CHEM232 Practical Exam
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
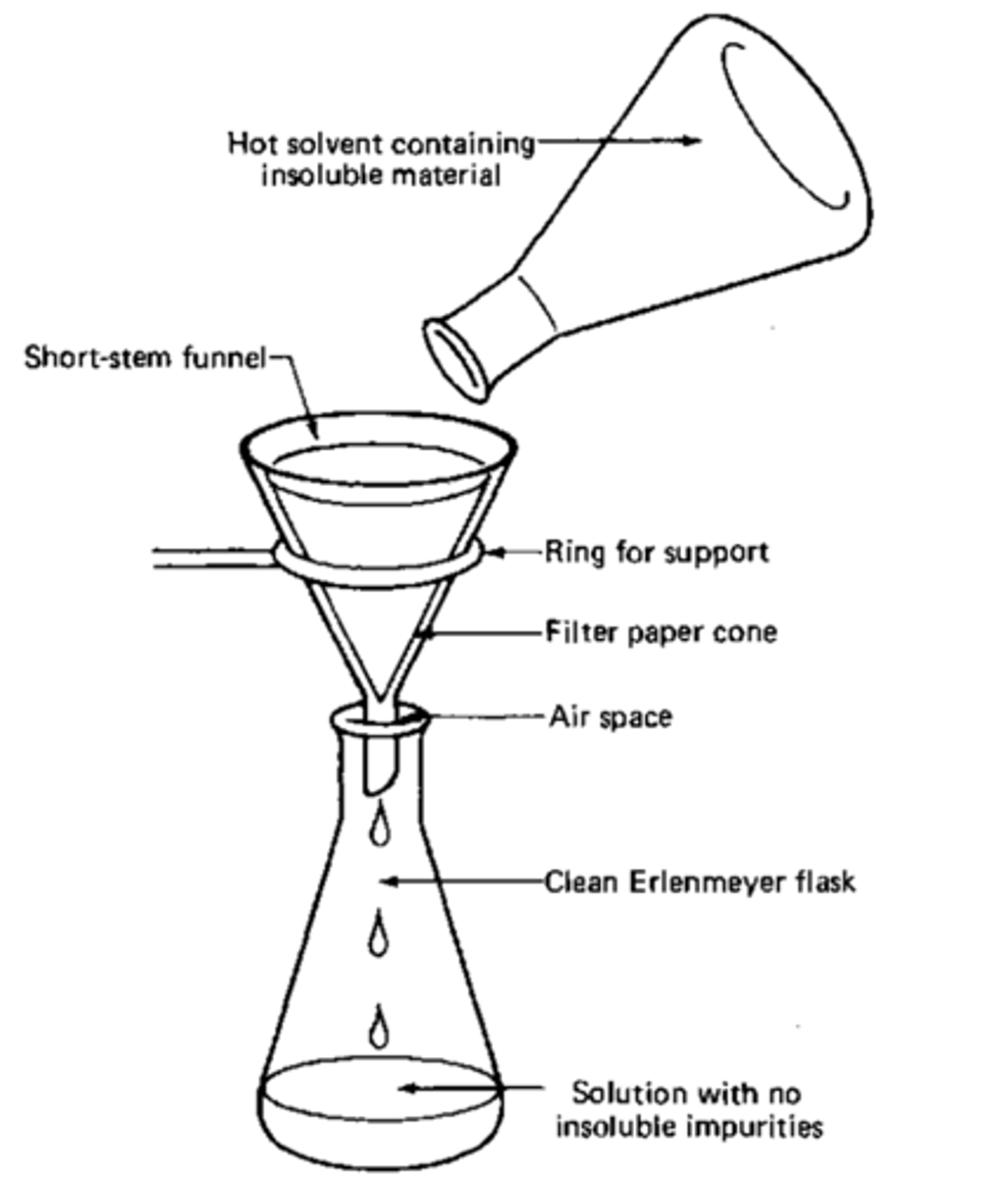
Hot Gravity Filtration
Used in Recrystallization of Impure Solids.
Filters out solids from the solvent (impurities that did not dissolve in the hot liquid)
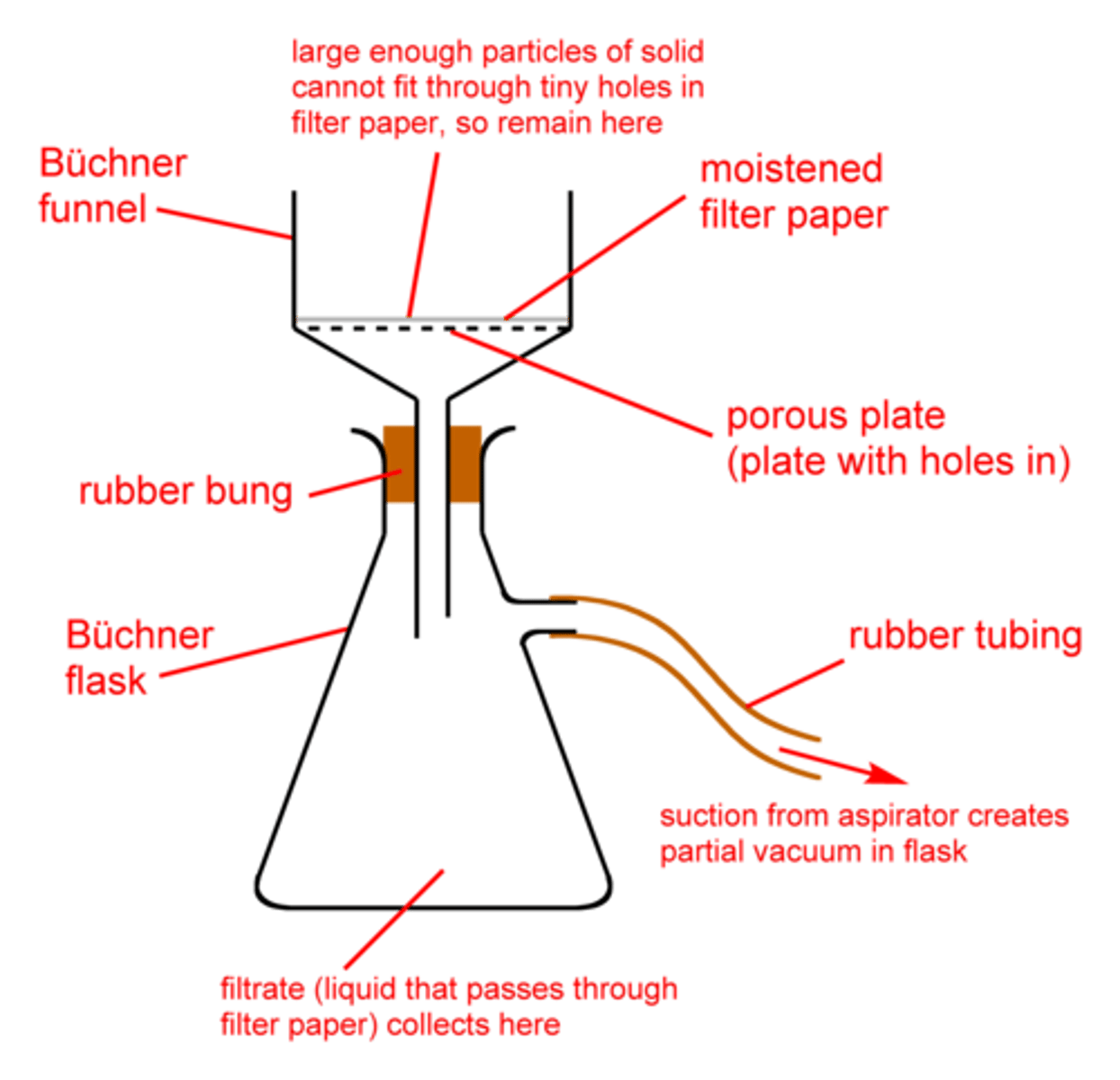
Vacuum Filtration
Used in Recrystallization of Impure Solids.
Remove solvent from recrystallized solid sample
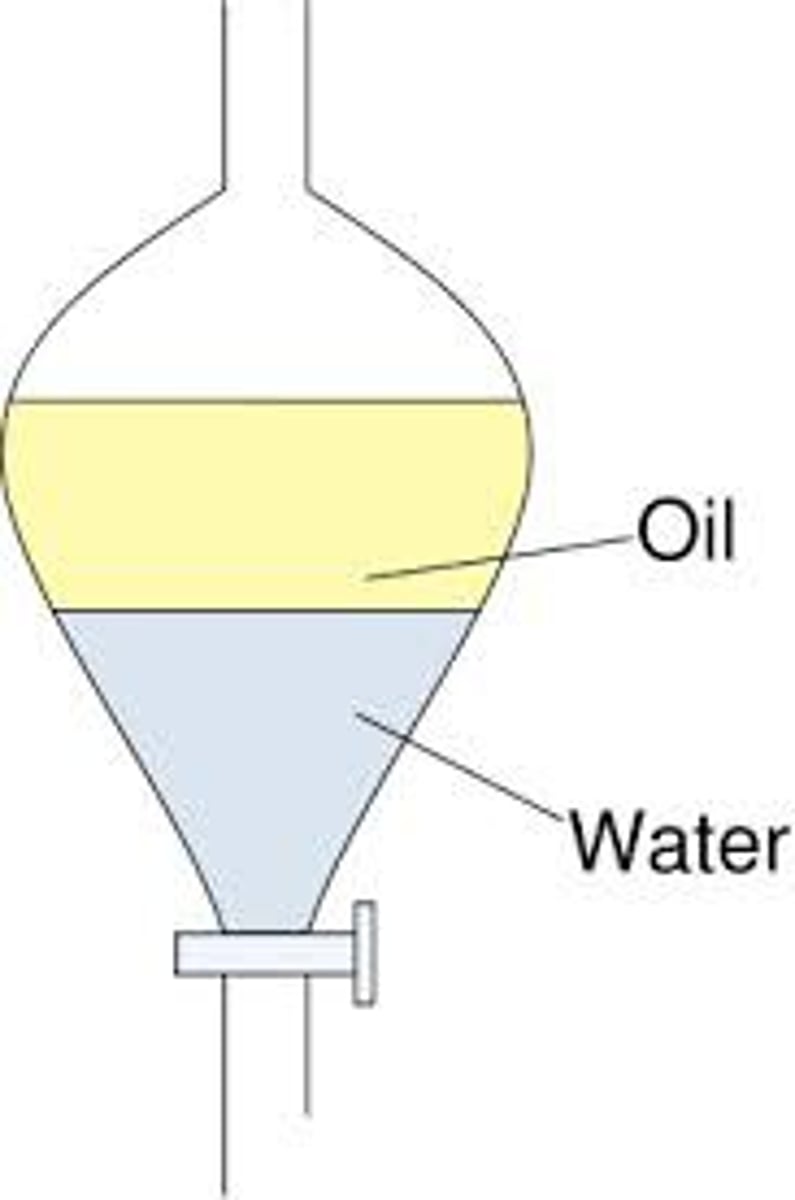
Separatory Funnel
Used in Extraction.
Separates liquids of different densities (organic solvents from polar solvents)
Reduced Pressure Solvent Removal
Used in Extraction.
The reduced pressure allows for quicker evaporation of the solvent, allowing the solid to be left behind.
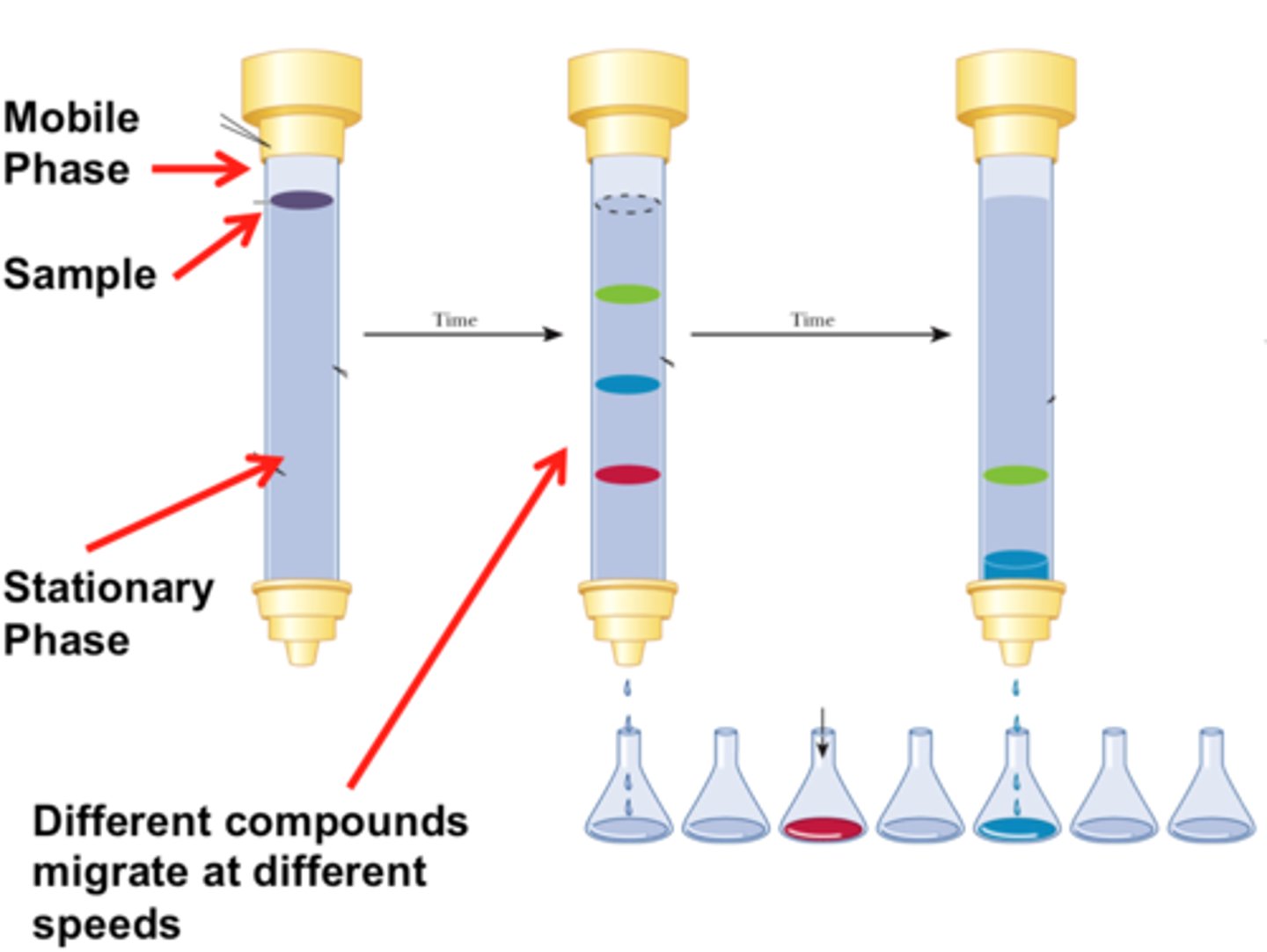
TLC plate
Used in Thin Layer Chromatography.
The plate is dotted and placed in the mobile phase.
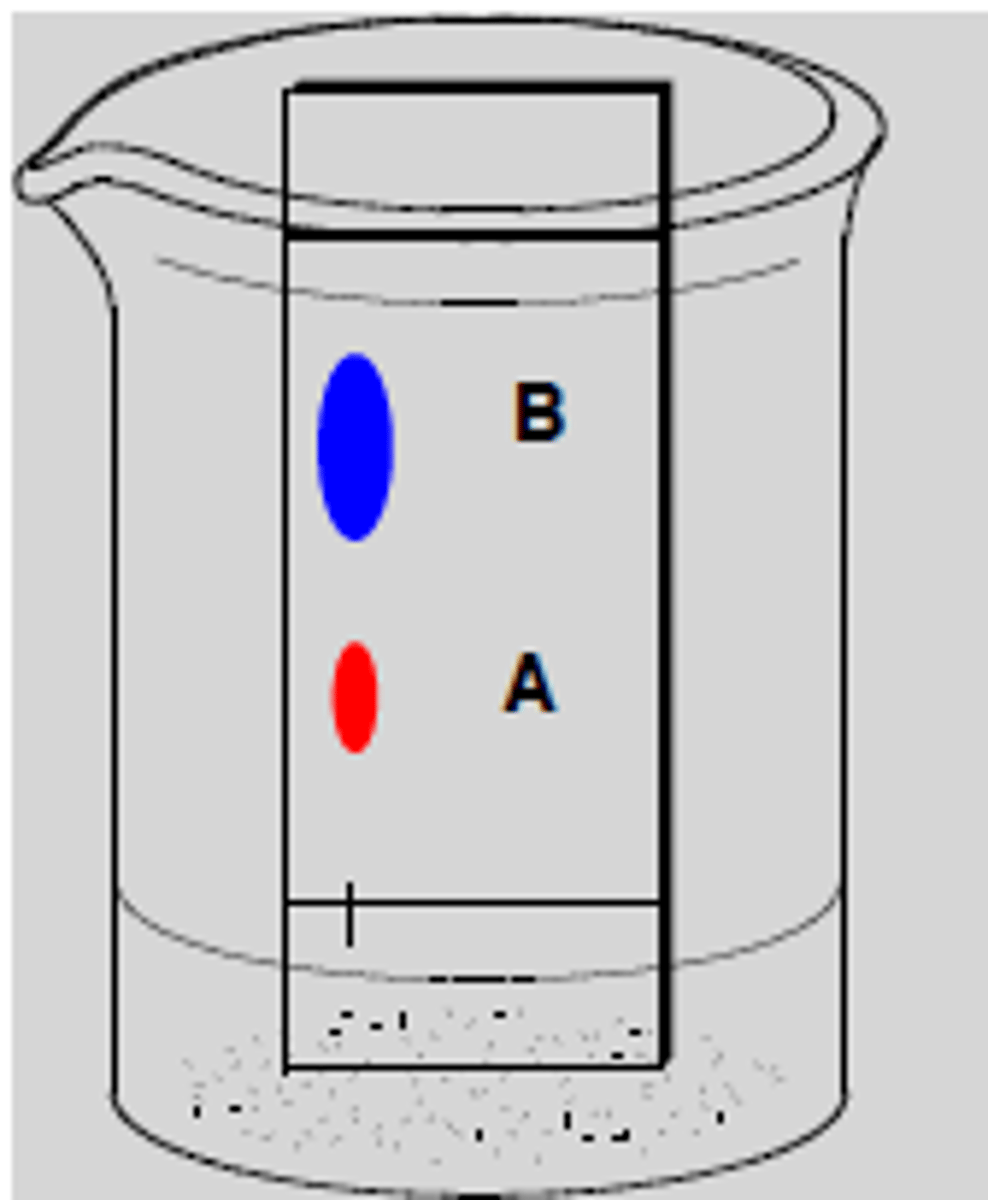
Silica Gel Column
Used in Column Chromatography.
The sample is added at the top, and the different affinities for SiO2 or Al2O3 (stationary phases) will cause differing retention times.
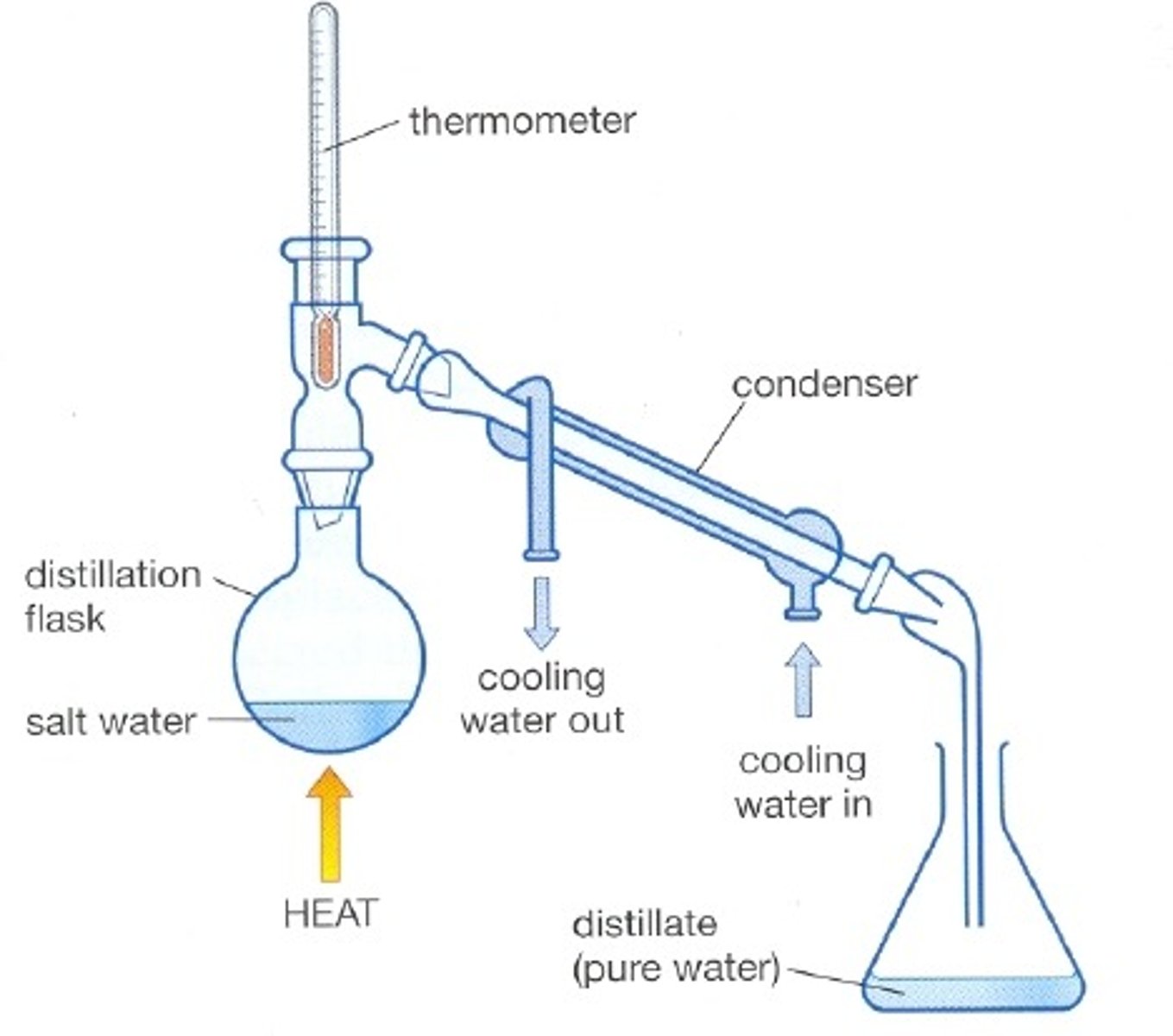
Simple Distillation
Used in Distillation.
The solution is heated to different temperatures, so that it evaporates and condenses into its separate fractions. Simple distillation is best used with fractions more than 50-100 degrees apart in boiling point.
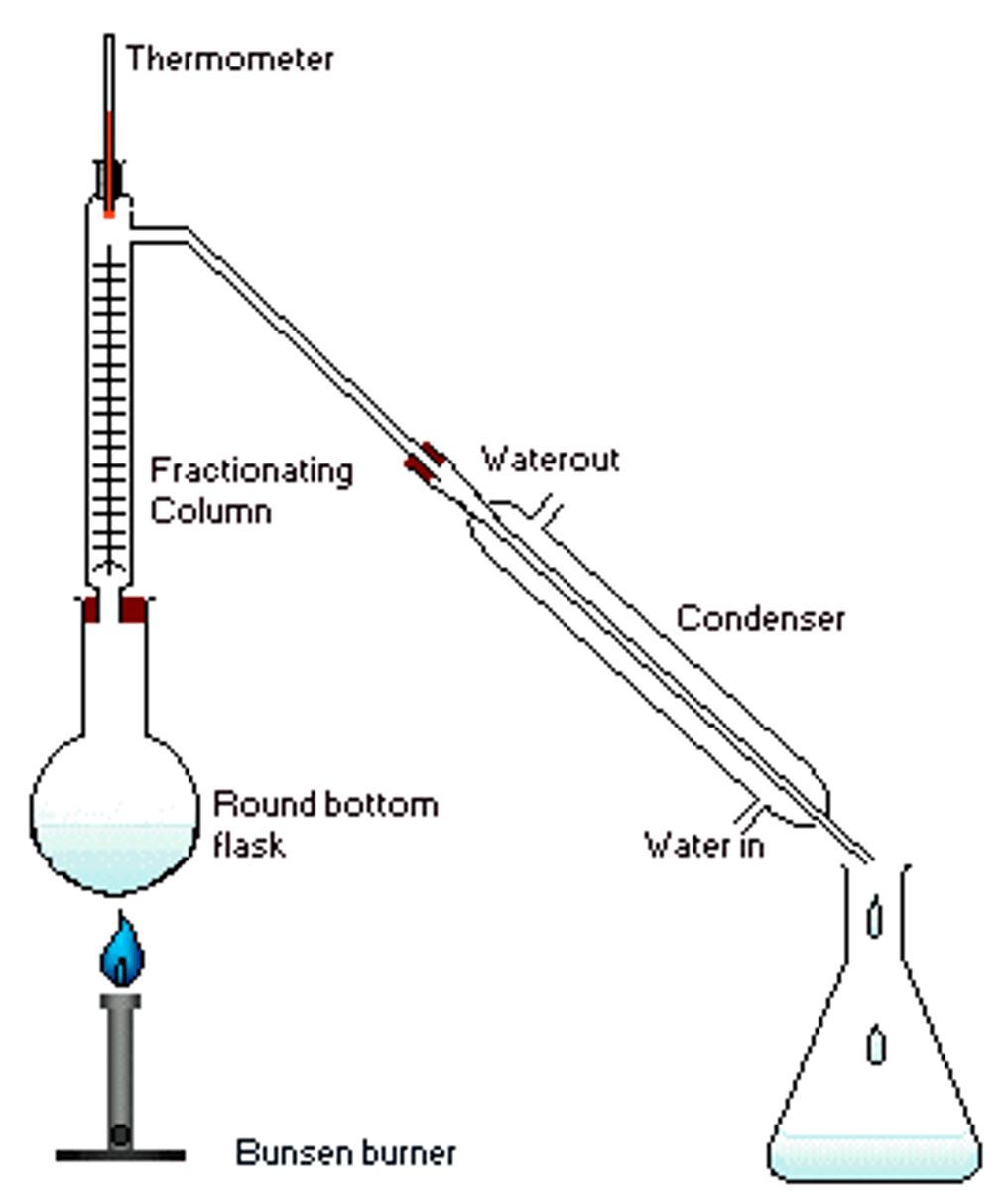
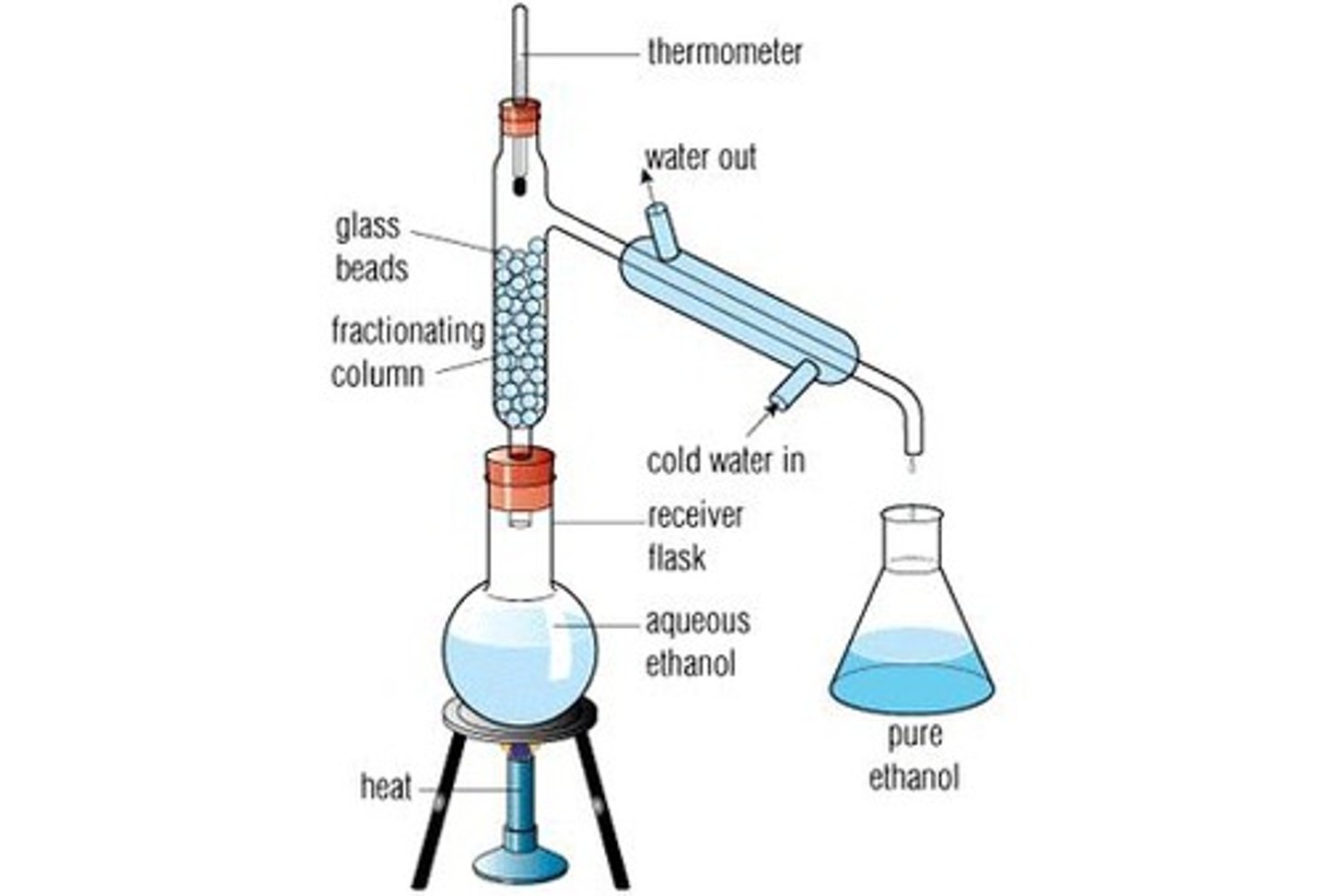
Fractional Distillation
Used in Distillation.
The fractioning column is equipped with extra surface area in the form of glass beads, which allows greater separation accuracy. It must be insulated using insulating material and foil.
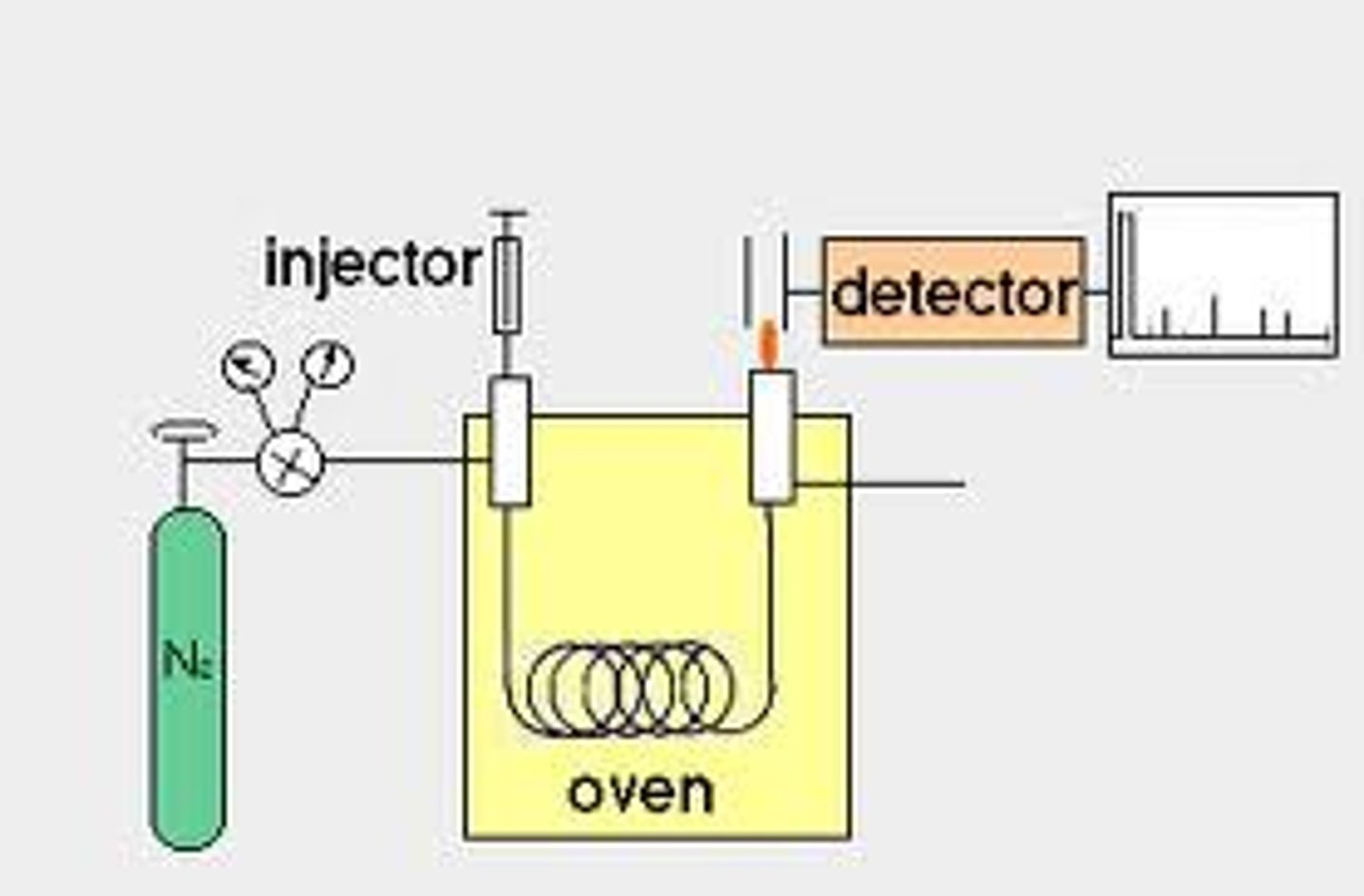
GC Column and Flame Ionization Detector
Used in Gas Chromatography.
The stationary phase is a coating of silicone on the column walls. The mobile phase is an inert gas (He). The method of separation is boiling point (more volatile compound is pushed more quickly by the mobile phase). The FID burns samples in a Hydrogen flame, producing ions which produce a current proportional to the amount of sample.
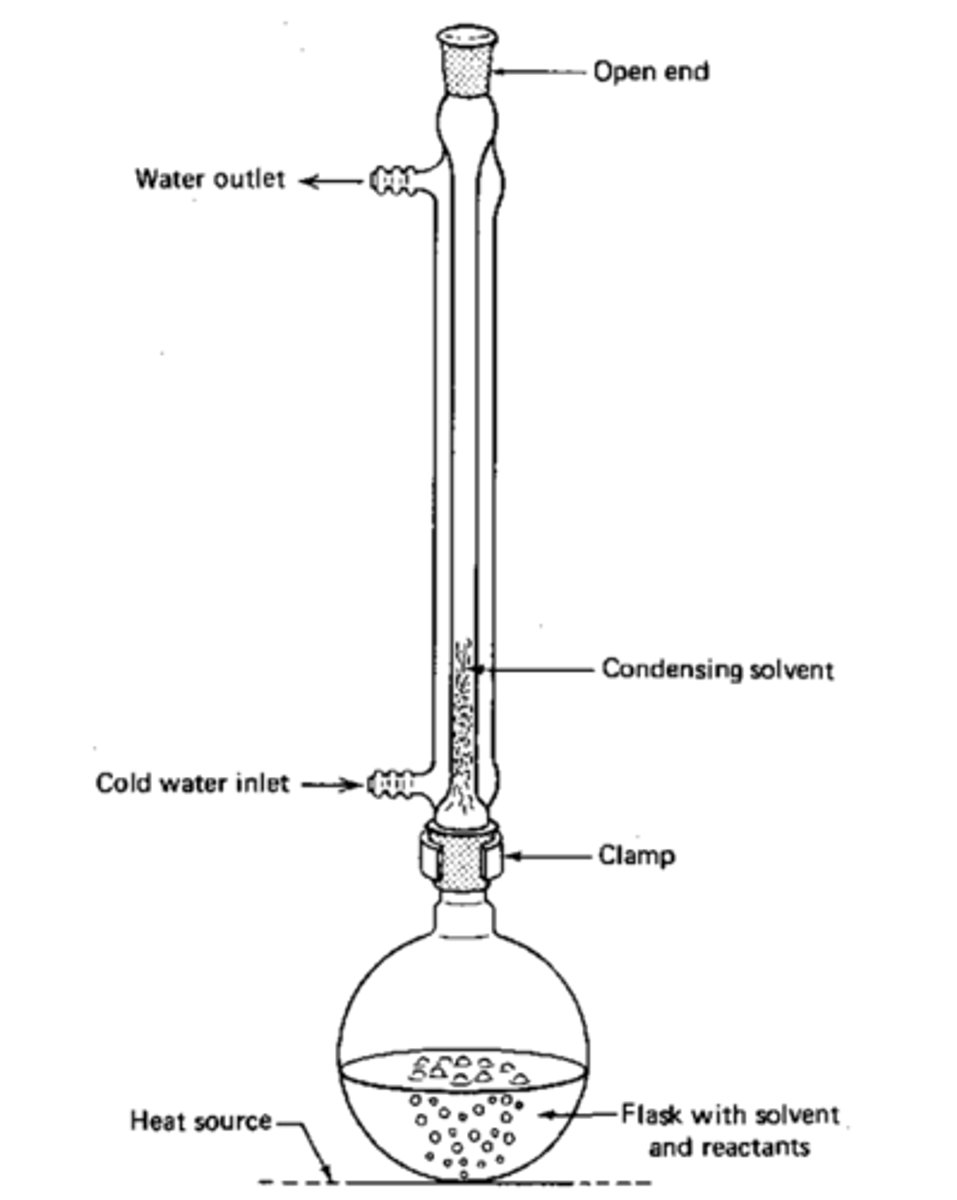
Reflux
Used in Nucleophilic Substitution Reactions.
This allows the reaction to be run at a high temperature without boiling the solvent off, by continually condensing the solvent and allowing it to drip back into the flask.
Fractional Distillation and Reflux combination
Used in Elimination of Alkyl Halides.
During the reaction, the column is used to perform reflux. Following 75 minutes, the column is used for fractional distillation, with the water being disconnected from the column, and it being insulated, to collect the sample.