Pericyclic Reactions
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
How does your very generalized Diels-Alder reaction look like?
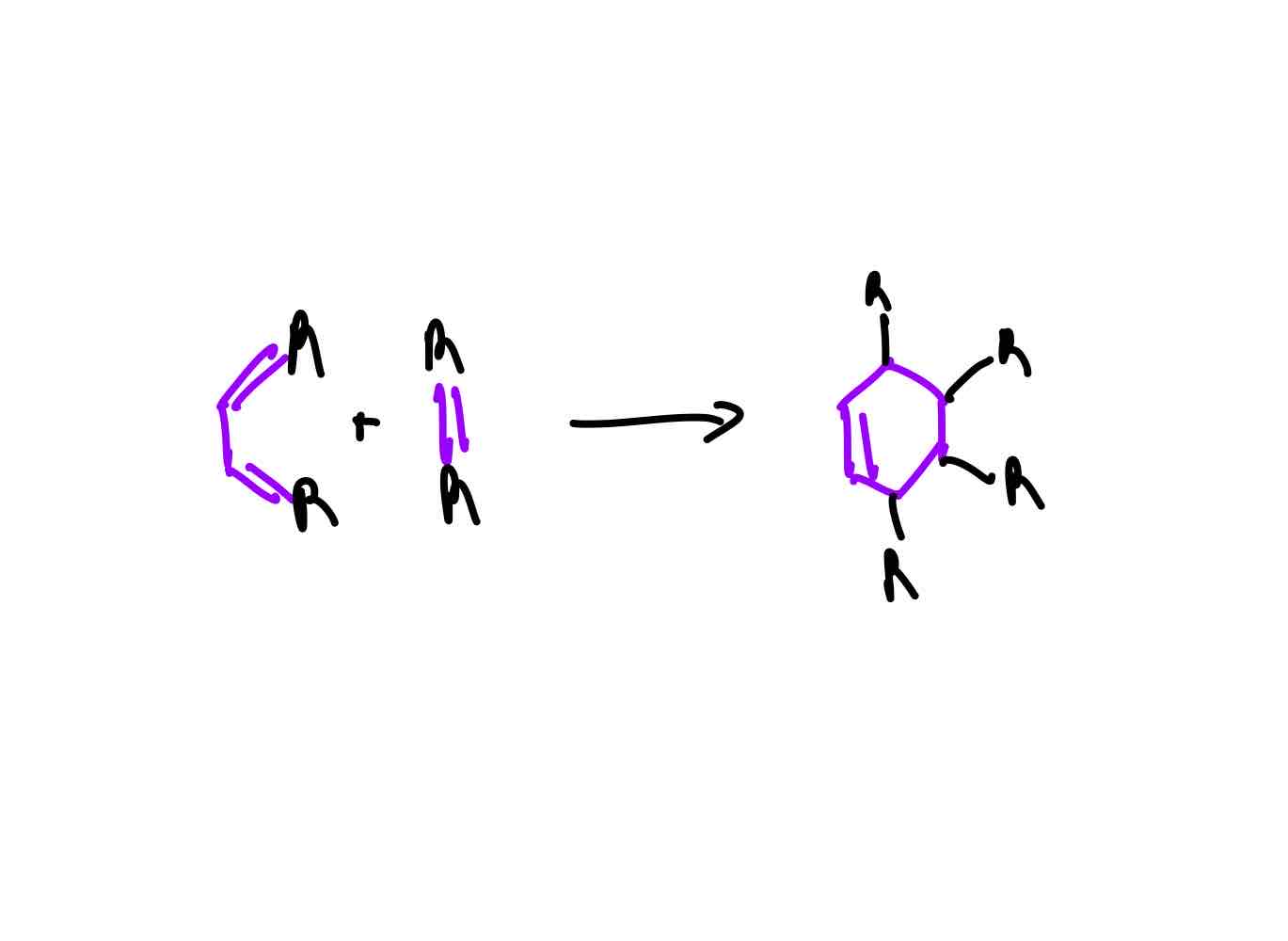
For a Diels-Alder (DA) reaction, what moiety do we need attached to the diene and what moiety do we need attached to the dienophile? Why for both cases?
The diene must have electron-donating groups because it raises the energy of the molecular orbitals, specifically the HOMO, because of its increasing repulsion via donation of electrons.
The dienophile should have electron-withdrawing groups because it lowers the energy of the molecular orbitals, specifically the LUMO, because its decreasing repulsion via withdrawal of electrons.
This raising of MOs from diene and lowering of MOs from dienophile provide better energy overlap and efficient bonding.
What should you remember, regioselectively, in regards to bonding in DA?
The delta positive of the dienophile and delta negative of the diene or full positive of the dienophile and full negative of the diene have to match.
What should you remember, stereochemically, without an Evans aux., in regards to bonding in DA?
Endo is always preferred over exo.
When there is an “outer” R on C1 of butadiene, what’s the Krel if R was CH3, or C(CH3)3, with R as H as a reference point for Krel, where Krel = 1? What explains these relative values?
If R was CH3, K would be 4.2 because of electron-donation.
If R was a t-butyl group, K would be 0.05 because of steric hinderance, i.e. less trajectories to attack.
When there is an “inner” R on C1 of butadiene, what’s the Krel if R was CH3, with R as H as a reference point for Krel, where Krel = 1? What explains this relative values?
If R = Me, then K would 1E-3 because of allylic strain that forces the butadiene to go cis instead of trans. If it goes trans, then the reaction can’t proceed because we need cis conformation.
When there is an R on C2 of butadiene, what’s the Krel if R was t-butyl, with R as H as a reference point for Krel, where Krel = 1? What explains this relative values?
If R was t-Butyl, then K would be 27 because of electron donation.
How do Lewis acids assist in the catalysis of DA reactions?
You would form a full positive charge on the dienophile, which lowers the energy of the LUMO for efficient bonding. Also makes the positively-charged resonance structure more pronounced as a call for bonding at its electrophilic carbon.
Generally, uncatalyzed results in 70% yield at 200ºC for 6 weeks, while catalyzed results in 95% yield at -78ºC for 10 seconds.
What are some useful DA products for synthetic utility?
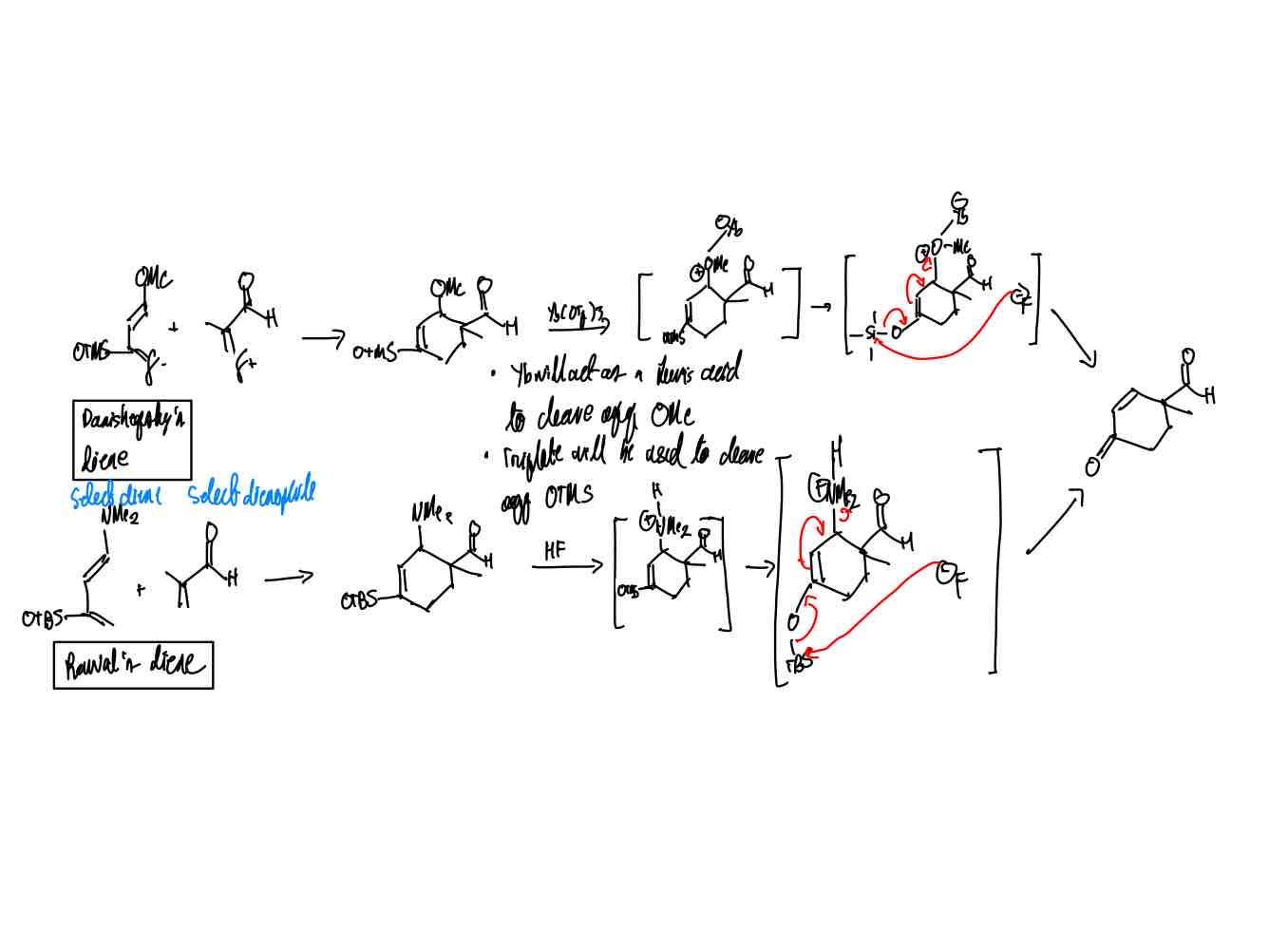
Danishefsky and Rawal’s product.
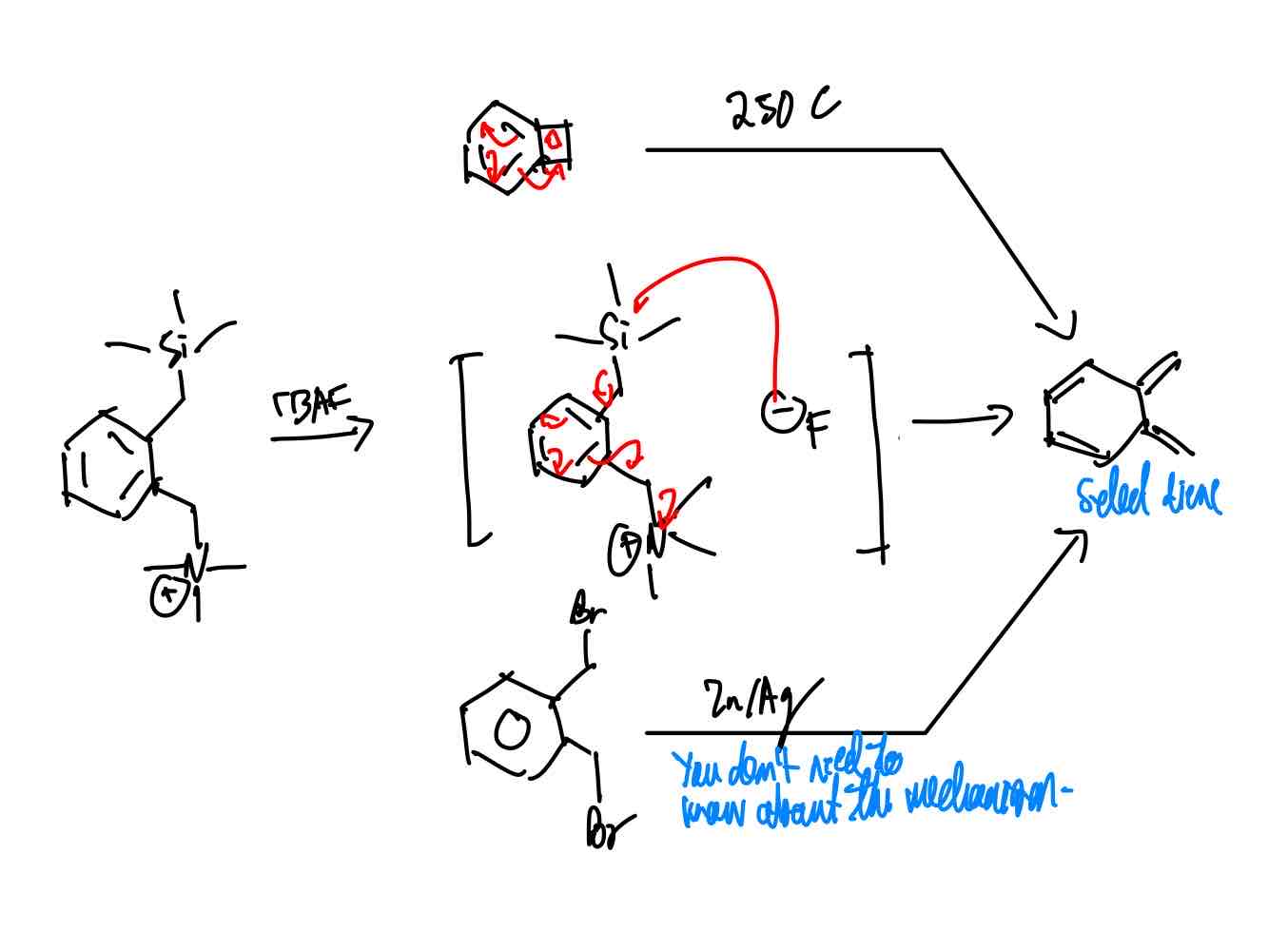
“Walsh’s product.”
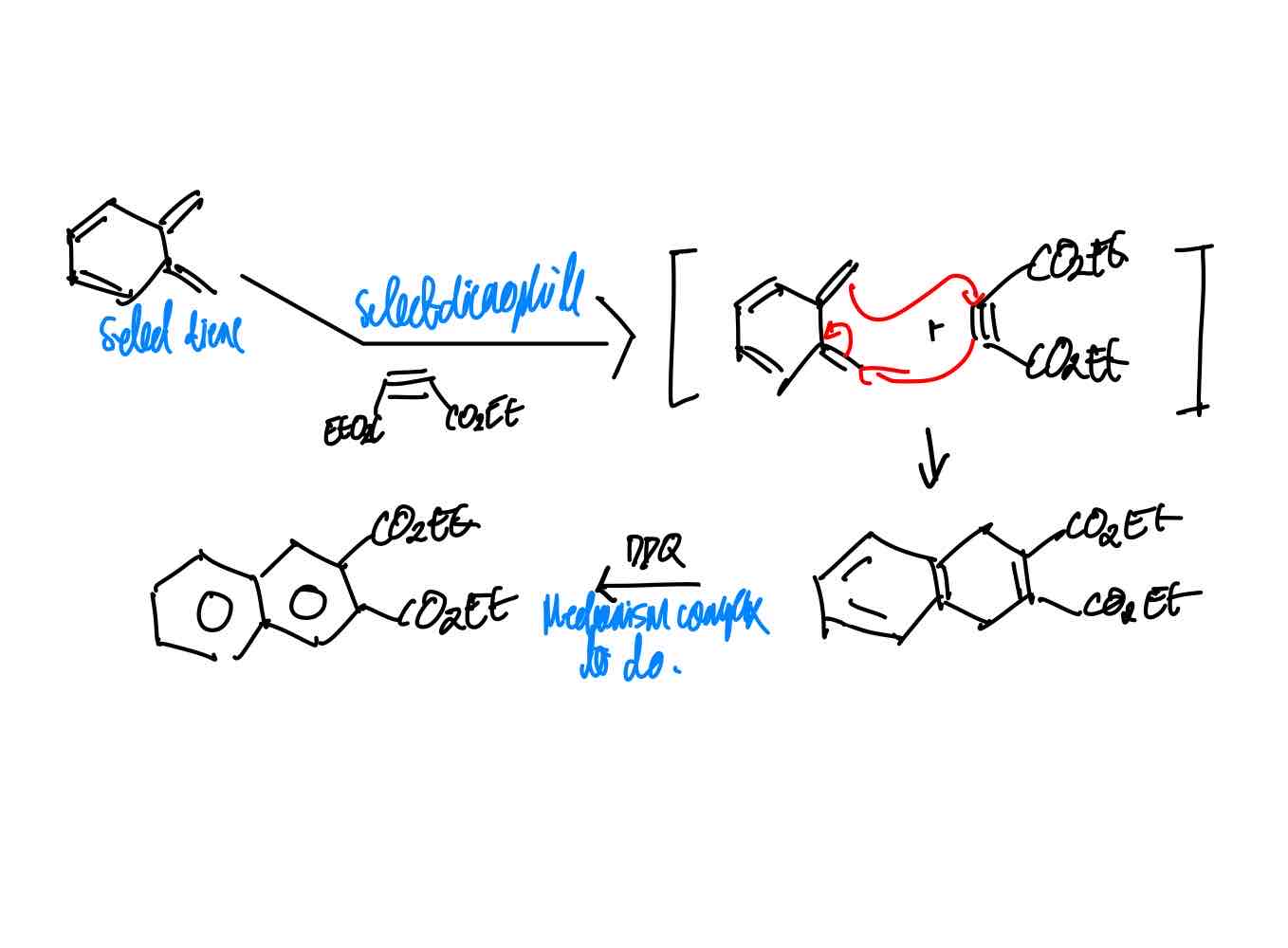
“Sorensen’s product.”
“Davis’ product.”
Ketene equivalent product.
Ethene equivalent product.
Acetylene equivalent product.
Wittig product.
How do you synthesize Danishefsky and Rawal’s product?
How do you synthesize Walsh’s product?
How do you synthesize Sorensen’s product?
How do you synthesize Davis’ product?
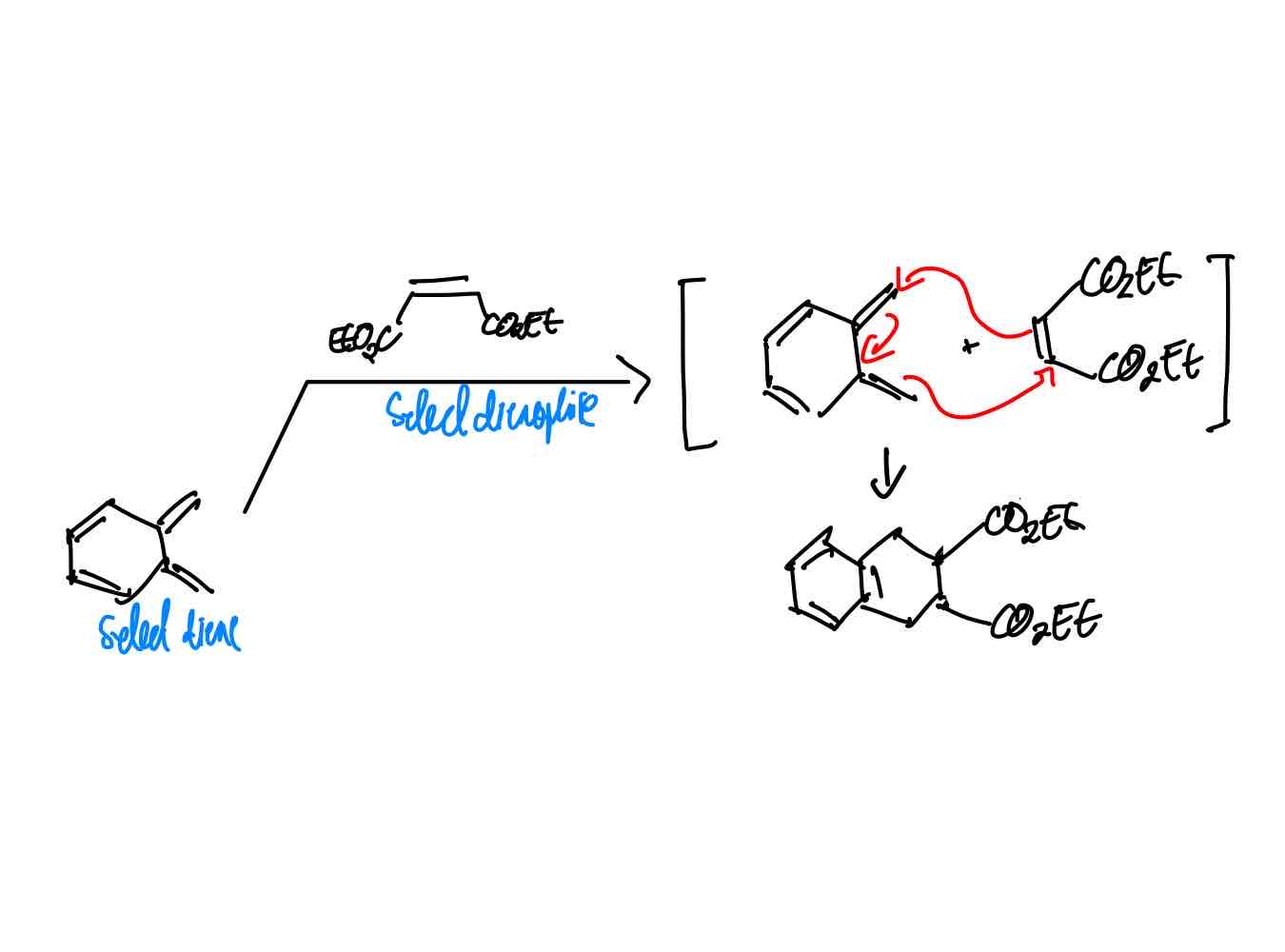
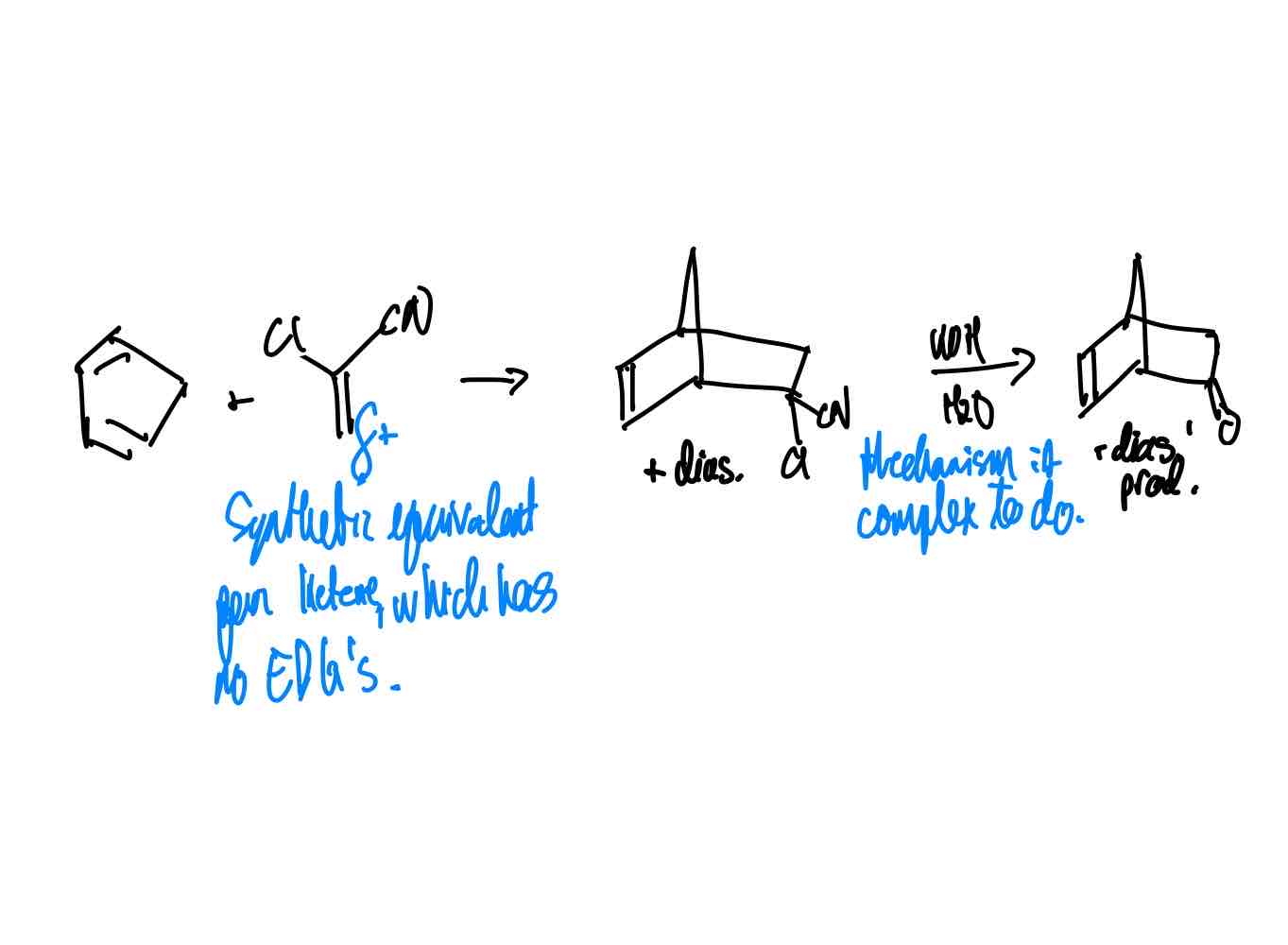
How do you synthesize the ketene equivalent product?
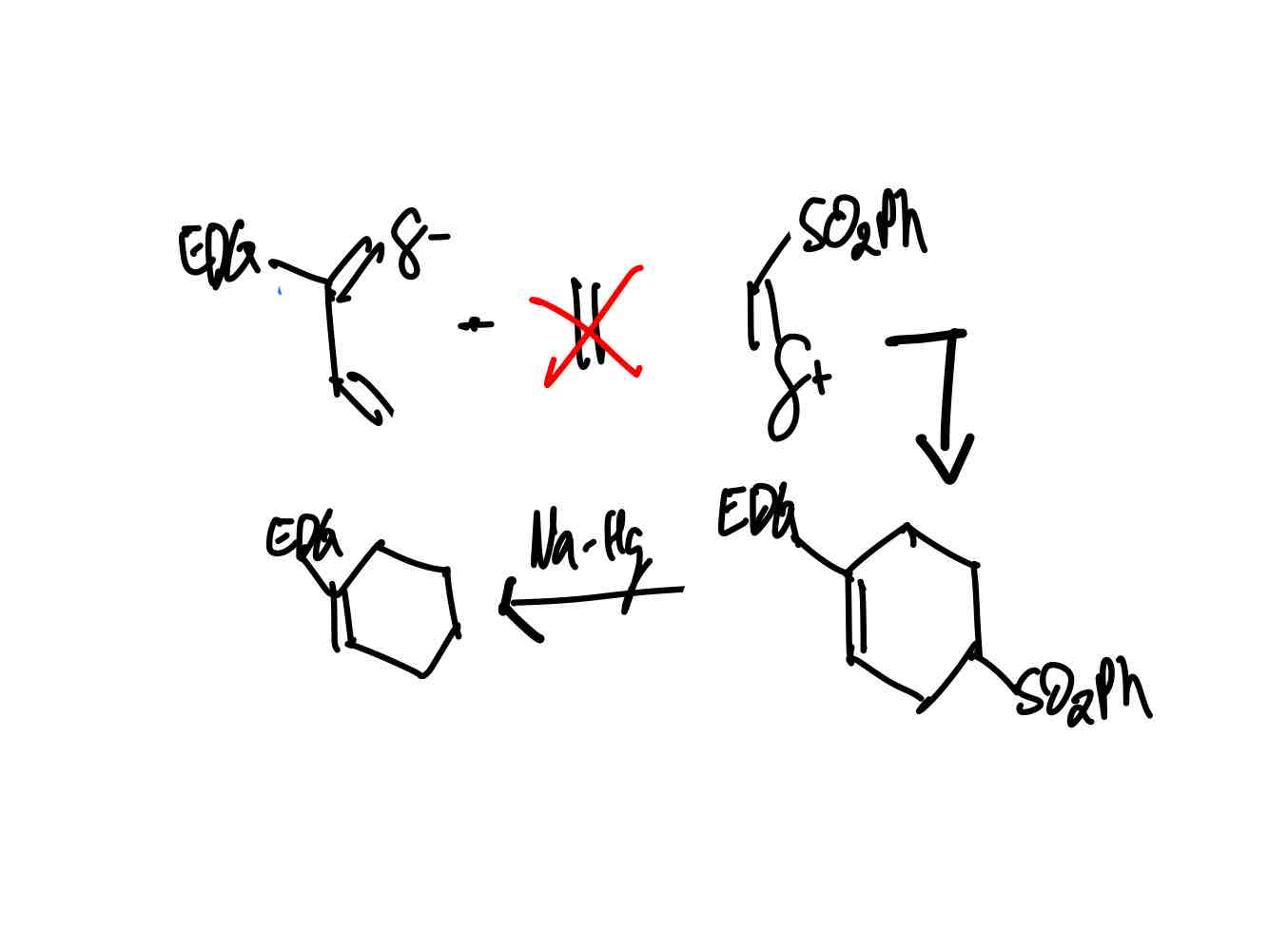
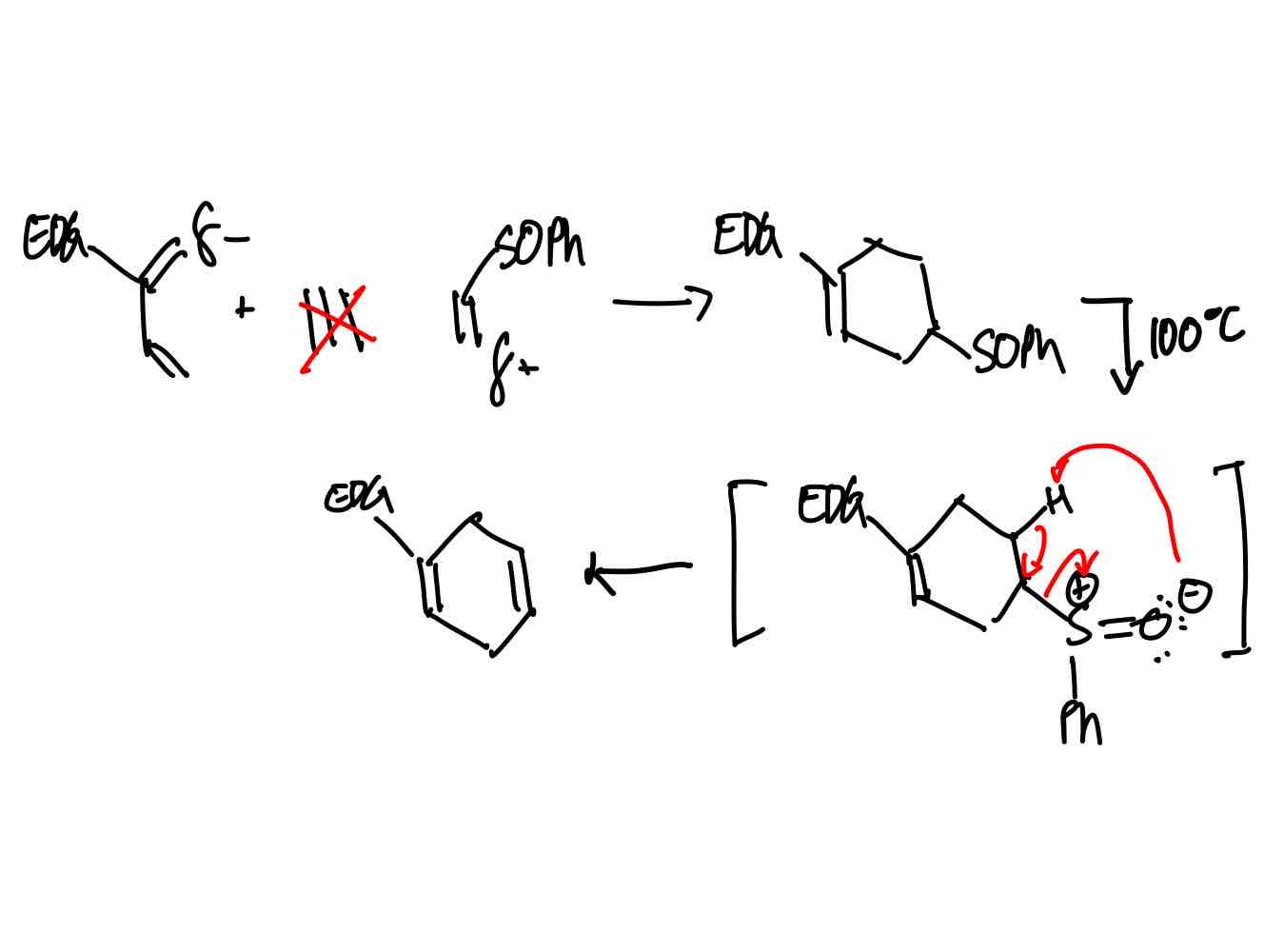
How do you synthesize the ethene equivalent product?
How do you synthesize the acetylene equivalent product?
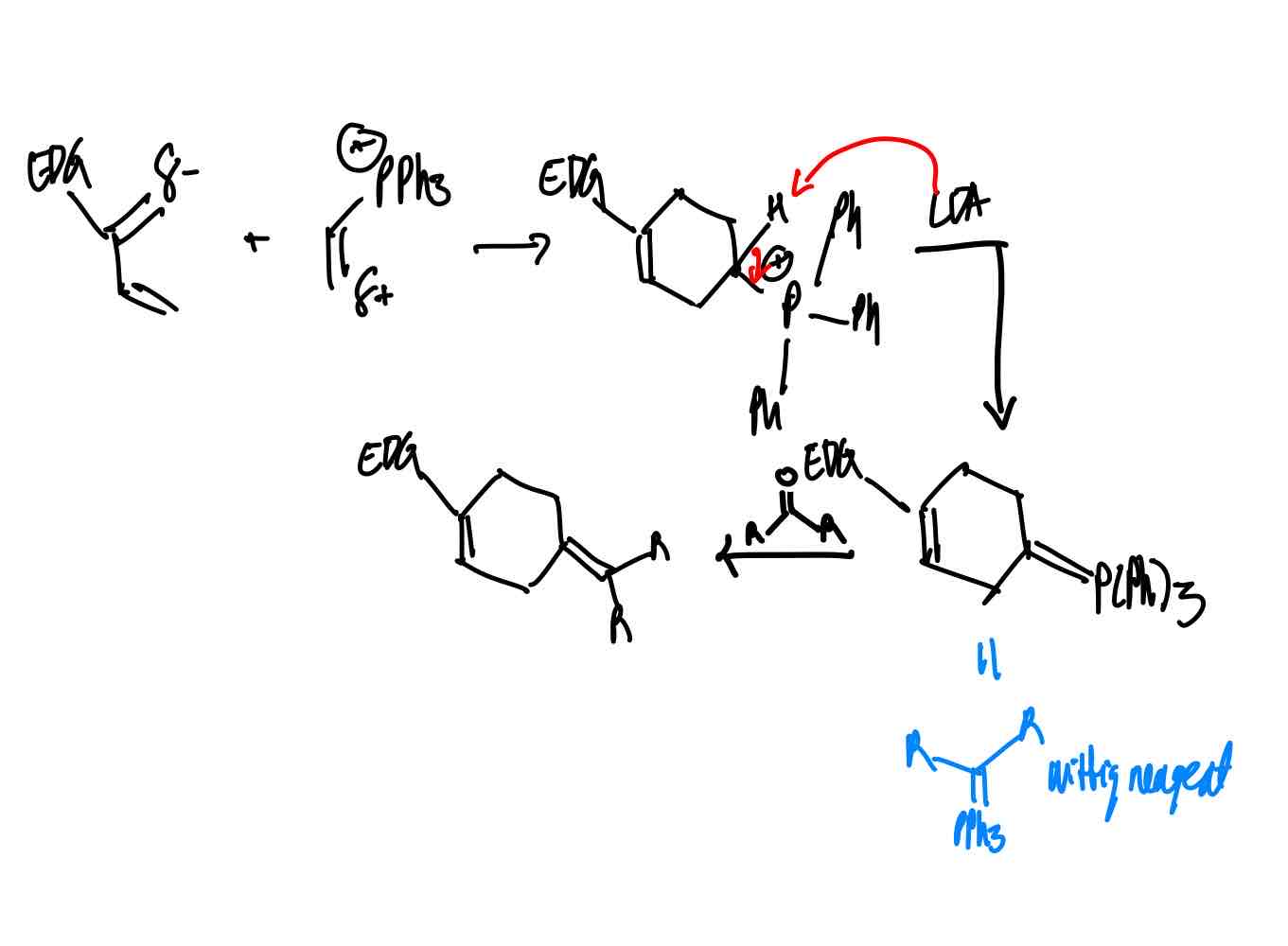
How do you synthesize the Wittig product?
How do you ensure stereochemistry of DA products?
Use Evans aux. and Lewis acids on the dienophile. The Lewis acids will activate the dienophile and make the whole dienophile as planar as possible to understand diene attack, a la re/si model, increasing endo selectivity further as well due to the cyclic lock, and endo 1 vs. endo 1 selectivity.
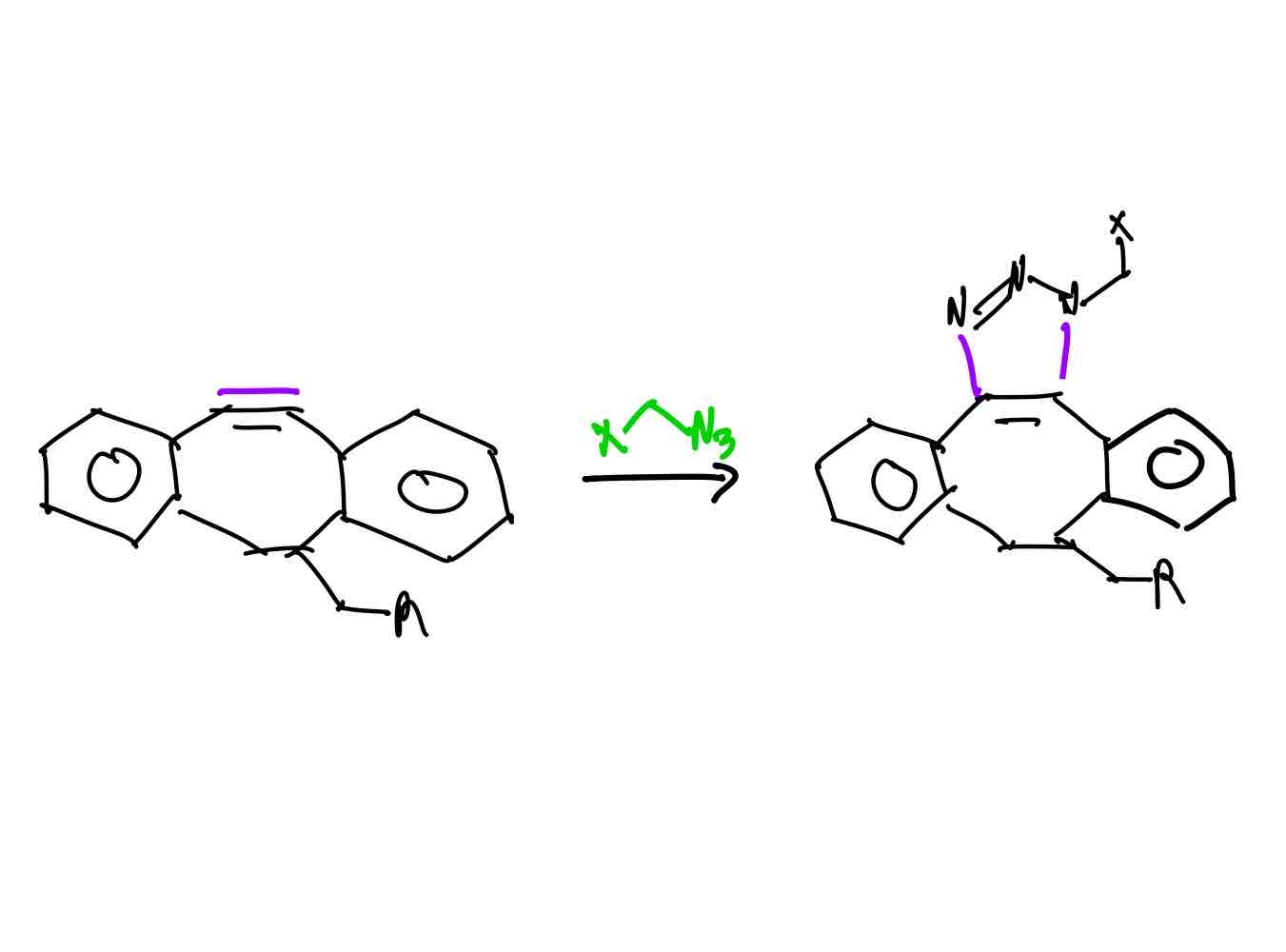
If you wanted to attach to molecules together like lego pieces, how would you proceed?
What are the five discussed 3,3-sigmatropic rearrangements in lecture?
Cope.
Oxy-cope.
Anionic oxy-cope.
Claisen.
Claisen of allyl phenyl ethers.
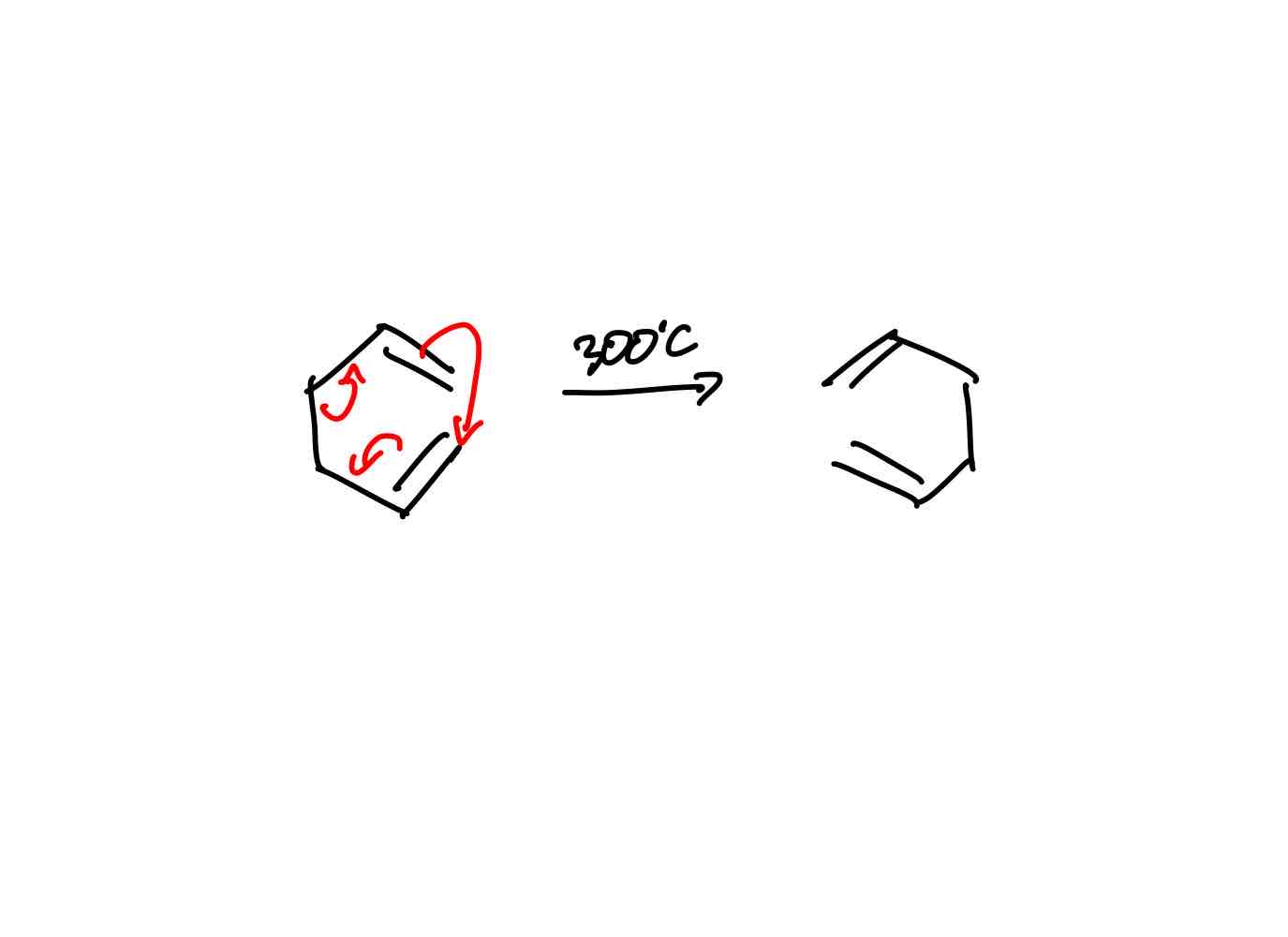
How does the cope rearrangement work?
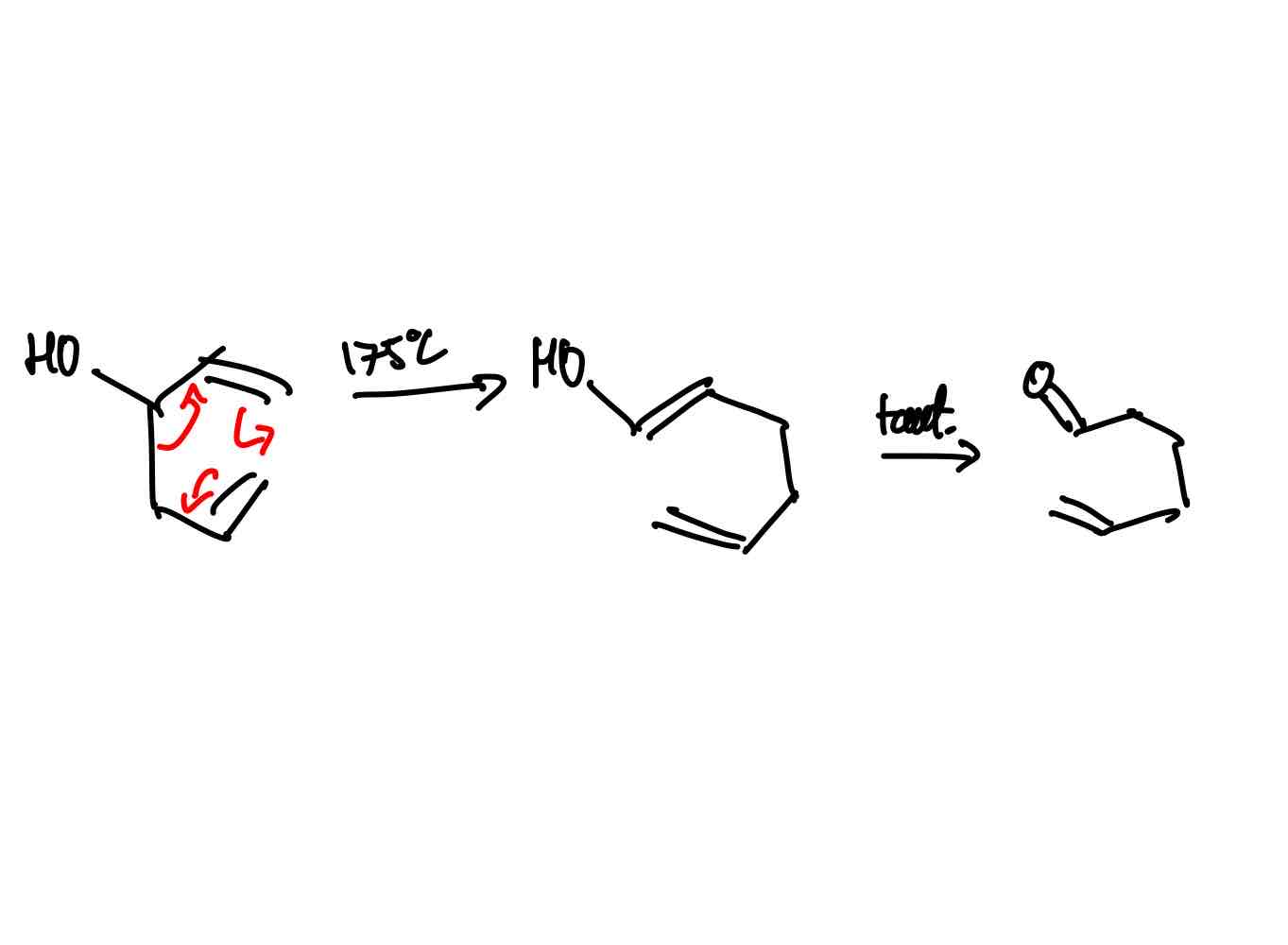
How does the oxy-cope rearrangement work?
How does the anionic oxy-cope rearrangement work?
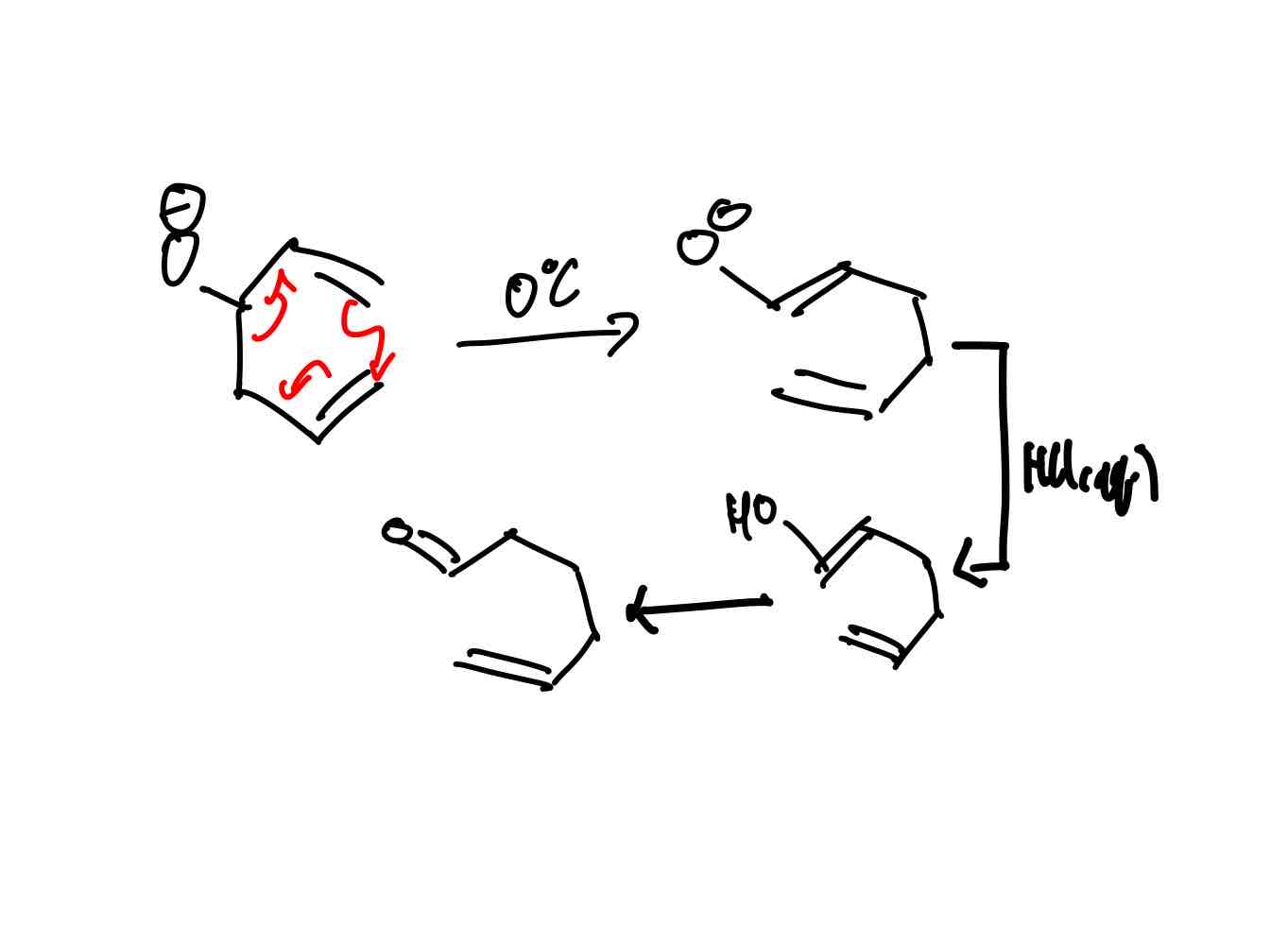
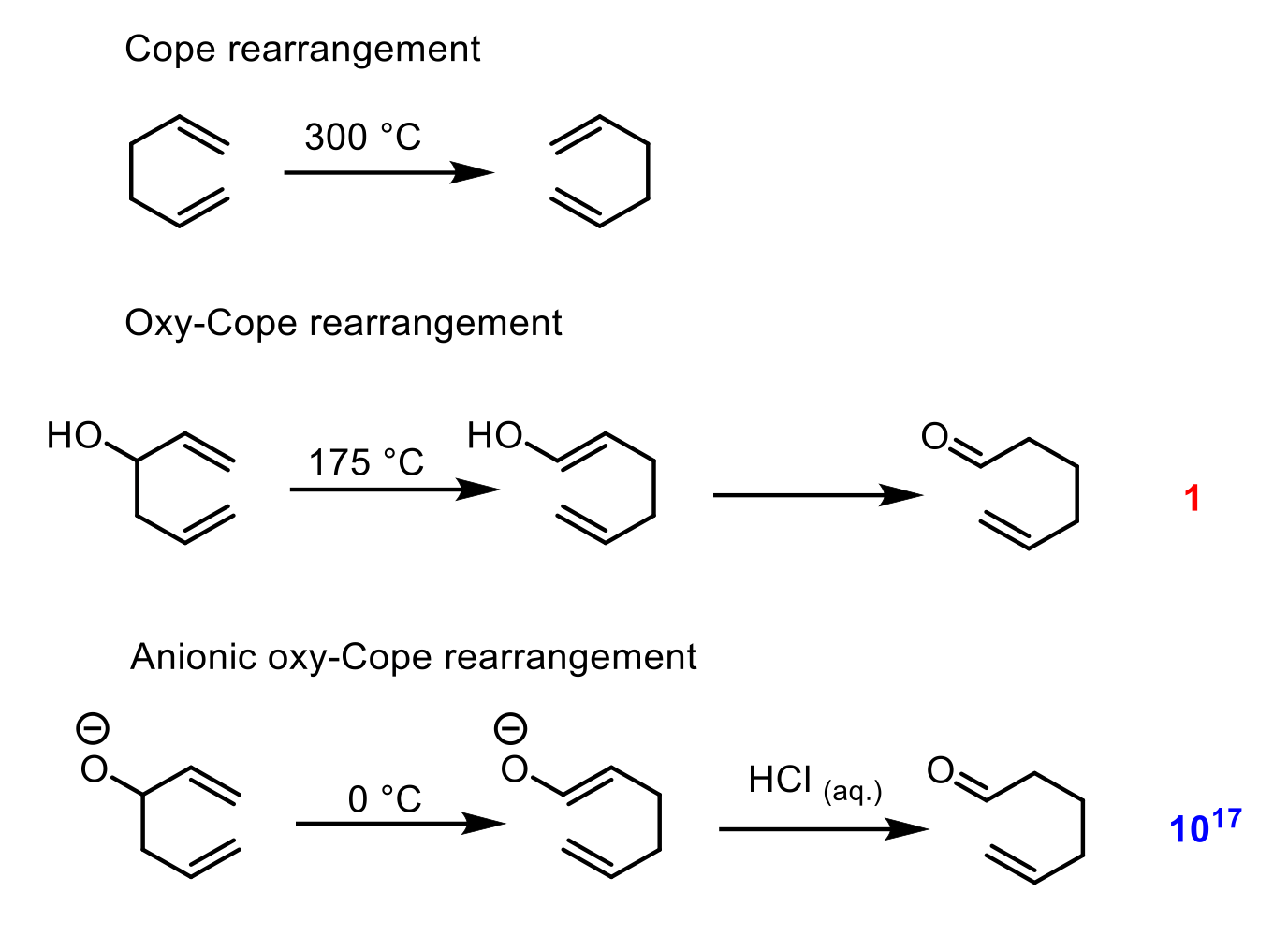
How does the cope and oxy-cope rearrangement explain the super-efficiency of the anionic oxy-cope rearrangement?
Cope rearrangement: Synthetically useless - same product as reactant. Uses a lot of energy 300ºC. In equilibrium too.
Oxy-cope rearrangement: Still not the best synthetically. Does give you keto product via taut with no full return to alcohol. In equilibrium regardless. Still needs energy - 175ºC.
Anionic oxy-cope rearrangement. The best synthetically here. The creation of the intermediate with a resonance-stabilized enolate is downhill in energy and more stable than the starting material, compared to the “downhill” in energy with the oxy-cope where there is no stabilization. Anionic is so stable to the point that it wouldn't be in equilibrium or want to return to non-resonance-stabilized-enolate form. Can taut. into keto once treated with proton source.
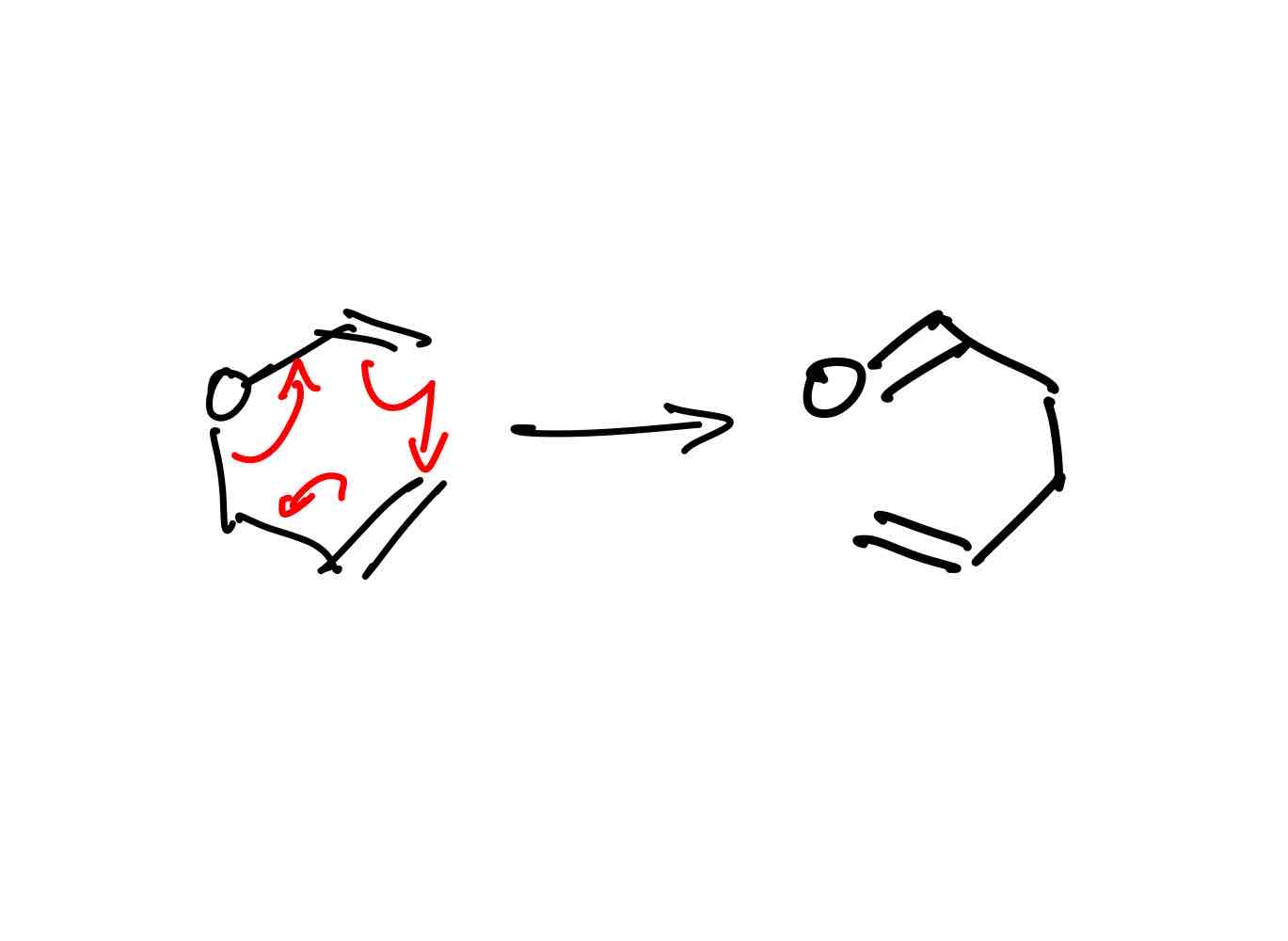
How does the Claisen rearrangement work?
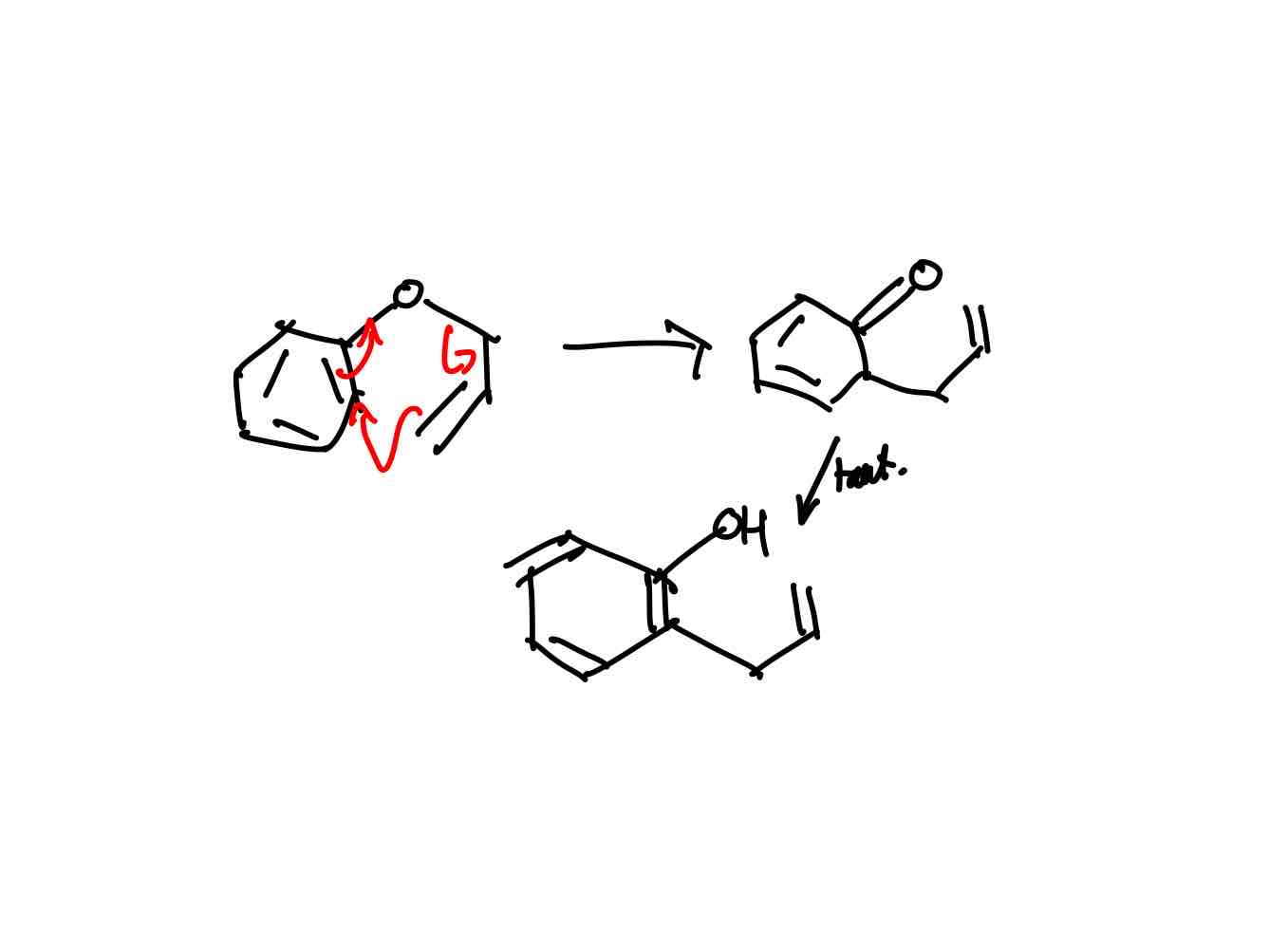
How does the Claisen of allyl phenyl ethers rearrangement work?
How does cheletropic elimination work?
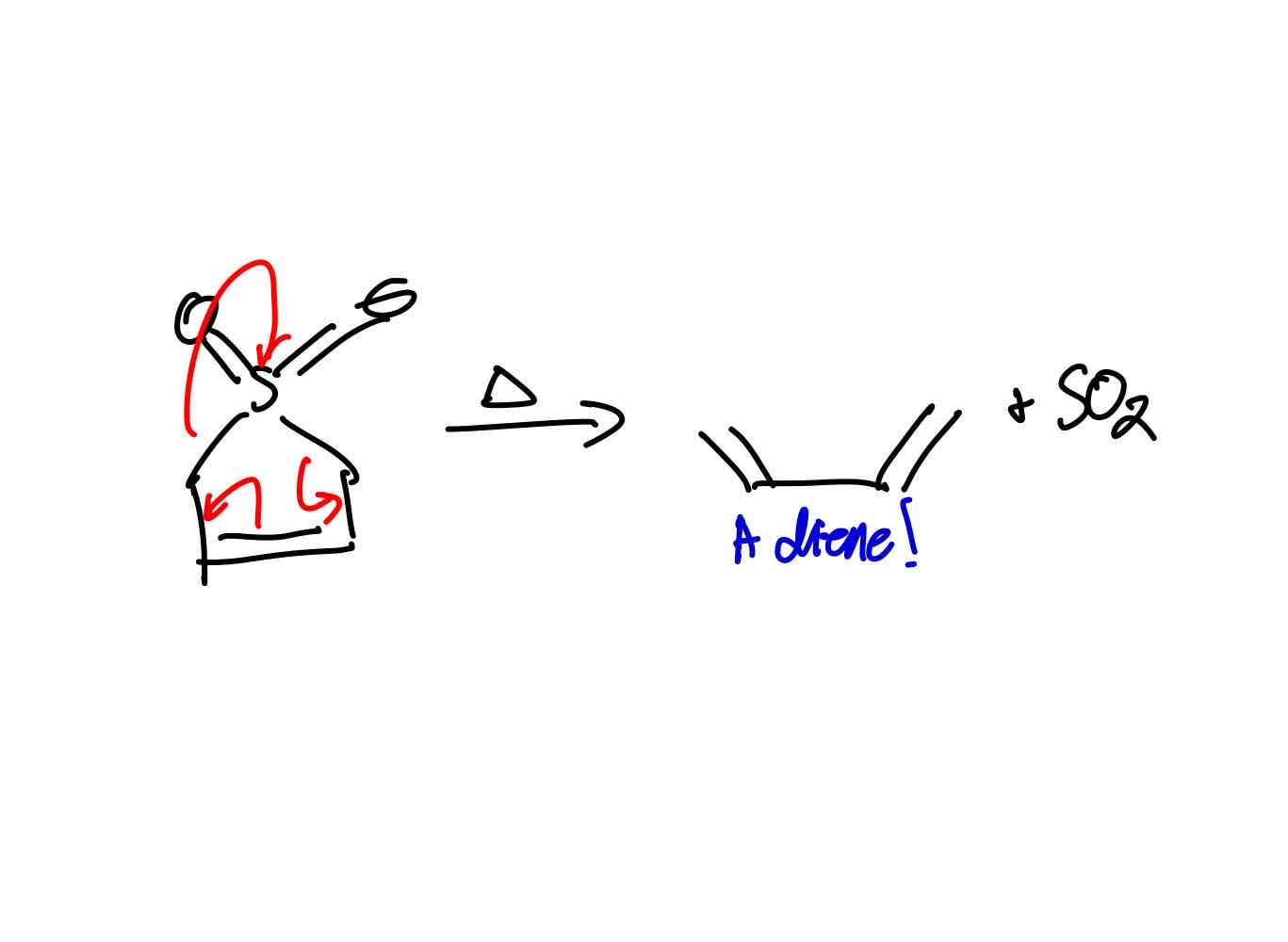
Why is the cheletropic elimination of SO2 beneficial for Diels-Alder reactions?
The generation of the cis diene is slower than Diels-Alder reactions, allowing Diels-Alder to occur with such dienes produced.
Does the reaction for rearrangement of phenyl ethers favour the enol form over the keto form? If so, why?
Phenol prefers enol form over keto form because of aromaticity.
What are the Woodward-Hoffman rules? What explains these rules?
4n / ∆ / con
4n+2 / ∆ / dis
4n+2 / hv / con
4n / hv / dis
Electrocyclic reactions occur at the highest occupied MO. Using heat, the HOMO is used as the orbitals in rotations. Using light, the electron is excited to the LUMO, which is now the SOMO (singly-occupied molecular orbital), and our new highest MO is the SOMO for orbital rotations.