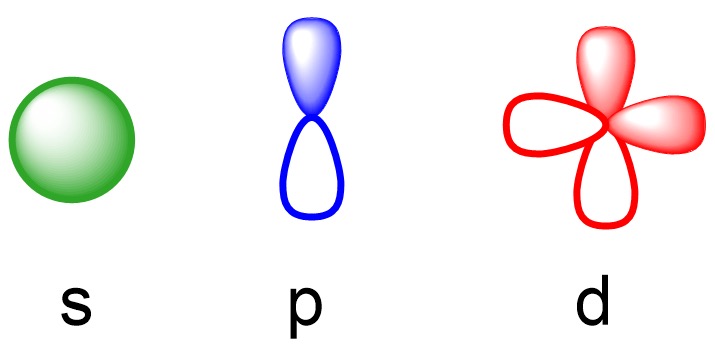
Diagram of the s, p, d orbitals
A way to remember the names and shapes: (Thank Annabelle’s older sister guys)
s= sphere
p= peanut
d= double peanut
f= flower
What are the n and L values for electrons in the 3s suborbital
n= 3, the main energy level
L=0, the value that corresponds to the shape of the S orbital
UV radiation has a wavelength of 4E-9. What is it’s frequency
Describe atomic radius and it’s behavior
Atomic radius the distance from the center of the nucleus to the end of the electron cloud and it decreases moving left to right across a period but increases going down a group. As you move left to right, while staying in the same period, you increase the protons without changing the energy level of the electrons. So the positive nucleus will pull in the cloud, making it smaller, while as you move down, the number of energy levels increases, making the atom bigger
Describe Ionization Energy and its behavior
The energy required to remove an electron. It increases going up a group and going right across a period. As atomic radius increases, the distance between the nucleus and the valence electrons increase, and the nucleus will exert less force on the electrons, making them easier to remove. Additionally, the energy levels between the nucleus and valence electrons will shield the shell from nucleus, making it even easier. As atomic radius decreases, the nucleus will hold onto the electrons more tightly, so its more difficult to remove
Electron Affinity and Electronegativity
Both very similar, they both increase up a group and right across a period. However, noble gases have basically no electron affinity/electronegativity because their valence shell is full. Electron Affinity is the energy gained as an atom absorbs an electron. It is quantifiable and has a unit; Joules (J). Electronegativity is the relative pull on electrons that one atom has over another. As atomic radius shrinks, the nucleus will more easily attract electrons
Melting point
The temperature at which a substance transitions from a solid to liquid. Generally, metals have high melting points and nonmetals have low melting points. Carbon’s melting point is the highest by a large margin. Melting point has to do with bond strength: As the bond gets stronger, the more energy is needed to break them and the melting point will increase.
Metallic Character
The reactivity of a metal. Increases going right to left and period and increases down a group. The easier is it to remove an electron, the more readily a metal will react