Alveolar Ventilation and gas exchange
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Why is the Total pressure @ lungs is 713 mmHg but the pressure at the atmosphere is 760?
This is because of water vapor. At the lung (wet surface), water vapor exists and displaces the gases. In this example, @ 37 ̊C (body temp), PH2O = 47; Thus 760-47=713
Given that the partial pressure @ lung surface:
PO2 = 149.7
PCO2 = 0.28
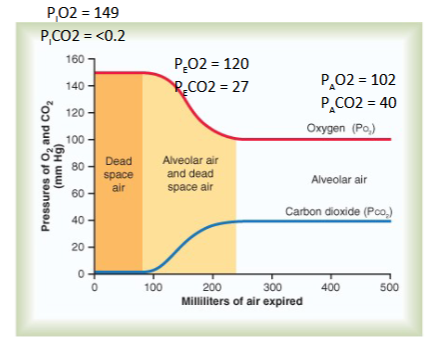
Explain the Partial Pressures of O2 and CO2 @ Alveolar and why these concentrations are different when Expired (@mouth):
Alveolar Air: PO2 = 104 mmHg PCO2 = 40 mmHg
Expired Air: PO2 = 120 mmHg; PCO2 = 27 mmHg
Alveolar Air:
PO2 is lower then atmosphere b/c:
Alveolar air is only partially replaced by atmospheric air
O2 is constantly diffusing from the alveoli into the blood
PCO2 is higher then atmosphere b/c:
CO2 is constantly diffusing into the alveoli from the blood
Expired Air:
PO2 is Higher then @ Alveolar Air b/c:
Alveolar air is mixed with dead space atmospheric air
O2 concentration is higher is atmospheric air than alveolar air
PCO2 is Lower then @ Alveolar Air b/c:
CO2 concentration is lower in atmospheric air than alveolar air
What determines Pgas in a fluid (ie. blood)?
Using this logic, why is PO2 > PCO2
Henry's law: Pgas (in fluid) = [gas]/solubility
CO2 is 20X more Soluble then O2; thus will have a lower pressure in fluid.
Describe the change in partial pressure in O2 and CO2 as you go from the mouth (atmosphere) down to the alveolar and explain why this occur.
Mixing of atmospheric air with dead space air and alveolar air creates a fall in PO2 and a rise in PCO2 by the time air reaches the alveoli
What is VE and what is the formula
What is VA and why is it lower then VE
How can you estimate VA ?
What can reduce/enhance VA
Minute ventilation (VE):
Total air volume exchanged (inhaled or exhaled) by the entire lung per minute
VE = VT x f
VT= ml of air per breath
f = breaths/min
Ex: 500 ml/breath x 15 breaths/min = 7500 ml/min (average 70kg male)
Alveolar ventilation (VA):
total air volume exchanged by the alveoli per minute
VA < VE b/c of Anatomic Dead Space (VD)
Anatomic Dead Space: conducting airways do not contain alveoli and do not participate in gas exchange*
Estimations:
VD is roughly: ~ 1 ml VD /lb body weight
VA = VT - VD x f
Rapid & shallow breathing pattern reduces VA
Slow and deep breathing pattern enhances VA
Compare and Contrast Anatomical/Physiological Dead Space
Anatomical Dead Space: refers to the fact that the conducting portion of the lungs can’t engage in gas exchange;
Physiological Dead Space: when pulmonary flow to a portion of alveoli is obstructed (Ex: pulmonary embolism) → thus that affected alveoli can’t exchange gas → dead space
What factors influences PAO2
What is the formula for PAO2?
PAO2 = [O2] entering - [O2] leaving; Determined by:
inspired oxygen, PIO2
alveolar ventilation rate VA
PAO2 is directly proportional to PIO2 and VA
PAO2 = PIO2 – PACO2/R
R = respiratory gas quotient (VCO2/VO2)
If Carbs: R = 1 b/c tissues produce 1 CO2 for each O2 consumed
If Fat: R = 0.70 b/c ~ 16 CO2 are produced for every 23 O2
Under normal circumstances R is estimated at 0.8
If R > 1; More CO2 is produced then O2 consumed
If R < 1; less CO2 is produced then O2 consumed
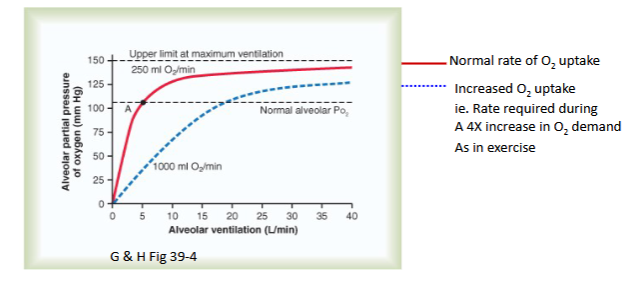
Draw out the graph depicting Alveolar Partial Pressure of O2 and Ventilation Rate given a O2 uptake of 250 vs 1000 ml O2/min
Explain the graph
As you can see, there is a direct relationship between ventilation rate and alveolar partial pressure of Oxygen;
Also, if you increase the O2 uptake for some reason (in this case,
b/c you’re exercising and need more O2); then you need to increase the ventilation rate in order to get back down to physiological O2 partial pressure. This is b/c w/ more O2 , you need to increase frequency of ventilation to diffuse more O2 (into blood)
What is the formula for PACO2?
What is PACO2 determined by?
PACO2 = VCO2/ VA:
directly proportional to CO2 production by the tissues
inversely proportional to VA
during ventilation, CO2 leaves lung/alveoli
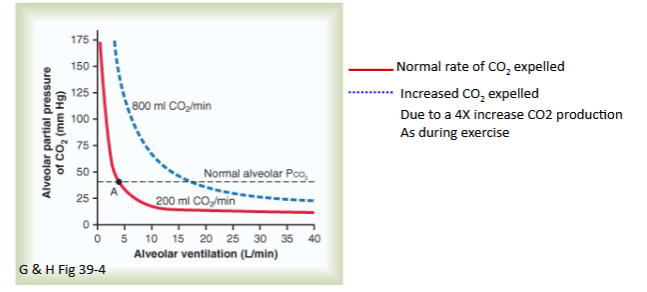
Draw out the graph depicting Alveolar Partial Pressure of CO2 and Ventilation Rate given a CO2 uptake of 200 vs 800 ml O2/min
Explain the graph
As you can see, there is an inverse relationship between Partial Pressure of CO2 and Ventilation rate
Also, the graph is shifted to the right in the presence of more CO2 in the Alveoli/min; This is because ventilation has to increase to match the increased CO2 in the alveoli
Describe how metabolism affects O2 and CO2?
How can Gas transport imbalances occur?
Metabolism:
↑ metabolism → ↑ O2 consumption → ↑O2 delivery
↑metabolism → ↑CO2 production → ↑CO2 removal
Gas transport imbalances:
amount of O2 and CO2 used and produced by tissues must exactly match the amount of O2 and CO2 that enters and exits the lungs
Imbalance results from lung disease or exercise
↓Blood [O2] = hypoxemia
↑Blood [CO2] = hypercapnia (acidosis)
Describe the layers that gas has to diffuse through in the respiratory system
Layers of the membrane
Layer of fluid mostly surfactant
Alveolar epithelium
Epithelial basement membrane
Thin interstitial space between alveoli and capillary
Capillary basement membrane
Capillary endothelial membrane
What are three factors that affect equilibration?
Diffusing properties (Fick’s law)
(membrane and gas)
Ficks equation in the lung
Equilibrium time
Blood carrying properties (ie Hemoglobin)
Describe Fick’s Law
DeltaP: partial pressure difference of the gas across the membrane
Area: surface area available for diffusion
Thickness: thickness of the membrane.

Describe how properties of the membrane varies during respiration cycle
Area/Thickness:
Inspiration: Stretch maximizes Surface Area and minimizes Thickness
Vice Versa
Pressure Gradient:
@ end of inspiration: influx of air → maximizes PO2 and minimizes PCO2
Why is the equilibrium time for O2 and CO2 same despite O2 having a much steeper pressure gradient?
Although partial pressure differences for CO2 are less, due to its
solubility it diffuses about 20 times more rapidly, therefore, it reaches
equilibrium in the capillary bed at about the same time as O2
Describe the consequences of:
Hypoventilation
Hyperventilation
Hypoventilation:
hypercapnia and can lead to acidemia (↑H+ ion)
Hyperventilation:
results in hypocapnia and can lead to alkalemia (↓H+ ion)
What are the three forms of O2 in blood? How much O2 is dissolved in plasma?
Forms:
Dissolved O2
15 mlO2/min
PO2
Bound O2
If O2 can travel through any membrane, Why do we need Hb?
This is b/c O2 has low solubility in plasma (15 mlO2/min); In theory, we can/do use this dissolved O2, but the average 70K human needs 250 mlO2/min at rest; Hb solves this.
[REVIEW] Properties of Hb

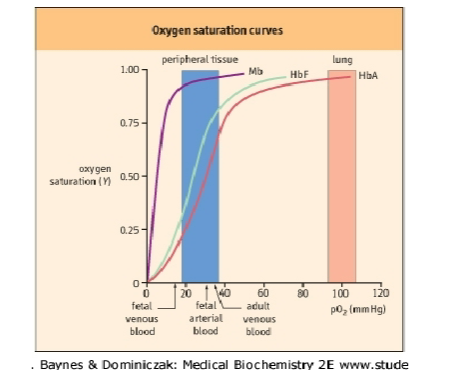
Differentiate between myo and hemoglobin
Hb:
restricted to erythrocytes
heterotetrameric protein
four O2 binding sites
O2 transport from lung to tissues
Mb:
found in striated and skeletal muscle
Monomeric – one O2 binding site
stores O2 in cytoplasm
delivers O2 to mitochondria on demand
Note the difference in saturation curves; Hb has a sigmoidal curve b/c of its cooperative characteristics
A person is offering you a can of pure O2, stating that this extra O2 will greatly benefit your body. Why is this BS?
you can have as much O2 as you want, but at some point, Hb will be saturated to 100% and that excess O2 can’t be put to actual use.
List the 4 mechanisms that alters O2 transport
Blood flow (HR x SV)
a-v O2 difference
O2 capacity (Hb content)
Anemia: a deficiency of Hb
Polycythemia: an increase in Hb
Shifting of the curve (Bohr effect)
Partial pressure of CO2
pH or H+
Temperature
2,3 diphosphoglycerate (2,3 DPG)
Describe the positive/negative allosteric effector that affects the oxyhemoglobin dissociation curve
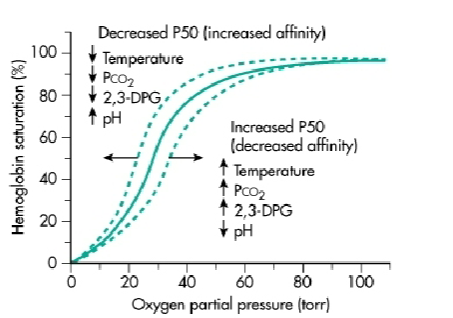
Describe the Bohr effect and mechanism
Bohr Effect: decreasing pH → decreaes Hb’s Affinity to O2 (right shift):
Mechanism:
@ lung Alveoli: O2 binding to Hb releases H+
This is b/c when O2 binds, Hb transition from Taut (T) → Relaxed (R) state → This releases H+ which used to stabilized the Taut State
This H+ then combines w/ HCO3- to make CO2 to be exhaled
@ Tissue capillary beds: Increased [H+] → enhances O2 release for aerobic metabolism
Describe the effect of CO2 and mechanism
CO2: decreases affinity of Hb for O2
Mechanism:
Increased PCO2 in venous capillaries
Hb-NH2 + CO2 ↔ Hb-NH-COO- + H+
carbamino-Hb (Hb-NH-COO) decreases O2 affinity
CO2 → Lungs as Carbamate + HCO3-
erythrocyte carbonic anhydrase → Carbamic Acid (H₂N-COOH) → provides a source of H+ for Bohr Effect → further decreases O2 affinity
Decreased PCO2 at the lungs
CO2 released from N terminus of Hb (increases O2 affinity)
Reversal of carbonic anhydrase rxn
Describe the effect of 2,3-bisphosphoglycerate and mechanism
What happens in hypoxia?
erythrocyte 2,3-BPG is negative allosteric effector of Hb that increases P50 (decreases Hb-O2 affinity)
without 2,3-BPG, Hb has Mb O2 saturation curve
Mechanism:
negatively charged 2,3-BPG binds to central site between B1 and B2 subunits → stabilized T-state → low O2 affinity
Increase in P02 → central pocket collapses, releasing 2,3-BPG
Hypoxia:
decrease PO2 → increases in 2,3-BPG → stabilizes deoxyHb → more O2 released to hypoxic tissues
Why is carbon monoxide dangerous?
Dangers of CO:
Affinity:
CO can bind to Hb w/ an affinity 250X stronger then O2 → Cooperativity causes other sites to have an even stronger affinity to CO → O2 can’t bind
Hb-CO liberates CO very slowly.
Makes it Hard for O2 to liberate as well
Left-Shift
Hard to Detect:
symptoms of CO poisoning are those of any type of hypoxia, especially headache and nausea
oxygen content of blood is greatly reduced but PO2 of the blood may be normal.
the normal PO2 → carotid and aortic chemoreceptors does not illicit a respiratory reaction
blood is bright red and there are no obvious skin signs of hypoxemia
***Death results when about 70-80% of the circulating hemoglobin is converted to Hb-CO.***
Describe the regulation of Hb Production
Kidney senses local tissue hypoxia → releases erythropoietin (EPO*) → travels to bone marrow → stimulate differentiation of
hemotapoietic stem cells
*= a peptide hormone that works through tyrosine kinase receptors
How is CO2 transported?
Gaseous and dissolved CO2 in plasma
Carbamino compounds: Hb-NH-COO- + CO2
Bicarbonate (HCO3-)
Describe the Haldane Effect
O2 bonds Hb at lungs it becomes a stronger acid → Displaces CO2 b/c:
Acidic Hb has less affinity for CO
Increased acidity causes release of excess H+ ions which bind with HCO3 → carbonic anhydrase rxn
Describe the steps of gas transfer from tissue to plasma
Oxidative metabolism in tissue
O2 unloading to meet demand
CO2 in tissue moves into plasma
Formation of bicarbonate
slow in plasma; Fast in RBC via carbonic anhydrase
Setting up gradients
more HCO3- diffuses out compared to H+
sets up an electrical gradient
Haldane and Bohr effects
formation of carbamino-Hb with continued desaturation of Hb
reduced Hb is strong proton acceptor, binds H+
forming acid Hb
Chloride shift
to maintain electroneutrality Cl- moves from
plasma into RBCthis results in greater ion concentration within
RBC therefore H2O also enters RBC
All steps reversed @ Lung