Organic Chemistry (MCAT)
1/71
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
nomenclature
methane, ethane propane, butane, pentane, hexane, heptane, octane, nonane, decane
methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, t-butyl
longest carbon chain, numbered based on lowest carbon substituent or OH, alphabetize substituents (ignore prefixes except iso),
functional groups
memorize them pg. 34
alkane, alkene, alkyne, alkyl halide
alcohol, phenol, thiol, epoxide, ether
aldehyde, ketone, hemiacetal, acetal, ester
amine, imine, enamine, amide
lactone, lactam
acetate
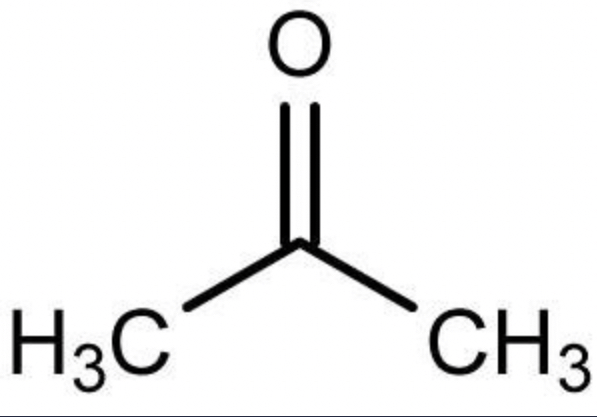
ethanoic acid
acetic acid
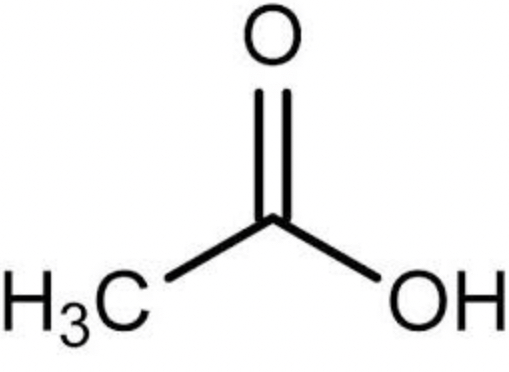
formic acid
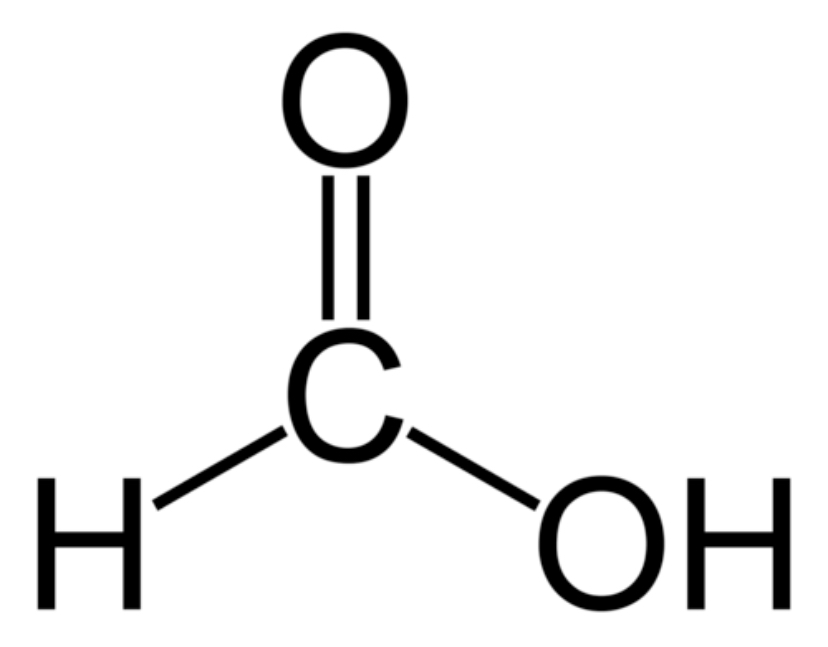
formaldehyde
degree of unsaturation
U = (2C - H + N + 2)/2
molecule is saturated if it contains no pi bonds or rings
saturated alkaline has a formula of CnH2n+2
ring strain
ideal bond angle is 109.5, ring strain creates reactive molecules with high heats of combustion
carbocations/carbanions
positive/negative charge on carbon
more substituted carbocations are more stable
less substituted carbanions are more stable
since alkyl groups are EDG
inductive effect
EDG stabilize carbocations, EWG stabilize carbanions
the stronger acid has the more stable conjugate base, what groups will stabilize the charge?
resonance stabilization
delocalization- electrons allowed to interact with orbitals on adjacent atoms
conjugated systems give rise to strong colors
electrons in unhybridized p orbitals can participate in resonance, atoms (N) can switch from sp3 to sp2 to free up a p orbital for resonance
major resonance contributor is determined by:
- octet rule
- minimized separation of charge
- charges on electronegative atoms
the stronger acid has the more stable conjugate base, what groups can delocalize the charge?
functional group acidity
strong acids > sulfonic acids > carboxylic acids > phenols > alcohol and water > aldehydes and ketones > sp CH > sp2 CH > sp3 CH (more s character is better at stabilizing conjugate base)
electronegative groups or positive charges can have inductive effect to increase acidity, stronger the close they are to the acidic proton
on benzene rings, para EWG can stabilize conjugate base, para EDG can destabilize conjugate base
acidity determined by stability of conjugate base:
- electronegativity of adjacent atom
- resonance stability
- induction
nucleophilicity
increases from top right to bottom left (opposite of electronegativity)
nucleophilicity increases with:
- increased negative charge
- going down a group, increased polarizability for larger atoms since they want to donate their electrons
- across a period, decreased electronegativity since less protons means they want to donate their electrons
leaving groups
good LGs:
- resonance stabilized
- weak bases
- neutrals (start with positive charge)
bad LGs:
- strong bases
- but protonating them can make them good
ugly LGs:
- hydrogens
- alkanes
constitutional isomers
different atomic connectivity, same molecular formula
different properties
stereoisomers
same molecular formula and atomic connectivity, different spatial orientation
umbrella term for conformational, enantiomers, diastereomers
conformational isomers
same atomic connectivity, differ by rotation about sigma bond
identical properties
Newman projection
anti- two groups that are 180 apart
gauche- two groups that are 60 or 120 apart
syn- two groups that are eclipsing each other
anti is lowest energy state, but gauche can be more stable if some hydrogen bonding is going on
ring stability
chair is more stable than boat
equatorial position for substituents is more stable than axial position
1,3-diaxial interactions- sterics that are less stable
chiral center
chiral center is a sp3 carbon with four different substituents
of possible isomers = 2^n, where n = # of chiral centers
chiral molecules are not superimposable on its mirror image, no plane of symmetry
absolute configuration- assign priority with lowest in the back, heavier isotopes have higher priority
enantiomers
nonsuperimposable mirror images
opposite configuration at all chiral centers
each enantiomer has optical rotation of equal magnitude, but different signs
all other properties are identical
optical activity
chiral molecules are optically active
d = (+) = dextrorotatory = clockwise
l = (-) = levorotatory = counterclockwise
this cannot be predicted by structure, must be measured by polarimeter
diastereomers
nonsuperimposable nonmirror images, always compares two
opposite configuration at least one chiral center, but not all
unrelated optical activity, different properties, can be physically separated
geometric isomers
subset of diastereomers, restriction in rotation about a double bond or ring
do not require chiral centers, different properties
cis (Z) vs. trans (E) compares the two highest priority groups
epimers
subset of diastereomers, differ only at one chiral center
anomers
subset of epimers that are sugars
meso compounds
subset of diastereomers, symmetrical chiral centers have opposite R/S
achiral, have internal plane of symmetry
chromatography vs. spectroscopy
chromatography is used to separate compounds (including distillation and resolution as well)
spectroscopy is used to analyze compounds (including MS and polarimetry)
chromatography
stationary phase supports the mixture and retains compounds
mobile phase carries mixture while traveling
two big questions:
- what's the best way to separate two compounds?
- what compound will be isolated first?
size-exclusion chromatography
porous beads, smaller compounds enter beads and elute last
separate proteins from peptide fragments
can be used to estimate molecular mass
exclusion limit- compounds with sizes too large will all pass through at the same time
longer column leads to better separation
TLC
stationary phase is polar (paper), so polar travels slower
mobile phase is solvent bath in development chamber, can be polar or non-polar
moves from down to up
retention factor (Rf) = spot distance/solvent distance
eluting strength- how strong the mobile phase is at pulling stuff away from the stationary phase, the more polar the solvent, the stronger its eluting strength
column chromatography
stationary phase is polar (silica gel), so polar elutes last
same as TLC, but upside down
move from up to down
HPLC
most common form is reverse phase, stationary phase is nonpolar, so nonpolar elutes last
moves from left to right
ion exchange chromatography
separates based on overall net charge, stationary phase is anionic/cationic resin, with counterions attached
mobile phase is buffer solution, keeps pH constant
cation exchange retains cations (positive charge)
need to be flushed out by high concentration of counterions or to change the pH of the solution
affinity chromatography
stationary phase is resin linked to antibody-binding protein
elute target protein with competitive antibody-binding protein
separate proteins from cell lysate or blood
metal ion affinity chromatography
stationary phase is resin linked to Ni2+ ions
mobile phase is protein tagged with his-tag, which binds Ni
elute target protein by changing pH
gas chromatography
separates based on boiling point
gas chromatogram- plot of intensity over time
3 pieces of information:
- number of compounds is number of peaks
- relative quantity of each compound is determined by area each peak
- higher BP compound (lower volatility) will elute later
distillation
separate large amounts based on boiling point
boiling chips- provide nucleation sites for bubbles to prevent superheating
two methods:
- simple distillation- remove impurities, difference in BP is > 30 C
- fractional distillation- separate diastereomers, difference in BP is < 30 C
vacuum distillation- lower Patm to lower boiling points
boiling point
higher BP molecules:
- are larger and heavier (more IMFs)
- have less branching (more compact)
determined generally by 3 intramolecular forces: H-bonds, dipole-dipole, Van der Waals
solubility in water
factors that determine solubility:
- polar dissolves in polar, nonpolar dissolves in nonpolar (organic)
- compounds with less than 5 carbons and a polar group are water soluble (exception, sugars are water soluble)
- charged functional groups are soluble in water
separatory funnel, extraction
aqueous layer and organic layer (contains compounds you want to separate, use an ether)
organic layer- ether is not very polar, so amides and other ethers will go there
aqueous layer- water is polar, so carboxylic acids, amines, and alcohols will be there
- to extract amine: add strong acid to protonate amine and turn into a salt to extract
- to extract carboxylic acid or phenol: add weak base to deprotonate carboxylic acid or phenol and turn into a salt to extract
add weak acid to extract strong base
add strong acid to extract weak base
add weak base to extract strong acid
add strong base to extract weak acid
resolution
enantiomers have same physical and chemical properties, separate racemic mixture
- add a chiral resolving agent (acid) to convert enantiomers to diastereomer salt
- separate with regular means (recrystallization, distillation)
- revert salt to enantiomers by adding a base
make sure they don't become meso compounds!
mass spectrometry
determine the molecular weight of compounds, determines elemental and isotopic composition
cannot separate isomers
UV/Vis spectroscopy
indicates presence of conjugated pi system
red shift- more resonance means more red
complementary wavelength- wavelength absorbed is opposite the one that is perceived (Christmas, Portal, Lakers)
red is 700 nm, violet is 300 nm
IR spectroscopy
IR light causes bonds to vibrate at different frequencies, shows which functional groups are present
peaks expressed as wavenumbers (cm-1)
does not show where the functional group is or how many, good for separating constitutional isomers but not stereoisomers
O-H is 3200-2600
C=O is 1700
C=C is 1600
CN/alkyne is 2100-2260
C-H appearance/disappearance does not help!
NMR spectroscopy
Hs are equivalent when there is free rotation or symmetry, each set of non-equivalent Hs give one NMR signal
splitting pattern indicate number of Hs on adjacent carbons, subtract one
multiplet for multiple splitting patterns
integration indicates number of equivalent Hs represented by peak, can be a multiple of the number of Hs
chemical shift is in ppm:
- EDG shifts right/upfield/low ppm (alkyl groups)
- EWG shifts left/downfield/high ppm (halogen, vinyl, aromatic, aldehyde, etc.)
common NMR peaks:
- carboxylic acid- 11 ppm
- aldehyde- 9 ppm
- aromatic- 7 ppm
- vinyl- 5 ppm
- alcohol/halide/ketone- 3 ppm
- alkyl- 1 ppm
SN1/SN2
SN1 involves carbocation formation which is the rate-limiting step, nucleophile can attack on either plane of sp2 C, so both enantiomers can form
SN2 involves backside attack, so inversion of configuration with pentavalent transition state
SN1 on 2/3 degree carbons only (carbocation stability!), SN2 on 0/1/2 degree carbons only
SN1 considerations:
- doesn't consider nucleophile size/conc. in rate law
- polar, protic solvent
- 2/3 degree carbons only
SN2 like polar, aprotic solvents: acetone, DMF, DMSO
alcohols and ethers undergo substitution only with an acid catalyst or conversion to a better leaving group (something resonance stabilized maybe)
tautomerism
keto form to enol form, goes through enolate, tautomers are constitutional isomers
keto form is more stable
since tautomerism occurs, adding D2O will cause deuterium exchange until all Hs are Ds, no signal on NMR, this shows that those Hs are acidic
carbonyl chemistry
carbonyl carbon is electrophilic
alpha hydrogen is acidic
resonance can occur
nucleophilic addition
weak nucleophile needs an acid catalyst to make the carbonyl C more electrophilic
hydride reduction (nucleophilic addition)
NaBH4 or LiAlH4 are reducing agents that donate an H to attack a carbonyl, reducing it to an alcohol
cannot produce tertiary alcohols
organometallic reagents (nucleophilic addition)
act as carbanions to attack a carbonyl, grinard reagent uses a halide with essentially a carbanion next to it
hemiacetals/acetals (nucleophilic addition)
alcohol is nucleophile, forms hemiacetal first (with one alcohol one ether group), then forms acetal (two ether groups)
acid catalyst for both steps so weak nucleophile can attack
imine/enamine formation (nucleophilic addition)
primary amine is nucleophile, reaction is similar to acetal formation, except instead of 2 attacks, carbonyl C is just double bonded to N to form imine
secondary amine is nucleophile, positive charge is left on N, so alpha H is eliminated to form an enamine
enamines are nucleophilic at their alpha-carbon, can do SN2 reaction to add something to the alpha position
adding H2O to enamine will reform carbonyl
aldol condensation (nucleophilic addition)
enolate reacts with ketone or aldehyde
SN2 reaction to add at alpha carbon of enolate
elimination to form double bond on newly attached ketone or aldehyde
enolates (kinetic vs. thermodynamic)
use a base on a ketone, which alpha proton will be taken to form which enolate?
kinetic enolate- forms faster, less steric hindrance for accessing the acidic H
you can select the kinetic enolate using a bulky base (LDA) and cold temperatures (-78 C)
thermodynamic enolate- forms more substituted double bond, more stable
select thermodynamic enolate using small base (MeOH) and high temperatures
carboxylic acids
3 quick points:
- highly polar, form many hydrogen bonds
- can be reduced to primary alcohol by LiAlH4
- reactions involve nucleophilic addition-elimination with a tetrahedral intermediate
esterification (ester formation)
- alcohol attacks carbonyl carbon on carboxylic acid
- proton transfer
- eliminate OH, form ester
a compound with both carboxylic acid and alcohol groups can form a cyclic ester if the ring is stable
saponification (ester hydrolysis)
- OH attacks ester
- ester piece is eliminated, nabs an H before leaving
- form carboxylate anion
hydrolysis
cyanohydrins are prone to hydrolysis
ATP -> ADP + P hydrolysis can either activate or inactivate the enzyme its bound to
pretty much everything can be broken down with hydrolysis
carboxylic acid derivatives
derivatives from most reactive to least:
- acid halide (add SOCl2 or PBr3 to carboxylic acid)
- acid anhydride (add another carboxylic acid)
- ester (add an alcohol)
- carboxylic acid
- amide (cannot be made, adding amine to carboxylic acid just does a little proton transfer lol)
Strecker amino acid synthesis
- imine formation from aldehyde and NH4Cl
- nucleophilic addition by KCN
- convert to carboxylic acid with acid
nonstereoselective!
simpler than gabriel synthesis
Gabriel malonic ester synthesis
- deprotonate phthalimide with KOH
- SN2- phthalimide attacks alpha carbon in malonic ester
- deprotonate center carbon and attack a new alkyl halide (which will have the desired R group)
- hydrolysis of esters and imide with acid
- decarboxylation with heat
nonstereoselective
charge on amino acids
when pH > pKa, group is more than 50% deprotonated
when pH > pI, the amino acid is net negative
- pI is the isoelectric point, zwitterion
- pI = the pH at which amino acid has no net charge
- average the two nearby pKas
pI help you determine amino acid separation in a gel
peptide bond formation
amide bond formed by amino group attacking carboxyl group and eliminating the OH
non-spontaneous process that needs enzymes!
characteristics of peptide bond:
- planar and cannot rotate!
- partial double bond character due to resonance
- amide H slightly acidic
peptide bond hydrolysis
also non-spontaneous process that needs proteases
denaturation
changes 3D structure without breaking peptide bonds
denaturing agents:
- high temperature
- high/low pH
- changing salt concentration
- urea
carbohydrate nomenclature
In Fischer projection (horizontal is out of page, vertical in into page, end OH is at the bottom):
OH on the right- D (+), penultimate carbon is R
OH on the left- L (-), penultimate carbon is S
penultimate carbon- second carbon from the end OH
anomeric carbon- carbonyl carbon, alpha/beta
carbohydrate cyclization (mutarotation)
OH on penultimate carbon attacks anomeric carbon
alpha anomer- OH on anomeric carbon is down
beta anomer- OH on anomeric carbon is up
these are epimers
Benedict's test, Tollen's test, silver deposition (mutarotation)
positive test (red, silver deposited) indicates reducing sugar present (hemiacetal/aldehyde present, all monosaccharides have hemiacetals)
negative test (no precipitate, no silver) indicates no reducing sugar (disaccharides usually have acetals)
maltose is a reducing sugar
sucrose is a nonreducing sugar
glycosidic linkage
acetals connecting two sugars
glycogen usually has an alpha-1,4-glycosidic bonds
but branching of glycogen occurs with alpha-1,6-glycosidic bonds
notation involves alpha/beta of left most sugar and carbon numbers
change in alpha/beta tells you an SN2 reaction occurred at the anomeric carbon
fatty acids
fat fun facts:
- triacylglycerol can be converted to glycerol and 3 fatty acids by saponification
- fatty acids are amphipathic, forming micelles in aqueous environment
- phospholipids have two hydrophobic tails with a hydrophilic head, form lipid bilayers
- saturated fatty acids pack better (form solids)
- unsaturated fatty acids increase fluidity (form liquids)